Abstract
A major limitation in lens gap junction research has been the lack of experimentally tractable ex vivo systems to study the formation and regulation of fiber-type gap junctions. Although immortalized lens-derived cell lines are amenable to both gene transfection and siRNA-mediated knockdown, to our knowledge none are capable of undergoing appreciable epithelial-to-fiber differentiation. Lens central epithelial explants have the converse limitation. A key advance in the field was the development of a primary embryonic chick lens cell culture system by Drs. Sue Menko and Ross Johnson. Unlike central epithelial explants, these cultures also include cells from the peripheral (preequatorial and equatorial) epithelium, which is the most physiologically relevant population for the study of fiber-type gap junction formation. We have modified the Menko/Johnson system and refer to our cultures as dissociated cell-derived monolayer cultures (DCDMLs). We culture DCDMLs without serum to mimic the avascular lens environment and on laminin, the major matrix component of the lens capsule. Here, I review the features of the DCDML system and how we have used it to study lens gap junctions and fiber cell differentiation. Our results demonstrate the power of DCDMLs to generate new findings germane to the mammalian lens and how these cultures can be exploited to conduct experiments that would be impossible, prohibitively expensive and/or difficult to interpret using transgenic animals in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preface
It is a pleasure and privilege to be invited to contribute to this special issue in honor of Ross Johnson. I have used this opportunity to write about the contribution that Ross has made to the gap junction field that has had the most direct impact on my career as a principal investigator.
In 1979, Willingham et al. 1979 reported that pp60src from avian sarcoma virus is concentrated at gap junctions in NRK cells. Sue Menko had studied v-src as a postdoctoral fellow with David Boettiger and approached Johnson about working in his laboratory to investigate the potential functional significance of its association with gap junctions. The logical system to conduct such studies was the lens because of the abundance of gap junctions between lens fiber cells. Unfortunately, at the time there was no good culture system that met the criteria required for Menko’s intended studies. During her stay in the Johnson lab, Menko developed a methodology to make primary epithelial cell cultures from E8-11 chick lenses. Although the choice of species was made in part because of practical considerations (fertilized eggs could be obtained at the University of Minnesota for free), in retrospect it was an excellent choice given that the chicken embryo has been one of the most useful and powerful systems in developmental biology (Stern 2005). In 1984, Menko and Johnson (Menko et al. 1984) published the initial characterization of this culture system, describing the remarkable extent to which these cells undergo the morphological and biochemical changes associated with lens epithelial-to-fiber cell differentiation in vivo. A subsequent paper (Menko et al. 1987) focused on gap junctions and showed, using both thin section and freeze-fracture electron microscopy, how closely these cultures recapitulate the in vivo process of fiber-type gap junction formation. I learned a variation of the Menko/Johnson prep from Dan Goodenough when I was a postdoctoral fellow in his laboratory and used these cultures to carry out the first study of the biosynthesis and posttranslational processing of a connexin (Cx43) in lens cells (Musil et al. 1990). In my own laboratory, we have modified this preparation, which we refer to as dissociated cell-derived monolayers (DCDMLs) to distinguish them from related, but functionally distinct, systems such as central epithelial explants and immortalized lens-derived cell lines. This article reviews key features of DCDMLs, summarizes what we have learned about the regulation and role of lens cell gap junctions and fiber differentiation using this system and discusses how these findings apply to the mammalian lens in vivo. Importantly, other lens researchers continue to use primary cultures of embryonic avian lens cells in their own work, with very recent examples from the labs of Drs. Jean Jiang (Liu et al. 2011) and Menko herself (Basu et al. 2012).
Introduction
Lens Structure and Development
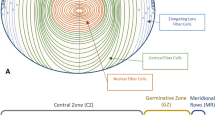
Let us begin with a brief primer on the lens. Although the time course and anatomical details differ between species, the general process by which the vertebrate lens develops is remarkably conserved between amphibians, birds and mammals including humans (reviewed by Piatigorsky 1981; Wride 1996; Robinson 2006). Following induction, the embryonic ectoderm overlying the optic vesicle thickens to form the lens placode. The lens placode invaginates and eventually pinches off as the lens vesicle, a hollow sphere of epithelial cells. The cells at the posterior of the lens vesicle then differentiate into the primary fiber cells, which elongate to fill the lumen of the lens vesicle. The cells at the anterior pole remain as a monolayer of undifferentiated epithelial cells. All subsequent growth of the lens is due to differentiation of epithelial cells into so-called secondary fiber cells and takes place at the border of the anterior and posterior faces of the organ in a region referred to as the lens equator (see Fig. 1 for a diagram of the lens). Secondary fiber differentiation is characterized by a dramatic increase in cell volume, extensive restructuring of the cell surface and cytosol and upregulation of fiber-specific proteins including crystallins and fiber connexins. Eventually, intracellular organelles are lost and synthesis of both DNA and protein ceases. Epithelial-to-(secondary) fiber differentiation continues throughout life, and lens cells do not turn over, resulting in the oldest and most differentiated fiber cells being located in the center (nuclear region) of the organ. The younger fiber cells that surround these mature fibers form the lens cortex and are referred to as cortical fiber cells. The lens is surrounded by a capsule of extracellular matrix that forms the thickest basement membrane in the body and is connected to the ciliary body via suspensory ligaments anchored to the lens capsule.
Schematic of the vertebrate lens, showing its orientation in the eye and identifying key features of lens anatomy. Lens epithelial cells differentiate into secondary fiber cells at the lens equator. Adapted from Garcia et al. (2005)
Lens Gap Junctions
The unique optical properties of the lens are due in part to the absence of blood vessels and to the extraordinarily tight cell-to-cell packing of the lens fibers. How, then, does this solid, ever-expanding mass of cells stay in ionic and metabolic homeostasis (and thus transparent) throughout a life span that can exceed 100 years? A major mechanism by which this is accomplished is an extensive network of gap junctional intercellular channels that physically and functionally link the cells of the lens (for review, see Goodenough 1992). Gap junctional intercellular coupling (GJIC) is much higher at the lens equator than at either pole (Baldo and Mathias 1992; Mathias et al. 1997). This pole-to-equator gradient in GJIC is believed to direct the overall pattern of current and solute flow in the lens, allowing metabolites and ions to be circulated between the peripheral (anterior epithelium and cortical fibers) and interior (nuclear region fibers) cell populations (Mathias et al. 1997; Donaldson et al. 2001). Others have suggested that the high level of GJIC at the lens equator is required to facilitate the uptake of essential substances (e.g., cysteine) into the organ (Sweeney et al. 2003).
In all species examined, lens epithelial cells express Cx43. During differentiation of epithelial cells to fibers at the lens equator, Cx43 disappears and two other connexins are upregulated, Cx46 and Cx50 in rodents and their avian orthologs Cx56 and Cx45.6 in the chick (Musil et al. 1990; Paul et al. 1991; White et al. 1992; Rup et al. 1993; Jiang et al. 1994). Mice with homozygous targeted deletions of either of the two fiber-type connexins (Gong et al. 1997; White et al. 1998) and humans with point mutations in these proteins (Shiels et al. 1998; Mackay et al. 1999) develop vision-destroying cataracts early in life (reviewed in Mathias et al. 2010).
The DCDML Prep
It is relatively easy to manually dissect out intact lenses from the eyes of E10 chick embryos. Any extralenticular tissue (e.g., suspensory ligaments, ciliary epithelium) is removed without destroying the capsular barrier by incubating the lenses in 0.08 % trypsin. The cleaned lenses are then broken by vigorous trituration into a single-cell suspension and filtered through three layers of lens paper to remove capsule material and any cell clumps. Mature nuclear and cortical fiber cells do not survive the dissociation process, leaving behind only less differentiated lens epithelial cells. These cells are then plated at 1.2 × 105 cells/well in 96-well tissue culture plates in M199 tissue culture medium plus BOTS (2.5 mg/ml BSA, 25 μg/ml ovotransferrin, 30 nM selenium) with penicillin G and streptomycin (Le and Musil 1998). The wells are precoated with laminin, the major component of the avian and mammalian lens capsule. As described by Menko et al. (1984), embryonic chick lens epithelial cells initially proliferate to form a flattened epithelial monolayer. Beginning at approximately 3–4 days of culture, discrete areas within these sheets differentiate into multilayered clusters of enlarged cells referred to as lentoids, which increase in number and size during the remaining culture period. Ultrastructural analysis by Menko et al. (1984, 1987) demonstrated that the cells contained in such lentoids have acquired many of the defining characteristics of fiber cells in the intact lens, including increased cell volume, loss of intracellular organelles and extensive formation of gap junctions. Thin-section electron micrographs of the lentoid cells closest to the substrate are indistinguishable from those obtained from the differentiated region of the embryonic lens, whereas the lentoid cells farthest from the plate closely resemble the elongating cells of the equatorial region. Lentoids also accumulate high levels of fiber-specific proteins including δ-crystallin, the beaded filament proteins CP49 and CP115 and aquaporin-0/MP28, the latter of which is expressed in vivo only by differentiating and differentiated primary and secondary lens fiber cells (Sas et al. 1985; Yancey et al. 1988).
Advantages of the DCDML System
-
(1)
DCDMLs consist of primary, unpassaged lens cells. Several investigators have succeeded in establishing permanent cell lines from mammalian lens epithelial cells (e.g., N/N1003A, αTN4, HLE B-3). Although some of these lines maintain features characteristic of lens epithelial cells in vivo and are valuable for certain types of studies (e.g., gene promoter analysis), to my knowledge none of them undergo appreciable epithelial-to-fiber differentiation as evidenced by their inability to synthesize more than nominal levels of β- and γ-crystallins and other fiber-specific proteins even when cultured under differentiation-promoting conditions (Krausz et al. 1996; Fleming et al. 1998; Wang-Su et al. 2003). As might therefore be expected, we have been unable to detect significant expression of fiber-type connexins in the lens cell lines we have tested.
-
(2)
DCDMLs consist of equatorial as well as central epithelial cells. It has been well documented that epithelial cells from the center of the anterior epithelial monolayer (i.e., those at the anterior pole of the organ) are biochemically, structurally and functionally distinct from more peripherally located populations (i.e., at or near the lens equator) (Ong et al. 2003). Notably, central epithelial cells require higher levels of growth factor to stimulate epithelial-to-fiber differentiation (Richardson et al. 1992, 1993) and do not express Cx46 (Rong et al. 2002). Because it is the cells at the lens equator that are the direct precursors of cortical fiber cells, it would follow that equatorial epithelial cells are the most physiologically relevant population in which to study fiber-type gap junction formation and fiber differentiation. DCDMLs contain cells from both the central and peripheral regions of the epithelial monolayer, with the latter predominating based on their greater abundance in vivo (Bassnett and Shi 2010) and on the number of cells in DCDMLs with early fiber-type features on day 1 of culture (e.g., low level expression of aquaporin-0/MP28) (Menko et al. 1984; Le and Musil 1998; Ong et al. 2003). In this regard, DCDMLs are very different from the other main primary lens cell culture system, central epithelial explants. Central epithelial explants are prepared by manually excising the intact central epithelial monolayer from the whole lens and consequently consist exclusively of cells from the region of the lens where fibers normally never form (Philpott and Coulombre 1968; Piatigorsky 1973). Central epithelial explants may therefore be best suited for investigations of the initiation of epithelial-to-fiber differentiation, whereas DCDMLs are a more appropriate system for the study of the subsequent steps in this process.
-
(3)
DCDMLs are cultured under serum-free, defined conditions. A key feature of the lens is the absence of blood vessels within the organ, a specialization essential for lens transparency. Lens cells produce their own survival factors (Ishizaki et al. 1993) and are stimulated to divide or to differentiate by factors in their local environment (e.g., either aqueous or vitreous humor). We therefore believe that culturing lens cells in more than trace amounts of serum (regardless of the source) can be considered nonphysiological/pathological. Moreover, exposing lens epithelial cells even temporarily to the myriad defined and undefined substances in serum inevitably confounds interpretation of experiments intended to study the response of these cells to individual growth factors. For these reasons, we culture DCDMLs in serum-free defined medium. In otherwise unsupplemented M199/BOTS, DCDMLs gradually increase the expression of fiber differentiation markers, although not to the extent obtained with vitreous humor or other differentiation-promoting factors (Le and Musil 1998). It is not yet clear whether this limited upregulation reflects initiation of fiber differentiation in response to autocrine/paracrine signaling by endogenously produced growth factors or is instead attributable to the time-dependent expression of fiber markers by a population of cells that became committed to fiber differentiation in vivo.
-
(4)
DCDMLs synthesize functional, fiber-type gap junctions. As demonstrated by Menko et al. (1984, 1987), using thin-section and freeze-fracture electron microscopy, lentoids in primary cultures of E9-11 chick lens cells form extensive (covering ~26 % of the lens cell membrane) gap junctions with fiber-type characteristics (e.g., loosely packed 9 nm particles on membrane P-faces). They also documented gap junction-mediated intercellular transfer of Lucifer yellow in both lentoid and monolayer epithelial cells. Later studies showed that these cultures synthesize, in addition to Cx43, the fiber cell connexins Cx45.6 (considered to be the ortholog of mammalian Cx50) and Cx56 (the ortholog of mammalian Cx46) (Le and Musil 1998; Berthoud et al. 1999; Jiang and Goodenough 1998). As in the lenses of all avian and mammalian species examined to date, Cx43 in DCDMLs is present at cell–cell interfaces throughout the epithelial monolayer. In contrast, Cx45.6 and Cx56 are most concentrated in lentoids, consistent with their accumulation in fiber cells in vivo. Based on the success of the chick system, several groups (including our own) have tried to develop a rodent lens culture system to study fiber-type gap junctions. Unfortunately, and for unknown reasons, these attempts have largely failed. Although dissociated cell-derived primary cultures of P21 rat lens cells form lentoid-like structures that express fiber cell markers such as crystallins and aquaporin-0, these otherwise differentiated lentoid cells rarely formed gap junctions and consequently did not mediate intercellular transfer of Lucifer yellow (FitzGerald and Goodenough 1986).
-
(5)
DCDMLs are amenable to many of the same tools and techniques routinely used to study gap junctions, signal transduction and cellular differentiation in mammalian systems. We have reported that transient transfection of DCDMLs is efficient (>70 %) and long-lasting (>6 days) (Boswell et al. 2009). Most signaling molecules relevant to the lens are highly evolutionarily conserved, thereby allowing mammalian forms to be functionally expressed in chick cells and antibodies raised against mammalian proteins to recognize their avian counterparts. Among the antibodies that were raised against mammalian signaling proteins that we routinely use to detect their chick orthologs are polyclonal and monoclonal reagents against total or phosphorylated forms of ERK, p38, AKT, GSK3β, MEK, Raf-1, JNK, FRS2, S6 kinase 1, Smad1/5/8, Smad2, Smad3 and CREB. Recently, we have developed techniques to carry out RNA interference in DCDMLs. Proof-of-principle experiments are shown in Fig. 2. DCDMLs were transfected on day 1 of culture with an EGFP-encoding plasmid in the presence of either a chemically synthesized 21-mer siRNA duplex (GFP-22 siRNA) (Castel et al. 2007) designed to silence EGFP (siGFP) or a mixture of four similarly sized scrambled (scr) siRNA duplexes. The anti-GFP siRNA very strongly knocked down expression of cotransfected EGFP, but not that of cotransfected LacZ, on day 3–6 of culture (Fig. 2a). We also conducted experiments in which DCDMLs were cotransfected on day 1 with a LacZ expression plasmid (pcDNA1.2/V5-GW/lacZ) and a plasmid (pENTR-GW/H1/TO-lacz2.1shRNA) encoding a shRNA (short hairpin RNA) designed to silence expression of LacZ, constructed using the Invitrogen (Carlsbad, CA) BLOCK-iT H1 RNAi entry vector kit (the RNA polymerase III promoter H1 effectively drives expression of shRNAs in primary chicken cells) (Yuan et al. 2006). Two to 5 days later, anti-LacZ immunostaining and immunoblotting (inset) demonstrated that expression of LacZ was much lower (by >85 %) in pENTR-GW/H1/TO-lacz2.1shRNA (shLacZ)-cotransfected cells than in cells cotransfected with an irrelevant pENTR-GW/H1/TO-based construct designed to silence human (but not chick) survivin (shSur) (Fig. 2b). All samples had the same protein content. Importantly, transfection of siRNA duplexes or shRNA-encoding plasmids into DCDMLs had no detectable effect on: (1) cell survival, morphology or proliferation; (2) basal or growth factor-induced expression of the fiber cell marker δ-crystallin or CP49; or (3) FGF-induced activation of ERK (not shown). We conclude that si- and shRNA-efficiently and specifically block expression of their target in DCDMLs under serum-free conditions, without any evidence of the toxic effects reported after electroporation of siRNAs into very early (<48 h) intact chick embryos (Mende et al. 2008). Using this methodology, we have also successfully and specifically knocked down the function of an endogenous protein in DCDMLs, the TGFβ-dependent transcription factor Smad3 (unpublished results).
Potential Limitations of the DCDML System
-
(1)
DCDMLs contain cells from the central as well as the peripheral regions of the lens epithelium. Because peripheral epithelial cells predominate in DCDML cultures, it is likely that central epithelial cells make only a small contribution to results obtained in most experimental paradigms. Nonetheless, it is conceivable that central epithelial cells could have a disproportionate influence on some aspects of DCDML culture behavior. In some cases, this possibility could be addressed by comparing results obtained in DCDMLs with those from experiments conducted in lens central epithelial explants. Methodology for the use of such explants for studies of GJIC has been developed by Tom White and colleagues (DeRosa et al. 2009).
-
(2)
DCDMLs are plated as a monolayer with a free apical surface. In vivo, the lens consists of concentric layers of cells, with the oldest, most differentiated fiber cells in the center. Thus, any aspects of lens cell biology that require the architecture of the intact lens cannot be recapitulated in DCDMLs. These cultures are therefore unlikely to be a good model system to study the final stages of fiber differentiation in which cortical fibers are transformed into mature (nuclear) fiber cells. Unfortunately, attempts to induce epithelial-to-fiber differentiation in cultured intact isolated lenses have failed (personal communication, Steve Bassnett, Washington University School of Medicine), eliminating them as an alternative system.
-
(3)
DCDMLs are derived from embryos. Although the processes of epithelial-to-fiber differentiation and fiber-type gap junction formation continue throughout life, it could in principle be argued that DCDMLs might behave differently from cells of postnatal origin.
-
(4)
DCDMLs are derived from chicken. Despite the similarities in their formation and function, the avian lens has at least one histological feature not present in mammals, the annular pad. This population of lens epithelial cells is located immediately anterior to the anatomic equator of the lens and is defined as “postmitotic cells committed to and undergoing initial stages of lens fiber formation” (Ireland and Mrock 2000). By these criteria, they are developmentally equivalent to other more differentiated epithelial cells in the equatorial region and would therefore be expected to have similar properties.
Most mammalian cells, including lens epithelial cells, synthesize both ERK1 and ERK2, whereas chick cells have only ERK2. FGF receptor 2 (FGFR2) is reportedly not expressed in chick lens, unlike in rodents (Walshe and Mason 2000). There could be concern that these species-specific differences in gene expression could have some bearing on lens cell differentiation and/or function. However, genetic ablation studies have shown that neither ERK1 nor FGFR2 appears to have a unique, essential role in mammalian lens formation that cannot be compensated for by coexpressed isoforms that are present in chick (i.e., ERK2, FGFR3 and/or FGFR1) (Reneker et al. 2007; Zhao et al. 2008).
Results
Role of Gap Junctions in Epithelial-to-Fiber Differentiation
In mammalian and avian lenses, both fiber-type gap junction formation and epithelial-to-secondary fiber differentiation take place at the lens equator. In other organs, gap junctions have been shown to play an important role in tissue development and differentiation, raising the possibility that this may also be the case in the lens. We examined the role of gap junctions in lens fiber formation in DCDMLs using βGA, one of several glycyrrhetinic acid derivatives that have been used to block gap junction permeability in cultured cells (Davidson et al. 1986; Goldberg et al. 1996; Guan et al. 1996). βGA has been shown (as were two other unrelated inhibitors of gap junction function) to inhibit cell–cell coupling and myogenesis of cultured rat L6 myoblasts and of primary embryonic chick myoblasts, illustrating the utility of this compound for addressing the role of gap junctions in differentiation processes in vitro (Mege et al. 1994; Proulx et al. 1997). As documented for other cell types, βGA rapidly (within 30 min) and continuously (if replaced every 2 days) suppressed gap junction-mediated intercellular transfer of Lucifer yellow and biocytin in DCDMLs without noticeable toxic effects (Le and Musil 1998). Inhibition of GJIC with βGA did not affect the expression of lens connexins or of molecules associated with other types of cell–cell junctions (N-cadherin, β-catenin, NCAM, aquaporin-0/MP28, ZO-1, occludin). Most importantly, there was also no effect on upregulation of markers of fiber differentiation (formation of aquaporin-0/MP28-positive lentoids and increased δ-crystallin synthesis). Gap junction blockade also failed to inhibit epithelial-to-fiber differentiation in E6 chick central epithelial explants as assessed by cell elongation, aquaporin-0/MP28 expression and δ-crystallin synthesis (Le and Musil 1998). This study provided the first evidence that secondary fiber formation is not dependent on the high level of GJIC characteristic of the lens equator. This concept has subsequently been supported by the phenotype of knockout mice in which expression of one, two or all three lens connexins has been eliminated (White et al. 1998, 2001; Xia et al. 2006). Upregulation of gap junctions at the lens equator may therefore primarily play a role in lens physiology.
Although proving that the massive upregulation of GJIC at the lens equator is not an absolute requirement for epithelial-to-fiber differentiation, our findings did not rule out the possibility that gap junctions could play a more subtle, secondary role in this process. Indeed, Rong et al. (2002) reported that fiber maturation is delayed in Cx50 knockout mice. A role for low levels of gap junctional coupling in facilitating optimal fiber differentiation would have been missed in our studies because βGA does not totally abolish all gap junction-mediated intercellular communication in DCDMLs, in keeping with results in nonlenticular cells (Martin et al. 1991; Goldberg et al. 1996).
Role of FGF in Epithelial-to-Fiber Differentiation
In addition to being the region in which fiber differentiation and GJIC are upregulated, the equator of the lens is where lens epithelial cells are first exposed to the high levels of FGF in the vitreous body (the vitreous body contains vitreous humor, a heparin sulfate–rich gel in which growth factors produced by other ocular tissues, especially the retina, accumulate). Over 20 years of research has led to the widely accepted concept that one or more FGFs play a central role in the early stages of epithelial-to-fiber differentiation in the mammalian lens and that the presumptive in vivo source of this FGF is the vitreous humor (reviewed by McAvoy et al. 1999; Lovicu and McAvoy 2005; Robinson 2006). FGFs have been purified from chick vitreous humor (Mascarelli et al. 1987), and avian lenses express FGFR1 and FGFR3 (Potts et al. 1993; Ohuchi et al. 1994). It is therefore remarkable that the prevailing view in the literature for over a decade had been that chick lens epithelial cells are unresponsive to FGF and are instead induced to differentiate by IGF-I or an IGF-like growth factor (Beebe et al. 1987; Caldes et al. 1991; Lang 1999). Using DCDMLs and central epithelial explants, we showed that chick lens cells in fact undergo differentiation when cultured in the presence of purified FGF1 or FGF2 for periods longer than the 5 h used in prior investigations (Le and Musil 2001a). Such longer-term treatments have been routinely used in studies with mammal-derived lens cultures and are physiologically relevant given that the lens is continuously exposed to growth factors in the ocular environment. We showed that a factor with the defining properties of an FGF is capable of diffusing out of the intact vitreous body and inducing fiber marker expression (Le and Musil 2001a). The importance of FGF in secondary fiber differentiation has largely been supported by the phenotype of mice in which FGF signaling has been altered at the lens equator by either dominant negative inhibition of FGF receptor function (Chow et al. 1995; Robinson et al. 1995; Stolen and Griep 2000; Govindarajan and Overbeek 2001), exogenous overexpression of various FGF isoforms (reviewed in Lovicu and Overbeek 1998) or conditional deletion of FGFR 1, 2 and 3 (Zhao et al. 2008). In contrast, overexpression of IGF-I in transgenic mice does not promote fiber differentiation (indeed, differentiation is delayed; Shirke et al. 2001) and lens defects have not been reported in IGF-I receptor knock-out animals. The in vivo significance of IGF-I in the lens remains unclear.
Role of FGF in Lens GJIC
As assessed by immunofluorescence microscopy, none of the known fiber connexins are markedly more concentrated throughout the equatorial axis of the lens than at the poles (Gruijters et al. 1987; Berthoud et al. 1994; Dahm et al. 1999). A study in human lens using freeze-fracture electron microscopy failed to reveal quantitative differences in gap junction channel content between equatorial region and polar fiber cells (Vrensen et al. 1992). There is therefore no compelling evidence that the estimated 14- to 335-fold increase in intercellular electrical conductance in the lens equatorial region (Baldo and Mathias 1992; Rae et al. 1996) is accompanied by a proportional increase in the number of channels assembled from either previously characterized or novel connexin species. Instead, the enhanced coupling at the equator appears to be due at least in part to greater flux through gap junctional channels in this region. The evolutionarily conserved response of lens cells to FGF and the high concentrations of this growth factor in vitreous humor led us to consider whether FGF might be involved in the upregulation of gap junctional function in the lens equatorial region thought to be essential for lens homeostasis and clarity (Le and Musil 2001b). We showed that FGF (either recombinant FGF 1 or 2 or purified from vitreous body-conditioned medium) upregulated gap junction-mediated intercellular communication in DCDMLs in a reversible manner that does not involve an increase in either connexin expression or gap junctional assembly. Upregulation of GJIC by FGF in DCDMLs occurs prior to, and is not a prerequisite for, fiber differentiation. Moveover, insulin and IGF-I, as potent as FGF at inducing lens cell differentiation, have no effect on gap junctional coupling in DCDMLs. These observations demonstrated that enhanced intercellular coupling in FGF-treated DCDML cultures is not a passive downstream consequence of increased fiber differentiation.
Which of the three connexin species expressed in DCDMLs (Cx43 and the fiber-type connexins Cx45.6 and Cx56) are functionally upregulated by FGF? Dong et al. (2006) reported that gap junction channels composed of Cx43, but not of Cx45.6, are permeable to the dye Alexa594 (Cx56 was not assessed). Unlike in fibroblastic cells expressing only Cx43, we found that cell-to-cell transfer of Alexa594 was very low in DCDMLs cultured either with or without purified FGF or vitreous body conditioned medium despite robust growth factor-stimulated intercellular transfer in the same cells of the less connexin type-specific, gap junction–permeant Lucifer yellow (Boswell et al. 2009). We concluded that Cx43 plays a minor role in GJIC in chick lens epithelial cells, in keeping with a report that most gap junction coupling in newborn mouse lens epithelium is due to Cx50 (the mammalian ortholog of Cx45.6) instead of Cx43 (White et al. 2007). FGF must therefore enhance GJIC in DCDMLs by acting on one or both fiber connexins. This is also likely to be true in the mammalian lens, given that FGF increases intercellular coupling mediated by Cx50 (Shakespeare et al. 2009; Cx46 was not assessed).
If FGF is responsible for upregulation of GJIC at the lens equator in vivo, then it would be expected that inhibiting FGF–FGFR interactions in the lens would disrupt the pole-to-equator gradient of cell coupling. Unfortunately, experimental manipulations that block FGF signaling in transgenic mice cause severe defects in lens development (including epithelial-to-fiber differentiation), thereby precluding a meaningful evaluation of gap junction function (Pan et al. 2006; Zhao et al. 2008).
Role of ERK in FGF Signaling in Lens GJIC and Fiber Differentiation
FGF is the main activator of the ERK MAP kinase in the lens in vivo (Govindarajan and Overbeek 2001; Zhao et al. 2008). At concentrations at which it upregulates GJIC and epithelial-to-fiber differentiation in DCDMLs and is able to diffuse out of the vitreous body, FGF induces sustained (≥24 h) activation of ERK as assessed by elevated levels of phospho-ERK. In contrast, FGF at the lower levels thought to be present in the aqueous humor activates ERK only transiently (<1 h) (Le and Musil 2001b). It has been well established in other cell types that one of the most important determinants of the biological outcome of MAP kinase signaling is the length of time that a stimulus activates ERKs (Marshall 1995). We therefore examined whether the duration of ERK phosphorylation played a role in the upregulation of GJIC in DCDMLs. In the first set of experiments, DCDMLs were exposed to GJIC-inducing levels of FGF for various periods of time before the addition of UO126, a highly specific inhibitor of the kinase immediately upstream of ERK in the MAPK cascade (MEK 1/2). In other experiments, DCDMLs were transiently transfected with a constitutively active form of MEK. Together, these studies revealed that sustained (≥12 h) activation of ERK is necessary for FGF to enhance gap junctional coupling in DCDMLs as well as sufficient to increase GJIC in the absence of FGF (Le and Musil 2001b).
The stimulatory effect of ERK on GJIC in DCDMLs was initially surprising given that lens epithelial cells express high levels of Cx43, a connexin that is phosphorylated and inactivated by ERK in other cell types (Hossain et al. 1998; Warn-Cramer et al. 1998; Zhou et al. 1999). This paradox was resolved when it was shown that lens cell coupling does not rely on Cx43 activity (see above) and is instead predominantly mediated by a fiber connexin whose channel function is apparently enhanced by FGF-stimulated ERK. Subsequently published studies by the White group (Shakespeare et al. 2009) reported that electrical coupling mediated by Cx50 (the mammalian ortholog of Cx45.6) between paired Xenopus oocytes is increased by coexpression of constitutively active MEK, whereas coupling by Cx46 (the ortholog of Cx56) is insensitive to ERK. In the same system, FGF enhanced GJIC mediated by Cx50 in an ERK-dependent manner. Although the mechanism by which ERK increases the function of Cx50/Cx45.6 is not known, it is not associated with an increase in connexin expression (Le and Musil 2001b; Shakespeare et al. 2009). It is also unlikely to involve direct modification by ERK, given that the avian, murine and human forms of Cx50 do not contain high-probability sites for ERK binding or phosphorylation (Obenauer et al. 2003; Shakespeare et al. 2009).
Studies with whole chick lenses demonstrated that FGF-induced activation of ERK is much higher in the equatorial region than in polar cortical fibers or in the lens core (Le and Musil 2001b), similar to the distribution of phospho-ERK reported in the mammalian lens (Lovicu and McAvoy 2001; Pan et al. 2010). These and additional results led to a novel model of the role of FGF in establishing the asymmetry in gap junctional coupling in the vertebrate lens believed to be required for lens clarity (Le and Musil 2001b): (1) cells in the central epithelium only have access to the low levels of FGF in the aqueous humor and consequently have relatively low levels of GJIC; (2) cells in the equatorial region of the lens respond to the FGF that diffuses out of the vitreous body by sustained activation of ERK and upregulation of GJIC, most likely at the level of gap junction channel gating; and (3) fiber cells at the lens posterior pole activate ERK in response to FGF only poorly, most likely because of downregulation of FGF receptors or of downstream signaling components.
Long-term activation of ERK by FGF was also shown to play a role in GJIC-independent processes in DCDMLs, including upregulation of expression of the beaded intermediate filament proteins CP49 and CP115. In contrast, synthesis of certain other markers of fiber cell differentiation such as crystallins is UO126-insensitive (Le and Musil 2001a). Similar studies conducted by others using rat central epithelial explants (Lovicu and McAvoy 2001) came to the same conclusion, demonstrating that the role of ERK in FGF-regulated gene expression in the lens is evolutionarily conserved. Genetic ablation of ERK1/2 signaling in the mouse lens blocks fiber differentiation and severely inhibits cell proliferation after E15, leading to lens degeneration and microphthalmia (Reneker 2008). Transgenic overexpression of constitutively active MEK in the lens also causes pleiotropic structural and developmental abnormalities that precluded an interpretable analysis of GJIC or epithelial-to-fiber differentiation in the postnatal lens (Gong et al. 2001).
Role of BMPs in Lens GJIC and Fiber Differentiation
First identified as inducers of ectopic bone formation, bone morphogenetic proteins (BMPs) have since been shown to be key regulators of the development and function of a wide variety of tissues and organs (reviewed by Whitman 1998). Both the ligands and their receptors are highly conserved across animal species. Although the components of the canonical FGF and BMP signaling pathways are distinct, cross-talk between the two classes of growth factors has been described in many systems. In the large majority of cases, FGF inhibits BMP signaling, regulating a myriad of processes including limb growth, neural induction and digit formation (Massague 2003). In contrast, we found that FGF and BMP positively cooperate in lens cells via a unique nonreciprocal interaction (Boswell et al. 2008a, 2008b; reviewed in Lovicu et al. 2011; Mathias et al. 2010). We found that the ability of FGF to upregulate gap junction-mediated dye coupling (Boswell et al. 2008a) or expression of markers of epithelial-to-fiber differentiation (Boswell et al. 2008b) is blocked by coincubation with the function-blocking anti-BMP2, -4 and -7 antibodies or with noggin, a highly specific protein antagonist of BMP2, -4 and -7 binding to BMP receptors (Fig. 3). This effect is attributable to inhibition of BMP4 and -7 produced by the lens cells themselves. Importantly, neither noggin nor anti-BMP antibodies induce cell death, block cell proliferation or prevent upregulation of GJIC or fiber marker expression by the nonphysiological activator fetal calf serum, demonstrating the specificity of their effect. In other studies, we showed that treating DCDMLs with relatively high levels of purified BMP2, -4 and -7 (≥4 ng/ml) upregulates GJIC (Boswell et al. 2008a) and fiber differentiation (Boswell et al. 2008b) in a process that does not require signaling from endogenously produced FGF. We are not aware of a precedent for this type of nonmutual interaction between FGF and BMP in any system. More recent work demonstrated that two types of mechanisms are operative. In the first, signaling from lens-derived BMPs is required to maintain lens cells in an optimally FGF-responsive state. In the second, FGF potentiates endogenous BMP signaling to a level approaching that obtained when BMP is added exogenously (Musil and Boswell 2010).
Inhibition of BMP signaling with noggin blocks FGF from enhancing expression of fiber differentiation markers and upregulating gap junction-mediated dye transfer. DCDMLs were cultured for 6 days (a, b) or 48 h (c) without additions (control), with 10 ng/ml BMP4 or with 15 ng/ml FGF2 in either the absence or the continuous presence of 0.5 μg/ml noggin. a Cells were labeled with [35S] methionine for 4 h, and SDS-solubilized lysates were analyzed by SDS-PAGE followed by phosphorimaging. b SDS-solubilized whole-cell lysates (2 μg protein/lane) were probed for CP49 by immunoblotting. c DCDMLs were assessed for their ability to mediate the intercellular transfer of the gap junction tracer Lucifer yellow using the scrape-loading/dye transfer assay. Each panel depicts a portion of the right half of the scrape/load wound. Although not shown, M r = 10 kDa rhodamine dextran remained confined to the cells at the wound edge into which dye had been directly introduced during the scrape-loading process
It has been well established that differentiation of the lens placode, as well as formation of primary lens fibers, requires BMPs (Furuta and Hogan 1998; Wawersik et al. 1999; Faber et al. 2002). Consequently, mice in which BMP signaling has been blocked at early stages of lens development are unsuitable to address the role of BMP in lens GJIC or in secondary fiber differentiation. We therefore used a previously generated strain of transgenic mice (OVE1196; Zhao et al. 2002) in which noggin is exogenously overexpressed under the control of a lens-specific promoter that becomes active after the formation of the lens placode and primary fibers. We found that these animals displayed a postnatal block of epithelial-to-fiber differentiation, with loss of the equatorial bow region and extension of the epithelial monolayer to the posterior pole of the organ (Boswell et al. 2008b). Morphological abnormalities were also obvious in other ocular tissues (e.g., ciliary body, absence of the vitreous body), likely due to the fact that noggin is a secreted protein and therefore has access to other organs. Because of these extralenticular effects, a direct cause-and-effect relationship between disruption of BMP signaling in the lens and inhibition of secondary fiber formation could not be drawn. Moreover, the rapid onset and severity of noggin-induced defects within and outside of the lens prevented a meaningful assessment of the role of lens-derived BMP in gap junctional coupling. These studies provide another example of the current limitations to the use of transgenic mice to study growth factor signaling in the lens. Although lacking the context of the whole animal, DCDMLs provide a system in which lens epithelial cells that developed within a wild-type eye can be manipulated in the absence of any confounding nonlenticular effects.
Conclusion
As illustrated by the aforementioned studies, results obtained using the DCDML system are consistent with (and, in several cases, predictive of) experimental findings acquired in the mammalian lens in vivo or with mammalian connexins expressed ex vivo. We conclude that DCDMLs are an appropriate and powerful system to study processes localized to the equatorial region of the lens in vivo, including epithelial-to-fiber differentiation and fiber-type gap junction formation, regulation and function. Importantly, there is no convincing justification for the concern that the embryonic or avian origins of DCDMLs preclude their use as a model system for the mammalian lens in vivo. In principle, the most direct test would be to compare results obtained in transgenic/conditional knock-out mice with those from comparably modified chickens. Unfortunately, current limitations in genetic engineering in poultry make such experiments untenable. The closest alternative would be to prepare dissociated cell-derived monolayer cultures from mice on day E16 (roughly equivalent to E10 in the chick). Given that an adult mouse lens contains ~40,000–50,000 epithelial cells (Bassnett and Shi 2010) and assuming that the number of epithelial cells in a mouse lens is proportional to lens diameter, one would have to harvest the lenses of ~400 mouse embryos to obtain as many cells as we do from eight dozen E10 eggs, even if the yield approached 100 %. The outlay of time and money required to generate such murine cultures on a weekly basis would exceed most NIH budgets (certainly mine). A potentially promising system is currently being developed by Ales Cvekl and colleagues (Yang et al. 2010). They reported that human embryonic stem cells can be differentiated in vitro to lens progenitor-like cells using a mixture of BMP4/7 and FGF2 and that these cells can then be induced to form lentoid bodies that express many of the key markers of fiber differentiation (e.g., CP49, CP115, aquaporin-0, β- and γ-crystallins). However, more than 28 days of culture was required for most of these proteins to become detectable by Western blot, and nonlens cell fates were also induced (mostly of neuroectodermal and mesodermal origin). We anticipate that DCDMLs will continue to be a useful system for the study of gap junctions and other fundamental aspects of lens cell biology for the foreseeable future.
References
Baldo GJ, Mathias RT (1992) Spatial variations in membrane properties in the intact rat lens. Biophys J 63:518–529
Bassnett S, Shi Y (2010) A method for determining cell number in the undisturbed epithelium of the mouse lens. Mol Vis 16:2294–2300
Basu S, Rajakaruna S, Menko AS (2012) Insulin-like growth factor receptor-1 and nuclear factor kappa B are crucial survival signals that regulate caspase-3 mediated lens epithelial cell differentiation initiation. J Biol Chem 287:8384–8397
Beebe DC, Silver MH, Belcher KS, Van Wyk JJ, Svoboda ME, Zelenka PS (1987) Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc Natl Acad Sci USA 84:2327–2330
Berthoud VM, Cook AJ, Beyer EC (1994) Characterization of the gap junction protein connexin56 in the chicken lens by immunofluorescence and immunoblotting. Invest Ophthalmol Vis Sci 35:4109–4117
Berthoud VM, Bassnett S, Beyer EC (1999) Cultured chicken embryo lens cells resemble differentiating fiber cells in vivo and contain two kinetic pools of connexin56. Exp Eye Res 68:475–484
Boswell BA, Lein PJ, Musil LS (2008a) Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell 19:2631–2641
Boswell BA, Overbeek PA, Musil LS (2008b) Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol 324:202–212
Boswell BA, Le AC, Musil LS (2009) Upregulation and maintenance of gap junctional communication in lens cells. Exp Eye Res 88:919–927
Caldes T, Alemany J, Robcis HL, de Pablo F (1991) Expression of insulin-like growth factor I in developing lens is compartmentalized. J Biol Chem 266:20786–20790
Castel D, Debily MA, Pitaval A, Gidrol X (2007) Cell microarray for functional exploration of genomes. Methods Mol Biol 381:375–384
Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA (1995) FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development 121:4383–4393
Dahm R, van Marle J, Prescott AR, Quinlan RA (1999) Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res 69:45–56
Davidson JS, Baumgarten IM, Harley EH (1986) Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun 134:29–36
DeRosa AM, Mese G, Li L, Sellitto C, Brink PR, Gong X, White TW (2009) The cataract causing Cx50-S50P mutant inhibits Cx43 and intercellular communication in the lens epithelium. Exp Cell Res 315:1063–1075
Donaldson P, Kistler J, Mathias RT (2001) Molecular solutions to mammalian lens transparency. News Physiol Sci 16:118–123
Dong L, Liu X, Li H, Vertel BM, Ebihara L (2006) Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol 576:787–799
Faber SC, Robinson ML, Makarenkova HP, Lang RA (2002) Bmp signaling is required for development of primary lens fiber cells. Development 129:3727–3737
FitzGerald PG, Goodenough DA (1986) Rat lens cultures: MIP expression and domains of intercellular coupling. Invest Ophthalmol Vis Sci 27:755–771
Fleming TP, Song Z, Andley UP (1998) Expression of growth control and differentiation genes in human lens epithelial cells with extended life span. Invest Ophthalmol Vis Sci 39:1387–1398
Furuta Y, Hogan BL (1998) BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12:3764–3775
Garcia CM, Yu K, Zhao H, Ashery-Padan R, Ornitz DM, Robinson ML, Beebe DC (2005) Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn 233:516–527
Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RR, MacPhee DJ, Zhang YC, Naus CC (1996) Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res 222:48–53
Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB (1997) Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91:833–843
Gong X, Wang X, Han J, Niesman I, Huang Q, Horwitz J (2001) Development of cataractous macrophthalmia in mice expressing an active MEK1 in the lens. Invest Ophthalmol Vis Sci 42:539–548
Goodenough DA (1992) The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol 3:49–58
Govindarajan V, Overbeek PA (2001) Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development 128:1617–1627
Gruijters WT, Kistler J, Bullivant S (1987) Formation, distribution and dissociation of intercellular junctions in the lens. J Cell Sci 88:351–359
Guan X, Wilson S, Schlender KK, Ruch RJ (1996) Gap-junction disassembly and connexin 43 dephosphorylation induced by 18 beta-glycyrrhetinic acid. Mol Carcinog 16:157–164
Hossain MZ, Ao P, Boynton AL (1998) Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. J Cell Physiol 176:332–341
Ireland ME, Mrock LK (2000) Differentiation of chick lens epithelial cells: involvement of the epidermal growth factor receptor and endogenous ligand. Invest Ophthalmol Vis Sci 41:183–190
Ishizaki Y, Voyvodic JT, Burne JF, Raff MC (1993) Control of lens epithelial cell survival. J Cell Biol 121:899–908
Jiang JX, Goodenough DA (1998) Phosphorylation of lens-fiber connexins in lens organ cultures. Eur J Biochem 255:37–44
Jiang JX, White TW, Goodenough DA, Paul DL (1994) Molecular cloning and functional characterization of chick lens fiber connexin 45.6. Mol Biol Cell 5:363–373
Krausz E, Augusteyn RC, Quinlan RA, Reddan JR, Russell P, Sax CM, Graw J (1996) Expression of crystallins, Pax6, filensin, CP49, MIP, and MP20 in lens-derived cell lines. Invest Ophthalmol Vis Sci 37:2120–2128
Lang RA (1999) Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci 40:3075–3078
Le AC, Musil LS (1998) Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol 204:80–96
Le AC, Musil LS (2001a) FGF signaling in chick lens development. Dev Biol 233:394–411
Le AC, Musil LS (2001b) A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol 154:197–216
Liu J, Ek Vitorin JF, Weintraub ST, Gu S, Shi Q, Burt JM, Jiang JX (2011) Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. J Biol Chem 286:16914–16928
Lovicu FJ, McAvoy JW (2001) FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development 128:5075–5084
Lovicu FJ, McAvoy JW (2005) Growth factor regulation of lens development. Dev Biol 280:1–14
Lovicu FJ, Overbeek PA (1998) Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 125:3365–3377
Lovicu FJ, McAvoy JW, de Iongh RU (2011) Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci 366:1204–1218
Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S (1999) Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 64:1357–1364
Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185
Martin W, Zempel G, Hulser D, Willecke K (1991) Growth inhibition of oncogene-transformed rat fibroblasts by cocultured normal cells: relevance of metabolic cooperation mediated by gap junctions. Cancer Res 51:5348–5351
Mascarelli F, Raulais D, Counis MF, Courtois Y (1987) Characterization of acidic and basic fibroblast growth factors in brain, retina and vitreous chick embryo. Biochem Biophys Res Commun 146:478–486
Massague J (2003) Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev 17:2993–2997
Mathias RT, Rae JL, Baldo GJ (1997) Physiological properties of the normal lens. Physiol Rev 77:21–50
Mathias RT, White TW, Gong X (2010) Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev 90:179–206
McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ (1999) Lens development. Eye 13:425–437
Mege RM, Goudou D, Giaume C, Nicolet M, Rieger F (1994) Is intercellular communication via gap junctions required for myoblast fusion? Cell Adhes Commun 2:329–343
Mende M, Christophorou NA, Streit A (2008) Specific and effective gene knock-down in early chick embryos using morpholinos but not pRFPRNAi vectors. Mech Dev 125:947–962
Menko AS, Klukas KA, Johnson RG (1984) Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol 103:129–141
Menko AS, Klukas KA, Liu TF, Quade B, Sas DF, Preus DM, Johnson RG (1987) Junctions between lens cells in differentiating cultures: structure, formation, intercellular permeability, and junctional protein expression. Dev Biol 123:307–320
Musil LS, Boswell BA (2010) Molecular mechanism of cross-talk between FGFs and BMPs in lens cell differentiation. Invest Ophthalmol Vis Sci 51; abstract 1213
Musil LS, Beyer EC, Goodenough DA (1990) Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 116:163–175
Obenauer JC, Cantley LC, Yaffe MB (2003) Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31:3635–3641
Ohuchi H, Koyama E, Myokai F, Nohno T, Shiraga F, Matsuo T, Matsuo N, Taniguchi S, Noji S (1994) Expression patterns of two fibroblast growth factor receptor genes during early chick eye development. Exp Eye Res 58:649–658
Ong MD, Payne DM, Garner MH (2003) Differential protein expression in lens epithelial whole-mounts and lens epithelial cell cultures. Exp Eye Res 77:35–49
Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X (2006) Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133:4933–4944
Pan Y, Carbe C, Powers A, Feng GS, Zhang X (2010) Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development 137:1085–1093
Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA (1991) Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115:1077–1089
Philpott GW, Coulombre AJ (1968) Cytodifferentiation of precultured embryonic chick lens epithelial cells in vitro and in vivo. Exp Cell Res 52:140–146
Piatigorsky J (1973) Insulin initiation of lens fiber differentiation in culture: elongation of embryonic lens epithelial cells. Dev Biol 30:214–216
Piatigorsky J (1981) Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19:134–153
Potts JD, Harocopos GJ, Beebe DC (1993) Identification of receptor tyrosine kinases in the embryonic chicken lens. Curr Eye Res 12:759–763
Proulx A, Merrifield PA, Naus CC (1997) Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet 20:133–144
Rae JL, Bartling C, Rae J, Mathias RT (1996) Dye transfer between cells of the lens. J Membr Biol 150:89–103
Reneker LW (2008) ERK1/2 signaling is required for epithelial-to-fiber differentiation in lens development. Invest Ophthalmol Vis Sci 49; abstract 1135
Reneker LW, Robinson ML, Ogata M, Sanjo H, Pages G (2007) The role of ERK mitogen-activated protein kinase (MAPK) in lens development. Invest Ophthalmol Vis Sci 48; abstract 1119
Richardson NA, McAvoy JW, Chamberlain CG (1992) Age of rats affects response of lens epithelial explants to fibroblast growth factor. Exp Eye Res 55:649–656
Richardson NA, Chamberlain CG, McAvoy JW (1993) IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest Ophthalmol Vis Sci 34:3303–3312
Robinson ML (2006) An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol 17:726–740
Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA (1995) Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development 121:3959–3967
Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X (2002) Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development 129:167–174
Rup DM, Veenstra RD, Wang HZ, Brink PR, Beyer EC (1993) Chick connexin-56, a novel lens gap junction protein. Molecular cloning and functional expression. J Biol Chem 268:706–712
Sas DF, Sas MJ, Johnson KR, Menko AS, Johnson RG (1985) Junctions between lens fiber cells are labeled with a monoclonal antibody shown to be specific for MP26. J Cell Biol 100:216–225
Shakespeare TI, Sellitto C, Li L, Rubinos C, Gong X, Srinivas M, White TW (2009) Interaction between connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol Biol Cell 20:2582–2592
Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S (1998) A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62:526–532
Shirke S, Faber SC, Hallem E, Makarenkova HP, Robinson ML, Overbeek PA, Lang RA (2001) Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech Dev 101:167–174
Stern CD (2005) The chick: a great model system becomes even greater. Dev Cell 8:9–17
Stolen CM, Griep AE (2000) Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev Biol 217:205–220
Sweeney MH, Garland DL, Truscott RJ (2003) Movement of cysteine in intact monkey lenses: the major site of entry is the germinative region. Exp Eye Res 77:245–251
Vrensen G, Van Marle J, Van Veen H, Willekens B (1992) Membrane architecture as a function of lens fibre maturation: a freeze fracture and scanning electron microscopic study in the human lens. Exp Eye Res 54:433–446
Walshe J, Mason I (2000) Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech Dev 90:103–110
Wang-Su ST, McCormack AL, Yang S, Hosler MR, Mixon A, Riviere MA, Wilmarth PA, Andley UP, Garland D, Li H, David LL, Wagner BJ (2003) Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci 44:4829–4836
Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF (1998) Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem 273:9188–9196
Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R (1999) BMP7 acts in murine lens placode development. Dev Biol 207:176–188
White TW, Bruzzone R, Goodenough DA, Paul DL (1992) Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell 3:711–720
White TW, Goodenough DA, Paul DL (1998) Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143:815–825
White TW, Sellitto C, Paul DL, Goodenough DA (2001) Prenatal lens development in connexin43 and connexin50 double knockout mice. Invest Ophthalmol Vis Sci 42:2916–2923
White TW, Gao Y, Li L, Sellitto C, Srinivas M (2007) Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest Ophthalmol Vis Sci 48:5630–5637
Whitman M (1998) Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev 12:2445–2462
Willingham MC, Jay G, Pastan I (1979) Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell 18:125–134
Wride MA (1996) Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation 61:77–93
Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X (2006) Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res 83:688–696
Yancey SB, Koh K, Chung J, Revel JP (1988) Expression of the gene for main intrinsic polypeptide (MIP): separate spatial distributions of MIP and beta-crystallin gene transcripts in rat lens development. J Cell Biol 106:705–714
Yang C, Yang Y, Brennan L, Bouhassira EE, Kantorow M, Cvekl A (2010) Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J 24:3274–3283
Yuan J, Wang X, Zhang Y, Hu X, Fei J, Li N (2006) Mammalian PolIII promoter H1 can transcribe shRNA inducing RNAi in chicken cells. Mol Biol Rep 33:33–41
Zhao S, Chen Q, Hung FC, Overbeek PA (2002) BMP signaling is required for development of the ciliary body. Development 129:4435–4442
Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML (2008) Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol 318:276–288
Zhou L, Kasperek EM, Nicholson BJ (1999) Dissection of the molecular basis of pp 60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol 144:1033–1045
Acknowledgements
This work was supported by grant R01 2EY014622 from the National Eye Institute (to L.S.M.). I thank Ross Johnson for generously providing antibodies to lens proteins and for many interesting and helpful discussions over the years.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musil, L.S. Primary Cultures of Embryonic Chick Lens Cells as a Model System to Study Lens Gap Junctions and Fiber Cell Differentiation. J Membrane Biol 245, 357–368 (2012). https://doi.org/10.1007/s00232-012-9458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9458-y