Abstract
The epithelium of the vertebrate lens plays a critical role in tissue homeostasis and maintenance of lens clarity. The epithelium is the most metabolically active region of the lens and contains all the mitotically active cells. Cell division in the epithelium occurs exclusively in the germinative zone, a swathe of cells encircling the lens just above the equator. Fibroblast growth factor, a molecule with a demonstrated role in lens fiber cell differentiation, may promote epithelial cell division although other growth factors likely contribute. The organization of cells within the lens epithelium has often been likened to a cobblestone pattern. However, recent three-dimensional imaging studies have revealed that individual epithelial cells have a complex, polarized anatomy, with morphologically distinct apical and basolateral domains. The apical membrane is delineated by a hybrid junctional complex consisting of adherens junctions and tight junctions. Adherens junctions play a critical role in epithelial organization and loss of nectins or cadherins, two core components of adherens junctions, has catastrophic consequences for lens organization and transparency. Tight junctions, the apical-most junctional element, restrict the paracellular flow of ions into the lens but also serve as scaffolds for an assemblage of important polarity proteins. Targeted disruption of the partitioning defective (Par) family of polarity proteins results in loss of apical cell junctions and promotes epithelial-to-mesenchymal transition.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The anterior hemisphere of the vertebrate lens is covered by a continuous monolayer of cells—the lens epithelium. In adult mice, the epithelium consists of approximately 40,000 cells [1]. The epithelium of the much larger human lens is believed to contain more than ten times that number [2]. Histologically, the lens epithelium is classified as a simple epithelium (i.e., every cell is in direct contact with the overlying basement membrane and extends across the full thickness of the epithelial layer). In the young lens, the epithelial cells are generally described as cuboidal (meaning that they are about as high as they are wide). Later in life, when in many species the epithelium is thinner and the cells less densely packed, the lens epithelium might be better described as a simple squamous epithelium.
The cellular organization of the vertebrate lens has been the subject of many investigations over the past 100 years or more. Only recently, however, have techniques been developed that allow the morphology of living lens cells to be visualized. One such approach is the induced expression of fluorescent proteins in individual lens cells. Expressing cells can be optically isolated from their nonfluorescent neighbors by confocal microscopy. Their structure can then be imaged at high resolution and in three dimensions [3–5]. Applied to cells in the epithelium, this methodology has provided a new conception of lens cell organization. Viewed in situ, living lens epithelial cells are shown to have a surprisingly complex and dynamic morphology (Fig. 2.1a). Although the apical membranes of the epithelial cells are polygonal in shape, their basolateral membranes are highly folded and irregular. Numerous lamellipodia-like processes extend from the cell body. In the lens literature, the epithelium is commonly described as having a “cobblestone” appearance and individual cells as being polygonal in shape. The discrepancy between this description and the morphology of cells as revealed in fluorescent labeling studies (Fig. 2.1a) arises because the junctional complex at the apicolateral border of the cells (see below) is particularly prominent under conventional phase contrast microscopy. Since the junctional complex delineates the shape of the (largely polygonal) apical membrane, conventional microscopy gives the impression that epithelial cells have a more regular structure than is in fact the case.
Morphology of individual mouse lens cells as revealed by expression of green fluorescent protein (GFP). Epithelial cells (a, viewed from the capsular surface) have a complex and irregular morphology. At the lens equator, epithelial cells differentiate into fiber cells (b). The differentiation process involves a radical restructuring of cellular morphology. Fiber cells are physically interlinked via a system of tooth-like membrane processes protruding from the lateral surfaces. The cells are too long to visualize in their entirety at high magnification. At the scale shown, the cell shown in b would extend for several meters in either direction
At the edge of the epithelium, near the lens equator, epithelial cells terminally differentiate into fiber cells. Fiber cells account for almost all of the lens volume and, as their name implies, have a highly elongated form (Fig. 2.1b), quite unlike the epithelial cells from which they are derived. During the initial stages of terminal differentiation, the epithelial progenitor is first reshaped into a flat, ribbonlike cell [4]. As differentiation proceeds, cell length and volume increase and the lateral membranes of the fiber cells are extensively remodeled (Fig. 2.1b).
Although only accounting for a small fraction of the lens volume, the epithelial layer contains all the mitotically active cells in the tissue and can, therefore, be thought of as the “growth engine” of the lens. Cell division within the epithelium ultimately provides both the fiber cells to fill the tissue volume and the additional epithelial cells necessary to cover its expanding surface. The mitotic index varies with latitudinal position. Cells in the central region of the epithelium have a relatively low mitotic index. This area lies within the pupillary space and is therefore exposed directly to light. Considerably higher rates of cell division are observed in the peripheral lens epithelium. In this location, the proliferating cells are sequestered in the shadow of the iris and thus protected from potentially mutagenic exposure to ultraviolet radiation [6].
As with all epithelia, the lens epithelium has an intimate association with its basement membrane. The basement membrane of the lens is called the lens capsule and completely envelopes the tissue. The lens capsule is synthesized by the epithelial cells themselves [7] and its detailed composition and properties will be discussed elsewhere in this volume. Here, we note merely that the capsule is particularly enriched in type IV collagen and contains components such as laminin and fibronectin that are common constituents of basement membranes throughout the body. Although its biochemical composition is not remarkable, the lens capsule is one of the thickest basement membranes in the body. A typical basement membrane might be 0.1 μm thick [8]. In contrast, the human lens capsule is approximately 10 μm thick [9]. Perhaps surprisingly, the lens capsule in mice is even thicker [10]. The elastic lens capsule helps mold the lens substance at rest and during accommodation. Near the equator, the capsule also serves as the anchor point for the ciliary zonule (Fig. 2.2), the rigging of fibrillin-rich fibrils that connects the lens to the adjacent ciliary body and thus centers the lens in the eye [11]. The zonular fibers insert into the lens capsule and in accommodating species, such as humans, transmit the forces that flatten the lens when the eye focuses on distant objects.
Attachment of the ciliary zonule (green, visualized with anti-Magp1) at the lens equator. The arrangement of lens epithelial nuclei is visualized with Draq5 staining (magenta). On the left-hand side of the figure, cell nuclei are aligned in rows, signifying the onset of fiber cell differentiation. Mitotic figures are visible among the interphase epithelial nuclei
2 Junctional Organization and Polarity in the Lens Epithelium
In common with all simple epithelia, the lens epithelium is highly polarized. As a result, the plasma membrane is partitioned into distinct apical and basolateral domains (asymmetry in the plane of the epithelium, so-called planar cell polarity, will be dealt with elsewhere). Because the lens epithelium arises from an invagination of the embryonic head ectoderm, epithelial cells are oriented inwards; their apical membranes apposed to the apical membranes of the underlying fiber cells. The highly folded basal membranes of the epithelial cells are in direct contact with the overlying capsule. The presence of an elaborate junctional complex at the apicolateral border of the cells allows the distinct compositions of the apical and basolateral membranes to be established and maintained. Examples of membrane proteins that show a segregated distribution in lens epithelial cell plasma membranes include ZO-1, which is restricted to the apical membrane domain [12, 13], and Cadm1, which is localized exclusively to the basolateral domain [14].
Contact points between neighboring cells in simple epithelia usually contain three distinct types of adhesive structures that can be recognized at the ultrastructural level. Proceeding from the apical to the basal surface these are tight junctions (zonula occludens), cadherin-based adhering junctions (zonula adherens), and desmosomes (macula adherens).
Desmosomes, which impart tensile strength to epithelial sheets, are only infrequently observed in the lens epithelium [15], although they may be somewhat more common in the lenses of primates than in those of other species [16].
Transmission electron microcopy reveals that adhering junctions (AJs) are numerous between lens epithelial cells (Fig. 2.3) [17]. AJs are also found between epithelial cells and the underlying fiber cells [16], particularly near the edges of the epithelium [18]. The transmembrane adhesive core of AJs consists of a mosaic of clustered cadherin and nectin molecules [19]. Lens epithelial cells express both epithelial cadherin (E-cadherin; Cdh1) [20] and neuronal cadherin (N-cadherin; Cdh2) [21]. E-cadherin and N-cadherin are single-pass membrane proteins with five extracellular cadherin domains and are thus defined as “classical” cadherins. E-cadherin, as its name implies, is widely expressed in epithelial tissues. In the lens, its expression is restricted to the epithelium, the fiber cell membrane proteome containing only trace levels of E-cadherin [22]. The presence of N-cadherin in the lens epithelium is less expected. Elsewhere in the body, N-cadherin expression is restricted largely to neuronal cell types, the lens being one of the rare epithelial tissues to express this membrane protein [23]. Cadherins mediate calcium-dependent cell-cell adhesion through trans-cadherin interactions between neighboring cells. Conditional deletion of either E- or N-cadherin in the lens leads to profound cellular disruption, testifying to the key roles that AJs play in maintenance of the epithelial phenotype [24].
Transmission electron micrograph of adhering junctions (AJs) between epithelial cells (filled arrow), between epithelial and fiber cells (arrowhead), and between fiber cells (open arrow) in the chicken lens. A band of actin microfilaments (asterisk) is associated with the fiber cell AJ. EC epithelial cell, FC fiber cell. Image courtesy of Ken Lo
The cytoplasmic domain of cadherins contains a juxtamembrane region and a C-terminal, catenin-binding domain [25]. The juxtamembrane region binds p120 catenin (and associated proteins), while the catenin-binding domain interacts with β-catenin and γ-catenin (plakoglobin). p120 is thought to regulate cadherin stability. The pool of AJ-associated β-catenin exists in equilibrium with cytosolic, membrane, and nuclear pools, reflecting the multifunctional nature of the β-catenin protein. β-Catenin interacts with α-catenin, an obligate component of AJs. It seems likely that AJs, which are invariably positioned adjacent to the circumferential actin belt (see Fig. 2.3), are physically connected to the actin cytoskeleton. Such a link would generate a transcellular actin network, allowing mechanical forces to be distributed across the epithelial sheet as a whole. Because it is a bona fide actin-binding protein in vitro, it has long been suggested that α-catenin represents the physical linkage between cadherins in AJs and the actin cytoskeleton. However, the precise role of α-catenin remains controversial [26]. In addition to its role in regulating cell adhesion, β-catenin also functions as a transcriptional co-activator in the canonical Wnt signaling pathway [27]. The role of β-catenin in the lens has been examined in mice by conditionally disrupting the gene using the Cre-lox approach [28]. Interestingly, specific disruption of the β-catenin (Ctnb1) locus in lens fiber cells has comparatively little effect on the lens. In contrast, when Ctnb1 is disrupted simultaneously in epithelial and fiber cells, the lens is profoundly disturbed, with loss of E-cadherin and disturbed apical/basal epithelial polarity. This observation underlines the importance of β-catenin in maintenance of the lens epithelial phenotype.
The second key group of adhesive proteins at AJs is the nectins. Nectins are members of the immunoglobulin superfamily and, in contrast to cadherins, mediate calcium-independent cell adhesion. Unlike the cadherins (which undergo only homophilic interactions in trans), nectins undergo both trans-homophilic and trans-heterophilic interactions at AJs. There are four members of the nectin family (nectin-1–4; encoded in humans by PVRL1–4), each of which have three extracellular domains, a transmembrane segment, and a cytoplasmic C-terminal domain that interacts with the PDZ domain of the scaffolding protein afadin. Afadin is a large, f-actin-binding protein that connects nectins to the actin cytoskeleton. The nectin–afadin complex is the first to be assembled at initial intercellular contact points and may help recruit cadherins to maturing AJs [29]. Both nectins and afadin are present in lens epithelial cell AJs [30]. Microarray analysis suggests that PVRL3 (which encodes nectin-3) is the most abundantly expressed of the nectin genes in the eye [23]. In humans, mutations in PVRL3 result in severe congenital cataracts [30]. Moreover, mice with targeted or spontaneous disruptions of the Pvrl3 locus exhibit multiple lens defects. These observations underscore the importance of nectin-3 in lens morphogenesis and homeostasis.
AJs are the first elements of the epithelial junctional complex to be assembled at sites of intercellular contact. Their assembly triggers the activation of polarity complexes (see below) that help establish the asymmetric compositions of the apical and basolateral membranes. Surprisingly, the polarized organization of cytoplasmic structures, including the nucleus, Golgi apparatus, and centrosome, also appear to depend on AJ formation [31].
Tight junctions (TJs) are the apical-most components of the classical tripartite epithelial junctional complex. By transmission electron microscopy, TJs appear as close membrane appositions or “kissing points” between neighboring cells. Viewed in three dimensions, TJs appear as belt-like structures that extend in an anastomosing network around each cell, connecting cells to their neighbors and forming a tight seal across the extracellular space. Thus, the central role of TJs is to regulate paracellular permeability (diffusion, via the intercellular space from one side of the epithelium to the other). In addition to this barrier function, TJs have also long been thought to act as a “fence,” preventing the intermingling of apical and basolateral components in the plane of the membrane, although recent evidence has cast some doubt on this latter role [32]. A great many proteins have been localized to the TJ, but the backbone of the TJ appears to be composed of claudins, a family of intrinsic membrane proteins. Hydropathy plots indicate that claudins have four transmembrane helices. Claudins interact with other claudins in the same cell through their N-terminal extracellular loops (cis-interactions) and with claudins in adjacent cells through their C-terminal extracellular loops (trans-interactions) [33]. Significantly, transfection of claudins into fibroblasts (a cell type that normally does not form TJs) is sufficient to trigger the formation of a network of TJ-like strands [34]. There are a number of other transmembrane protein components at TJs but these have less well-defined roles. This group includes occludin, tricellulin, JAM (junction adhesion molecule), and CAR (coxsackievirus and adenovirus receptor). At the cytoplasmic face of TJs, scaffolding proteins congregate, including ZO-1, ZO-2, and ZO-3.
For many years the existence of TJs in the lens epithelium was a contentious issue. Early freeze-fracture experiments gave conflicting results. The anastomosing strands indicative of TJs in freeze-fracture replicas were seen in some lens epithelia [35] but not others [36]. However, the use of electron dense tracers has since helped confirm the existence of TJs at the apicolateral border of lens epithelial cells [35]. Claudin-1 and occludin are expressed in lens epithelial cells [37]. Jam-1 is present in the lens, but mice deficient in F11r (the gene encoding Jam-1) do not have a lens phenotype [38]. In contrast, in humans, frameshift mutations in JAM-3 result in intracranial hemorrhage and congenital cataracts [39]. Similarly, in mice, disruption of the Jam-3 locus results in nuclear cataracts and other ocular pathology [40]. These findings are consistent with proteomic studies which show that Jam-3 is expressed particularly strongly in the lens [22].
Much of what is known about the establishment and maintenance of epithelial cell apical–basal polarity has come from studies of spindle orientation in Drosophila neuroblasts [41]. Remarkably, the protein complexes that determine the polarized distribution of cell fate determinants in dividing Drosophila neuroblasts are highly conserved and play equally pivotal roles in epithelial morphogenesis in vertebrates. Three distinct assemblages of polarity proteins are believed to control epithelial apical–basal polarity. These are the Crumbs complex (consisting of Crumbs/Pals1/Patj), the partitioning defective (Par) complex (consisting of Par3/Par6/atypical protein kinase C (aPKC)/Cdc42), and the Scribble complex (consisting of Scrib/Dlg/Lgl). Crumbs is localized to the apical side of the AJ and is required for its formation. Par is associated with the tight junction. Together Par and Crumbs help specify the apical membrane domain. Scribble localizes to and helps define the basolateral membrane. In general, the complexes function to promote the establishment and expansion of the membrane domains with which they are associated.
The expression of polarity proteins in the lens epithelium has been examined in a number of studies. The Scribble complex members, Dlg-1 and Scrib, are widely expressed in the lens, often colocalizing with E- and N-cadherin at AJs [13]. Conditional deletion of Dlg-1 in the lens results in multilayering of the epithelium and redistribution of ZO-1 and E-cadherin [42].
The Par complex members aPKC, Par3, and Par6β colocalize with claudin-1 and occludin at lens epithelial cell TJs [37]. Conditional deletion of aPKC disrupts apical cell junctions and promotes epithelial-to-mesenchymal transition (EMT). At the edge of the lens epithelium, aPKC is also necessary for the formation of the “lens fulcrum,” the region in which cells pivot through 180° as they begin the process of terminal cell differentiation [43].
During EMT, polarized epithelial cells adopt a fibroblastoid, motile phenotype. In the lens, this phenomenon is associated with posterior capsule opacification following extracapsular cataract surgery [44]. EMT involves loss of apical–basal polarity, reorganization of the cytoskeleton, and disassembly of the junctional complex. The Par polarity complex is targeted directly during TGFβ-induced EMT. During EMT, TGFβ receptors I and II (TGFβRI, II) associate with Par6 at TJs. Binding of TGFβ ligand to TGFβRII results in phophorylation of both TGFβRI and Par6. Phosphorylated Par6 mediates the destruction of RhoA at TJs and subsequent dissolution of the junctions [45]. Loss of E-cadherin, disintegration of AJs, and disappearance of the cortical actin ring are other characteristic early findings in EMT [46].
3 Proliferative Compartments in the Lens Epithelium
The lens increases in size and mass throughout life. In most species, growth is rapid initially but slows subsequently as, later in life, lens weight approaches some asymptotic maximum value [47]. Primate lenses appear to be an exception in that they follow a biphasic, growth pattern. During prenatal development in humans, for example, lens growth is rapid and asymptotic. After birth, however, growth is slow and linear across the remaining lifespan [48].
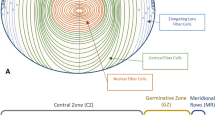
The ultimate driver of lens growth is cellular proliferation. Early investigators used the incorporation of tritiated thymidine by S-phase cells to visualize the distribution of mitoses in the lens. Such studies quickly established that mitotic cells are restricted to the lens epithelium [49–51]. In neonatal animals, S-phase cells are detected throughout the lens epithelium, but with age, cell division is increasingly concentrated in a band of epithelial cells that encircles the lens above the equator (Fig. 2.4). This region is called the proliferative or germinative zone (GZ) of the lens and contains most of the dividing cells. Between the germinative zone and the edge of the epithelium proper is a band of epithelial cells ≈ 10 cells wide. This transition zone (TZ) contains cells that have permanently withdrawn from the cell cycle. Epithelial cells in the TZ region express the cyclin-dependent kinase inhibitors p27Kip1 and p57Kip2 [52]. Together, Kip1 and Kip2 ensure timely exit of TZ epithelial cells from the cell cycle. Expression of both proteins persists in the fiber cell compartment, and combined knockout of Kip1 and 2 in mice causes an over-proliferation defect in the lens and attendant apoptosis [53].
Distribution of proliferating cells in 2-month-old mouse lens. S-phase cells (green) are labeled with the thymidine analog EdU. Mitosis is rare near the anterior pole (AP) of the lens but more common near the equator (Eq, left panel). High magnification, en face view of the lens equator (right panel) shows the proliferatively active germinative zone (GZ), the postmitotic transitional zone (TZ) at the epithelial margin, and the meridional rows (MR) formed as a result of nuclear alignment in differentiating fiber cells
The striking, lifelong growth of the lens has led some authors to suggest that the epithelium may contain a contingent of tissue stem cells. A characteristic of many stem cells (e.g., limbal stem cells in the cornea) is that they divide only infrequently. When pre-labeled with BrdU, such cells therefore behave as “label-retaining cells” because the BrdU staining intensity is not diminished by multiple rounds of cell division. Cells in the central lens epithelium act as label-retaining cells, prompting the suggestion that this region harbors lens stem cells [54]. Other studies, however, have examined the expression of various stem cell markers and concluded that lens stem cells might instead reside anterior to [55] or within the GZ [56]. To date, no empirical studies have convincingly demonstrated the existence of a stem cell niche in the lens. It is equally plausible that lens epithelial cells are of a single type and that the proliferative behavior of a given cell is dictated entirely by its latitudinal position on the lens surface and its exposure to local growth factors. Interestingly, cells in the central epithelium (which are thought to be arrested in the G0 phase of the cell cycle) reinitiate DNA synthesis following traumatic injury to the lens [57].
Despite the significant increase in the anterior surface area of the lens that accompanies postnatal growth, there does not appear to be a corresponding increase in the number of epithelial cells [55]. Instead, the area covered by individual epithelial cells increases significantly. To retain epithelial cell constancy, therefore, cells lost from the epithelium due to fiber cell differentiation (or cell death) must be continuously replaced by epithelial cell division. Given that in adult lenses the central cells rarely if ever divide, new cells can only be introduced within the proliferatively active GZ. Addition of cells to this zone will inevitably result in posterior displacement of cells situated between the GZ and the lens equator. We have called this pattern of cellular displacement the “penny pusher” model of lens growth. Among other predictions, the penny pusher model suggests that the speed at which lens epithelial cells move will increase as cells approach the equator. Consider, for example, a cell situated near the anterior border of the GZ. It moves only slowly because the chance of cells dividing in more anterior regions (i.e., within the quiescent central epithelium) is very small. In contrast, for cells located near the equator, many mitoses are likely to occur in the GZ region that separates the equatorial cells from the central epithelium. Consequently, equatorial cell displacement will be much more rapid. A second important prediction of the penny pusher model is that cells are resident in the GZ long enough for several rounds of cell division to occur. If this is the case, then clones of cells should be produced as cells traverse the GZ. This prediction has been validated using induced expression of GFP in individual lens cell as a lineage tracer [5]. When GFP expression is triggered in random lens epithelial cells using the Cre-lox system, individual cells are labeled initially. However, in the succeeding weeks, clusters (clones) of GFP-expressing cells emerge in the GZ, and clone size gradually increases (Fig. 2.5). The synchronous differentiation of such clones results in the deposition of a cuneiform group of fluorescent fiber cells [5].
Multiple rounds of cell division within the GZ result in formation of epithelial cell clones. GFP expression was induced in lenses of 3-week-old mice by tamoxifen treatment (see Shi and Bassnett [3]). Lenses were examined 3 days (a), 2 weeks (b), or 13 weeks (c) later. Note that although individual labeled epithelial cells are present initially, over time, only clusters of GFP-labeled cells (arrowheads) are detected within the GZ
In young rodent lenses, the labeling index (percent of S-phase cells) in the GZ is 3–5 % [58, 59]. This is >20-fold higher than in the central epithelium. What accounts for the relatively high mitotic rate in the GZ? An attractive early hypothesis was that low concentrations of FGF might serve to stimulate epithelial cell proliferation in this zone [60]. The ability of high levels of FGF to stimulate lens fiber cell differentiation had been amply demonstrated in previous studies [61]. In vitro experiments suggested that at lower concentrations, FGF stimulates cell migration and proliferation rather than differentiation [60]. FGF levels are known to be higher in the vitreous humor than in the aqueous humor. Conceivably, therefore, a gradient of a single growth factor, FGF, might account for both the proliferative and differentiation behavior of cells in various locations on the lens surface. More recent studies have suggested that although FGF may indeed be implicated in the control of lens cell proliferation, it is unlikely to act in isolation. Work in several laboratories has established that lens epithelial cells express many growth factor receptors including the PDGF and EGF receptors. The distribution of some of these receptors parallels the proliferative behavior of the cells (i.e., the receptors are most abundant in the equatorial epithelium). Functional imaging studies have also shown that otherwise evenly distributed receptors often show enhanced responsiveness in the GZ [62]. Explanted lens epithelial cells retain the capacity to proliferate in response to treatment with FGF-depleted aqueous humor or with a range of growth factor ligands in vitro [63]. Based on these and other studies, it seems likely that no single growth factor is responsible for epithelial cell proliferation. Finally, recent studies have visualized the attachment of the ciliary zonule to the lens surface and correlated this with the distribution of S-phase cells in the underlying epithelium. Significantly, the GZ is located in the region spanned by the zonular fibers [11]. Cells that exist in physically dynamic environments often both sense and transduce the forces acting upon them. Thus mechanical stress can be transduced into signaling cascades that activate transcription factors and stimulate cell proliferation [64]. It would, perhaps, be surprising if cells near the lens equator were not sensitive to the forces generated by the zonular suspension system, particularly in species like humans, where the lens tissue is physically distorted each time we focus our eyes.
References
Bassnett S, Shi Y (2010) A method for determining cell number in the undisturbed epithelium of the mouse lens. Mol Vis 16:2294–2300
Kuszak JR, Costello MJ (2004) The structure of the vertebrate lens. In: Robinson ML, Lovicu FJ (eds) Development of the ocular lens. Cambridge University Press, Cambridge, pp 71–118
Shi Y, Bassnett S (2007) Inducible gene expression in the lens using tamoxifen and a GFP reporter. Exp Eye Res 85:732–737
Bassnett S (2005) Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Exp Eye Res 81:716–723
Shi Y, Barton K, De Maria A, Petrash JM, Shiels A, Bassnett S (2009) The stratified syncytium of the vertebrate lens. J Cell Sci 122:1607–1615
Mallet JD, Rochette PJ (2011) Ultraviolet light-induced cyclobutane pyrimidine dimers in rabbit eyes. Photochem Photobiol 87:1363–1368
Haddad A, Bennett G (1988) Synthesis of lens capsule and plasma membrane glycoproteins by lens epithelial cells and fibers in the rat. Am J Anat 183:212–225
Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M (2013) Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adh Migr 7:64–71
Ziebarth NM, Manns F, Uhlhorn SR, Venkatraman AS, Parel JM (2005) Noncontact optical measurement of lens capsule thickness in human, monkey, and rabbit postmortem eyes. Invest Ophthalmol Vis Sci 46:1690–1697
Danysh BP, Czymmek KJ, Olurin PT, Sivak JG, Duncan MK (2008) Contributions of mouse genetic background and age on anterior lens capsule thickness. Anat Rec (Hoboken) 291:1619–1627
Shi Y, Tu Y, De Maria A, Mecham RP, Bassnett S (2013) Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest Ophthalmol Vis Sci 54:2504–2515
Nielsen PA, Baruch A, Shestopalov VI et al (2003) Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell 14:2470–2481
Nguyen MM, Rivera C, Griep AE (2005) Localization of PDZ domain containing proteins discs large-1 and scribble in the mouse eye. Mol Vis 11:1183–1199
De Maria A, Shi Y, Luo X, Van Der Weyden L, Bassnett S (2011) Cadm1 expression and function in the mouse lens. Invest Ophthalmol Vis Sci 52:2293–2299
Kuwabara T (1975) The maturation of the lens cell: a morphologic study. Exp Eye Res 20:427–443
Lo WK (1988) Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell Tissue Res 254:31–40
Lo WK, Shaw AP, Paulsen DF, Mills A (2000) Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp Eye Res 71:45–55
Kuszak JR, Novak LA, Brown HG (1995) An ultrastructural analysis of the epithelial-fiber interface (EFI) in primate lenses. Exp Eye Res 61:579–597
Indra I, Hong S, Troyanovsky R, Kormos B, Troyanovsky S (2013) The adherens junction: a mosaic of cadherin and nectin clusters bundled by actin filaments. J Invest Dermatol 133:2546–2554
Nose A, Takeichi M (1986) A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol 103:2649–2658
Hatta K, Takagi S, Fujisawa H, Takeichi M (1987) Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol 120:215–227
Bassnett S, Wilmarth PA, David LL (2009) The membrane proteome of the mouse lens fiber cell. Mol Vis 15:2448–2463
Lattin JE, Schroder K, Su AI et al (2008) Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res 4:5
Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA (2009) Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol 326:403–417
Etienne-Manneville S (2011) Control of polarized cell morphology and motility by adherens junctions. Semin Cell Dev Biol 22:850–857
Brieher WM, Yap AS (2013) Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 25:39–46
Cadigan KM, Peifer M (2009) Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1:a002881
Cain S, Martinez G, Kokkinos MI et al (2008) Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol 321:420–433
Takai Y, Ikeda W, Ogita H, Rikitake Y (2008) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342
Lachke SA, Higgins AW, Inagaki M et al (2012) The cell adhesion gene PVRL3 is associated with congenital ocular defects. Hum Genet 131:235–250
Dupin I, Camand E, Etienne-Manneville S (2009) Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol 185:779–786
Ikenouchi J, Suzuki M, Umeda K et al (2012) Lipid polarity is maintained in absence of tight junctions. J Biol Chem 287:9525–9533
Piontek J, Winkler L, Wolburg H et al (2008) Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22:146–158
Furuse M, Sasaki H, Fujimoto K, Tsukita S (1998) A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143:391–401
Lo WK (1987) In vivo and in vitro observations on permeability and diffusion pathways of tracers in rat and frog lenses. Exp Eye Res 45:393–406
Goodenough DA, Dick JS 2nd, Lyons JE (1980) Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol 86:576–589
Sugiyama Y, Prescott AR, Tholozan FM, Ohno S, Quinlan RA (2008) Expression and localisation of apical junctional complex proteins in lens epithelial cells. Exp Eye Res 87:64–70
Kang LI, Wang Y, Suckow AT et al (2007) Deletion of JAM-A causes morphological defects in the corneal epithelium. Int J Biochem Cell Biol 39:576–585
Mochida GH, Ganesh VS, Felie JM et al (2010) A homozygous mutation in the tight-junction protein JAM3 causes hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Am J Hum Genet 87:882–889
Daniele LL, Adams RH, Durante DE, Pugh EN Jr, Philp NJ (2007) Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol 505:166–176
Wodarz A (2005) Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol 17:475–481
Rivera C, Yamben IF, Shatadal S, Waldof M, Robinson ML, Griep AE (2009) Cell-autonomous requirements for Dlg-1 for lens epithelial cell structure and fiber cell morphogenesis. Dev Dyn 238:2292–2308
Sugiyama Y, Akimoto K, Robinson ML, Ohno S, Quinlan RA (2009) A cell polarity protein aPKClambda is required for eye lens formation and growth. Dev Biol 336:246–256
Eldred JA, Dawes LJ, Wormstone IM (2011) The lens as a model for fibrotic disease. Philos Trans R Soc Lond B Biol Sci 366:1301–1319
Viloria-Petit AM, Wrana JL (2010) The TGFbeta-Par6 polarity pathway: linking the Par complex to EMT and breast cancer progression. Cell Cycle 9:623–624
Tiwari N, Gheldof A, Tatari M, Christofori G (2012) EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol 22:194–207
Augusteyn RC (2008) Growth of the lens: in vitro observations. Clin Exp Optom 91:226–239
Augusteyn RC (2010) On the growth and internal structure of the human lens. Exp Eye Res 90:643–654
Harding CV, Hughes WL, Bond VP, Schork P (1960) Autoradiographic localization of tritiated thymidine in wholemount preparations of lens epithelium. Arch Ophthalmol 63:58–65
Hanna C, O’Brien JE (1961) Cell production and migration in the epithelial layer of the lens. Arch Ophthalmol 66:103–107
Thomson DS, Pirie A, Overall M (1962) Autoradiography of lens epithelium after parenteral injection of tritiated thymidine. Arch Ophthalmol 67:464–469
Nagahama H, Hatakeyama S, Nakayama K, Nagata M, Tomita K (2001) Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat Embryol (Berl) 203:77–87
Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ (1998) Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev 12:3162–3167
Zhou M, Leiberman J, Xu J, Lavker RM (2006) A hierarchy of proliferative cells exists in mouse lens epithelium: implications for lens maintenance. Invest Ophthalmol Vis Sci 47:2997–3003
Yamamoto N, Majima K, Marunouchi T (2008) A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Med Mol Morphol 41:83–91
Oka M, Toyoda C, Kaneko Y, Nakazawa Y, Aizu-Yokota E, Takehana M (2010) Characterization and localization of side population cells in the lens. Mol Vis 16:945–953
Harding CV, Donn A, Srinivasan BD (1959) Incorporation of thymidine by injured lens epithelium. Exp Cell Res 18:582–585
Mikulicich AG, Young RW (1963) Cell proliferation and displacement in the lens epithelium of young rats injected with tritiated thymidine. Invest Ophthalmol 2:344–354
Rafferty NS, Rafferty KA Jr (1981) Cell population kinetics of the mouse lens epithelium. J Cell Physiol 107:309–315
McAvoy JW, Chamberlain CG (1989) Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 107:221–228
Lovicu FJ, McAvoy JW (2005) Growth factor regulation of lens development. Dev Biol 280:1–14
Maidment JM, Duncan G, Tamiya S, Collison DJ, Wang L, Wormstone IM (2004) Regional differences in tyrosine kinase receptor signaling components determine differential growth patterns in the human lens. Invest Ophthalmol Vis Sci 45:1427–1435
Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ (2009) Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors 27:50–62
Mendez MG, Janmey PA (2012) Transcription factor regulation by mechanical stress. Int J Biochem Cell Biol 44:728–732
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Bassnett, S. (2014). Cell Biology of Lens Epithelial Cells. In: Saika, S., Werner, L., Lovicu, F. (eds) Lens Epithelium and Posterior Capsular Opacification. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54300-8_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-54300-8_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54299-5
Online ISBN: 978-4-431-54300-8
eBook Packages: MedicineMedicine (R0)