Abstract
The formation of lens progenitor cells and differentiated lens tissue in cell culture conditions presents a number of experimental challenges, even though lens lineage formation and lens fiber cell differentiation are among the best characterized model systems at both genetic and molecular levels. Lens differentiation from ES cells in vitro appears to be a feasible goal. This chapter describes the significance of using ES and iPS cells for better understanding of embryonic lens development and formation of congenital cataracts. A discussion of how iPS cells can help studies of age-related cataract is also included. The chapter summarizes the current data on lentoid body formation from human and primate ES cells, and the molecular basis of directed differentiation of human ES cells into lens progenitor cells and lentoid bodies. Finally, current gaps in lens research and future directions to address these problems are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Embryonic Stem Cell
- Human Embryonic Stem Cell
- Lens Epithelial Cell
- Congenital Cataract
- Retinal Pigment Epithelium
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The central premise of embryonic stem (ES) cell biology is an unlimited potential of ES cells to form every cell type of the whole organism [33, 42, 91, 120]. The potential is fulfilled during ontogenesis. The major question is if it is possible to differentiate ES cells into all transient (embryonic germ cell layers and common cell progenitors) and terminally differentiated cell types in vitro. A large body of work using mostly human and mouse ES cells conducted during the last decade has shown that it is a generally feasible goal with major implications for our understanding of embryonic development; modeling of human disease and treatment of a wide range of diseases that require cell-based therapeutics [50].
The human eye is an excellent organ for in vitro studies of its organogenesis, modeling of human eye diseases through the generation of disease-specific-induced pluripotent stem (iPS) cells via nuclear reprogramming [121], and for cell replacement and paracrine rescue therapies [66]. To harness the power of ES- and iPS-cell-based ideas of treating human eye diseases, the essential first step is to develop procedures to form ocular cells and tissues using in vitro conditions. The main challenge for this research originates from our limited knowledge of cell fate specification processes that occur normally in a three-dimensional (3-D) context in developing embryos and what specific cell culture conditions may favor simultaneous formation of multiple cell types that might both positively and negatively influence the development of the desired cell type. While the cells can achieve the desired cell type, their terminal differentiation into a status comparable with tissues generated during ontogenesis often requires additional conditions that have to be determined empirically.
The formation of lens progenitor cells and differentiated lens in cell culture conditions presents a number of experimental challenges, even though lens lineage formation and lens fiber cell differentiation are among the best characterized model systems at both genetic and molecular levels [16, 18, 20, 23, 35, 59, 69, 82]. It has been shown that cultured lens epithelial cells can be differentiated into primitive lens-like structures termed “lentoid bodies.” Lentoid bodies are 3-D structures that resemble the lens as they are both transparent and refract light. They can be generated in vitro either from primary, spontaneously transformed or viral oncogene-transformed lens epithelial cells [8, 45, 46, 51, 75, 76, 87, 112, 119]. Lentoid body formation can also be found in vivo in vertebrate embryos as a result of spontaneous or genetically engineered mutations in genes that operate in the pathways that control lens formation [56, 61, 101]. Finally, it is possible to transdifferentiate lentoids from retinal pigmented epithelium (RPE) cells [68, 72]. The formation of lentoid bodies in different experimental settings shows that the basic program to establish the 3-D structure of the lens is functional independently on the local environment such as in the absence of optic cup/retinal tissue [106]. Thus, lens differentiation from ES cells in vitro appears to be a feasible goal. This chapter first describes the significance of using ES and iPS cells for better understanding of embryonic lens development and formation of congenital cataracts. A discussion of how iPS cells can help studies of age-related cataract is also included in “New Model Systems Based on ES and iPS Cell Differentiation to Understand Lens Development and Disease,” section of this chapter. “Differentiation of ES Cells into Lens” summarizes the current data on lentoid body formation from human and primate ES cells, and the molecular basis of directed differentiation of human ES cells into lens progenitor cells and lentoid bodies. Finally, “Conclusions and Future Directions” provides a summary of current gaps in lens research and future directions to address these problems.
New Model Systems Based on ES and iPS Cell Differentiation to Understand Lens Development and Disease
Use of ES and iPS cells differentiated into lens cells offers a wide range of experimental approaches to better understand embryonic lens formation and lens fiber cell differentiation. Similarly, cataract-specific iPS cells offer a new array of approaches to evaluate various aspects of human lens homeostasis and identification of novel relationships between cellular processes and their impact on lens transparency.
Modeling of Embryonic Development
Although embryological studies on lens morphogenesis date to the beginning of the twentieth century, and have resulted in a comprehensive understanding of the origin of lens cell lineage, formation of the lens placode, formation of the lens vesicle, cell cycle exit regulation in the posterior compartment of the lens vesicle, lens fiber cell terminal differentiation, lens regeneration in specific amphibians, transdifferentiation of lens from other ocular and non-ocular tissues, and lens evolution in animal kingdom ([18–20, 30, 35–38, 48, 59, 63, 69, 82]), a number of important questions remain to be addressed, with three examples described below.
Based on studies in chicks and zebrafish, it has been proposed that lens progenitor cells originate from a common pool of pre-placodal cells [1, 38, 105]. Data to support this attractive model on mammalian lens development are still missing. A large body of data exists to support the role of FGF signaling at multiple stages of lens development [88]; however, little is known how the specificity of this signaling is established in the embryo in a 3-D space crowded with many signaling molecules, their agonists and antagonists [102]. The lens is also a unique tissue in terms of its terminal differentiation. To achieve transparency, lens fiber cells lose their subcellular organelles including the nuclei in a highly controlled process that ultimately preserves the lens fiber cells for the rest of the life [3, 4]. These questions can be addressed through the use of ES cell differentiation as described in “Differentiation of ES Cells into Lens” and future experiments outlined in “Conclusions and Future Directions” of this chapter.
Congenital Cataracts
Congenital cataracts are typically caused by mutations in genes that control lens development and by mutations in genes encoding key lens structural proteins [36, 40, 98]. Although molecular mechanisms for many of these genes were established using mouse models, the power to produce lens cells from human patients that carry these mutations is unique. The advantage of this system is that one can prepare human lens cell extracts from genetically defined material and study protein–protein interactions of mutant crystallins and lens membrane proteins in their native environment [17]. Similarly, it is possible to derive lens cells from patients with mutations in DNA-binding transcription factors such as FOXE3 [69], HSF4 [11, 26, 100], MAF [17, 123], PAX6 [43], and PITX3 [7, 10, 96] to study molecular mechanisms of these mutations in their native biological environment. This approach should identify those specific genes with disrupted expression due to specific missense mutations and/or by their haploinsufficiency [17].
Age-Related Cataract
Age-related cataract is a disease of the ocular lens that is responsible for just under half of blindness worldwide, and is expected to increase as a result of extended life spans in industrialized, emerging-market, and underdeveloped countries [71]. Age-onset cataract develops between the age of 40–50 years as a result of the progressive breakdown of the lens microarchitecture [97]. Age-onset cataract is a complex disease involving both genetic and environmental factors that affect 42 % of the population between the ages of 52–64, and 91 % of the population for ages 75–85 [54, 103]. Genetic studies of age-related cataract point to both multiple genes and environmental factors influencing the phenotype [71, 97]. The Beaver Dam Eye Study suggests that mutations in a single gene/locus could be responsible for as much as 35 % of nuclear and up to 75 % of cortical cataract incidence [39, 47, 55]. Other studies using siblings and twins also demonstrate significant genetic influence on age-onset cataract [41, 97].
Age-related (or senile) cataract is defined as cataract occurring in people over the age of 50 in the absence of known mechanical, chemical, or radiation trauma. At the molecular level of age-related cataract, lens structural proteins, the crystallins, become oxidized and water-insoluble, and form high molecular weight aggregates. The continual accumulation of crystallin aggregates and other lens proteins causes opacification and loss of lens transparency. The current treatment of senile cataract is surgery that replaces the opaque lens with an artificial intraocular lens. Although the surgery is routinely performed in the USA, numbering 1.5–2 million patients treated annually, it represents a major Medicare reimbursement category. It has been estimated that a 10-year delay in the onset of senile cataract could decrease the number of surgeries needed by almost one half, thus significantly decreasing vision care costs ([58]; www.nei.nih.gov/strategicplanning/np_lens.asp). Progress in human cataract research is hampered by the lack of genetically defined and abundant experimental materials as well as the absence of relevant animal models [6, 41]. The use of cataract-specific iPS cells offers a unique opportunity to develop well-defined human cell culture models to study cataract as a disease of lens protein homeostasis.
Differentiation of ES Cells into Lens
In this section, we will first summarize our knowledge about mammalian lens formation that is relevant to the design of experiments to differentiate lens cells from ES cells (“Mammalian Lens Development and Lessons for a Rational Design of ES Cell-Based Differentiation Systems”). We then provide examples of lentoid body formation in various ES culture systems (“Formation of Lentoid Bodies”) and describe a procedure to produce highly enriched lens progenitor cells and “immature” lentoid bodies from human ES cells (“Lens Differentiation from Human ES Cells in Chemically-Defined Conditions”). Finally, we will discuss different strategies to improve the differentiation of human lentoid bodies (“3-D Cultures of Lentoid Bodies to Improve Their Differentiation Status”).
Mammalian Lens Development and Lessons for a Rational Design of ES Cell-Based Differentiation Systems
Multiple signal transduction systems including BMP (bone receptor protein), FGF, Notch, TGF-β, and Wnt have been identified to control various stages of lens morphogenesis [18, 38, 59, 64, 102]. In addition, the origin of lens lineage from the pre-placodal region shown in chicken and zebrafish models suggests that early stages of the differentiation process require the formation of neuroectoderm and its subsequent “by-product,” the pre-placodal ectoderm [105].
Neuroectoderm formation in cell cultures can be induced by a variety of growth factors, inhibitors of BMP signaling including noggin, follistatin, cerberus, chordin, ventropin, and gremlin [2, 92] as well as small drugs such as SB431542 [15]. It has been found recently that noggin is produced by a subpopulation of MyoD-positive cells in the epiblast; their immunologically mediated ablation interfered with lens and optic cup morphogenesis [31].
Loss-of-function studies of BMP4 in mouse established a critical role of this growth factor for lens placode formation [27]. BMP7 knockout mice also develop ocular abnormalities that were linked to the abnormal lens induction [65, 116]. In addition, studies of lens formation through conditional knockouts of two BMP receptor genes, Acvr1 and Bmpr1a, further confirmed the essential roles of BMP signaling in lens induction, as reduced lens placode thickening and failure of lens invagination were observed [86]. In ex vivo explant assays using chicken embryonic tissues, BMPs have been shown to specify the formation of lens and olfactory placodes [80, 99]. BMP signaling not only plays a role in the formation of lens placode but also participates in lens fiber cell differentiation. BMP2, BMP4, and BMP7 have been shown to induce the expression of markers of fiber differentiation in primary chick lens cell cultures. In addition, expression of noggin, an inhibitor of BMP signaling, in the lenses of transgenic mice resulted in a postnatal block of epithelial-to-secondary fiber differentiation [9].
Numerous studies have shown multiple functions of the FGF (fibroblast growth factor) signaling pathway for the formation of the lens placode [105]. FGF signaling is well known as the key trigger for lens fiber cell differentiation [63]. The pioneering work conducted more than two decades ago showed that FGF2/bFGF is a potent inducer of lens fiber cell differentiation in vitro [14]. A recent study using conditional triple knockout mice with deletion of FGF receptors, Fgfr1, Fgfr2, and Fgfr3 provided evidence for the essential role of FGF signaling in lens fiber cell differentiation in vivo. The specific inactivation of these three FGF receptors at lens pit stage totally abrogated lens fiber cell differentiation, resulting in a hollow lens [126]. Transgenic mice expressing a dominant-negative Fgfr1 in the presumptive lens ectoderm showed many early stage defects including reduced lens placode thickness and delayed lens placode invagination [25]. Studies on two genes, Frs2 and Ndst1, also revealed that FGF signaling is critical for lens placode formation. Frs2α encodes a docking protein for linking FGFRs with a variety of intracellular signaling pathways. A mutation of this gene Frs2α 2F/2F led to the halt of the lens development at lens placode stage in severely affected mutant eyes [32]. Ndst1 (N-acetylglucosamine N-deacetylase-N-sulfotransferase 1 enzyme) encodes an enzyme for biosynthesis of heparan sulfate proteoglycans, which is low affinity co-receptor of FGFRs. Inactivation of Ndst1 in mouse resulted in invagination defects of the early lens [79]. The most recent study showed that inactivation of Fgfr1 and Fgfr2 at lens placode stage led to increased cell death and the formation of a thinner lens placode, suggesting that the primary role of autocrine or paracrine FGF signaling is to provide essential survival signals to lens placode cells [29].
Recent genetic experiments, lens-specific inactivation of Jag1 [60], Notch2 [93] and RBP-J [90] have established role of Notch signaling in primary lens fiber cell differentiation.
Both canonical Wnt signaling, via β-catenin, and planar cell polarity (PCP/Wnt) non-canonical Wnt signaling play a range of roles in lens morphogenesis [64, 67]. Wnt/PCP signaling is required for organization of lens fiber cell cytoskeleton and lens 3-D architecture.
In summary, studies of lens development suggest that active BMP and FGF signaling are required for lens cell formation. FGF signaling is sufficient to induce lens fiber cell differentiation in in vitro cultures, and modulation of this process via Notch, Wnt/β-catenin and Wnt/PCP signaling pathways could provide additional tools to recapitulate lens ontogenesis from ES cell cultures.
Formation of Lentoid Bodies
Three earlier procedures identified lentoid body formation in primate and murine ES cells cultures. These methods were limited to a production of a small percentage of lentoid bodies along with a number of other cells types such as retinal pigmented epithelium (RPE) [44, 77, 107]. The protocols used in these earlier studies employed mouse feeder cells, and differentiation was induced by co-culture with mouse PA6 stromal cells (“SDIA, or cultures”). External FGF2 was added to some cultures [77]. The yield of lentoid bodies was between 200 and 300 colonies/10-cm dish after 30 days in culture. Formation of lentoid bodies was also detected when both mouse and human ES cells were cultured on matrix components of the human amniotic membrane (“AMED system”) together with many other cell types including dopaminergic neurons, motor neurons, and RPE cells [111]. These experiments provided the “proof-of-principle” of lens cell formation from mammalian ES cells; nevertheless, they are not suitable for the standardized production of enriched lens cells and lentoid bodies.
Lens Differentiation from Human ES Cells in Chemically Defined Conditions
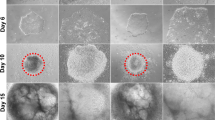
Using the information on normal lens formation (“Mammalian Lens Development and Lessons for a Rational Design of ES Cell-Based Differentiation Systems”), we established a new experimental three-stage protocol with defined growth factors to generate large quantities of lens progenitor cells and lentoid bodies from human ES cells as shown in Fig. 4.1. Inhibition of BMP signaling by recombinant noggin triggered differentiation of ES cells towards neuroectoderm. Subsequent reactivation of BMP and activation of FGF signaling elicited robust formation of lens progenitor cells marked by the expression of PAX6 and αA- and αB-crystallins (CRYAA and CRYAB). The formation of lentoid bodies required the presence of FGF2 and the total number of the lentoids increased in the presence of Wnt3a yielding approximately 1,000 lentoid bodies per a 30 mm well. Lentoid bodies expressed and accumulated lens-specific markers including αA-, αB-, β-, and γ-crystallins, filensin/BFSP1, BFSP2/CP49, and MIP/aquaporin 0 [122]. Nevertheless, morphological and scanning and transmission electron microscopic analysis of these lentoid bodies identified nucleated lens cells and only moderately elongated lens fiber cells. These data indicated that while specific pathways of the lens fiber cell differentiation program such as synthesis and accumulation of both αA- and αB-crystallins were turned on in the “immature” lentoid bodies; however, activation of the denucleation pathway was not achieved. We conclude that this procedure can be immediately used to probe various aspects of human lens lineage cell formation focusing on the function of specific DNA-binding transcription factors, chromatin remodellers, and extracellular signaling; nevertheless, follow-up studies are necessary to address the culture conditions to achieve formation of “mature” lentoid bodies comprised of elongated enucleated lens fiber cells.
Diagrammatic summary of a three-step procedure to differentiate human ES cells into lens progenitor-like cells and lentoid bodies. (a) Diagram of three steps: noggin treatment (days 0–6), BMP4/BMP7/FGF2 treatment (days 7–18), and differentiation in the presence of FGF2 (essential factor) and Wnt3a (modulatory factor) (days 22–35). Formation of putative cell populations including the neuroectoderm, pre-placodal region (PPR) and neural crest (NC) cells is indicated. (b) Sequential activation of PAX6, αB-crystallin (CRYAB) and αA-crystallin (CRYAA) indicates establishment of the lens progenitor-like cells around day 14 of the culture. At this time, the number of PAX6+ and CRYAA+ cells was 65 and 41 %, respectively [122]. Both αA- and αB-crystallins accumulate during the differentiation of lentoid bodies
3-D Cultures of Lentoid Bodies to Improve Their Differentiation Status
A number of potential improvements of the differentiation procedure described above should be considered and empirically tested. In principle, the system can be improved through testing of different 3-D gels and extracellular matrix proteins that are found in the lens capsule, growth of lentoid bodies on lens capsule, specific activators and inhibitors of differentiation, chemical libraries, 3-D scaffolds to generate a gradient of growth factor(s), and any combination of these procedures. In addition, genetically engineered human and mouse ES cells that carry fluorescent reporter genes, under the control of lens regulatory elements, can be used to aid in the analysis of the differentiation process.
There are at least three commercially available 3-D systems: ExtraCel hydrogel (Glycosan Biosystems), HyStem-C Cell Culture Scaffold kit (Sigma), and Cultrex 3-D Culture Matrix Extract (R&D Systems). Each system allows for the incorporation of variable amounts/ratios of laminin, collagen IV, entactin/nidogen, perlecan, fibronectin, collagen XVIII and sparc/osteonectin, extracellular matrix (ECM) proteins found in the lens [21, 117].
A number of drugs have been shown to promote cellular differentiation with some of the tested in lens cell cultures. These include specific inhibitors of DNA methylation such as 5-azacytidine and 5-deazacytidine [12, 49, 94], inhibitors of histone methyltransferases (cytarabine and decitabine [84]), inhibitors of histone deacetylases (valproic acid and sodium butyrate [22, 24, 34, 74, 78]), and inhibitors of cyclin-dependent kinases (olomoucine and roscovitine [70, 73, 89, 115]). Of particular interest are the rho-kinase (ROCK) inhibitors, Y27632 and PP-1, as the PP-1 drug has been successfully used to promote cell cycle withdrawal and commitment of lens cells to differentiate [113, 114].
Considering the specific roles of Notch and Wnt signaling pathways for lens fiber cell differentiation, and the role of Wnt signaling in the differentiation of lens epithelial cells described above (“Mammalian Lens Development and Lessons for a Rational Design of ES Cell-Based Differentiation Systems”), stimulation of ES cell differentiation may be considered. Recombinant Notch ligands, Jagged 1 and 2, can be added transiently during the thirds stage of the differentiation procedure. Concerning Wnt signaling, the situation is more complex as multiple Wnts and their receptors, the frizzled proteins, can regulate lens development both in the epithelial and fiber cell compartments. Nevertheless, inclusion of Wnt3a improved the quantitative parameters of the current procedure of lentoid body formation [122].
Ongoing experiments in the laboratory are aimed to improve differentiation of lentoid bodies using a combinatorial approach as outlined above. The procedure can be improved via genetically engineered ES cells [5] that carry fluorescent markers under the control of lens regulatory regions from genes known to control different stages of the lens lineage formation, cell cycle exit, and terminal differentiation. For this purpose, the EGFP, or enhanced green fluorescent protein marker can be inserted into a specific BAC clone with PAX6 (early marker), HSF4 (late marker), β-/γ-crystallins, DNase IIβ, MIP/aquaporin 0, paralemmin, and other genes expressed in terminally differentiated lens fiber cells as established for similar differentiation systems [83, 110].
iPS Cells and Cataract Research
For the first time in human lens research, we are about to establish a general strategy to model human lens development and diseases with an unlimited supply of lens cells that originate from genetically and phenotypically defined human source(s). In addition, these materials can be shared between multiple laboratories to accelerate research. The pioneering work of S. Yamanaka at the Institute for Frontier Medical Sciences, Kyoto University, Japan, to establish the reprogramming procedure using skin fibroblasts provided proof-of-principle that the iPS cell can be established from somatic terminally differentiated cells, and these iPS cells behaved like authentic ES cells in a series of functional tests [108, 109]. A large follow-up effort in a number of laboratories worldwide resulted in expansion of the reprogramming procedures and cell types suitable for these manipulations. The majority of currently existing procedures are summarized in Table 4.1. It has been shown recently that iPS cells can be produced from a cataract patient using lens epithelial cells as the starting material [85]. Most importantly, these iPS cells were differentiated into lentoid bodies using the procedure described here (see Fig. 4.1) [85]. Nevertheless, whether iPS cells, generated through other reprogramming protocols and cell types, are capable of producing lentoid bodies similar to those generated from human ES cells, remains to be formally proven.
Conclusions and Future Directions
One of the most pressing objectives of medical research today is to develop novel approaches to model formation of human organs, tissues and diseases. Use of human ES and iPS cells differentiated into individual tissues provides the highest possible promise to achieve this objective as it is now possible to understand the contribution of genetic and environmental factors in various diseases including those related to aging such as age-onset cataract.
Thus, the present cell culture system can be used to modulate these common signaling pathways during lens formation [62] via siRNA technology and through the use of small drug molecules, inhibitors of FGF and BMP signaling (e.g., SB431542—an inhibitor of the Alk1 receptor, SU5402—an inhibitor of FGFR and U0126—an inhibitor of MEK) to study formation of lens lineage and formation of alternate cell fates that originate from the common pre-placodal region [105].
It is now possible to produce iPS cells from human patients that carry heterozygous mutations in regulatory genes such as PAX6, FOXE3, MAF, HSF4, PITX3, and others and to identify those genes that are not properly regulated during early stages of lens development. In contrast, studies of cataractogenesis using the system of ES/iPS cells seems be premature until procedures to generate enucleated lentoid bodies with distinct epithelium/fiber cell compartments are established. The long-term benefits of the research to model human cataract using iPS cells should stimulate our efforts to achieve this challenging goal.
References
Baker CV, Bronner-Fraser M (2001) Vertebrate cranial placodes I. Embryonic induction. Dev Biol 232(1):1–61
Balemans W, Van Hul W (2002) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250(2):231–250
Bassnett S (2009) On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res 88:133–139
Bassnett S, Shi Y, Vrensen GF (2011) Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci 366(1568):1250–1264
Ben-Dor I, Itsykson P, Goldenberg D, Galun E, Reubinoff BE (2006) Lentiviral vector harboring a dual-gene system allow high and homegenous expression in selected polyclonal human embryonic stem cells. Mol Ther 14:255–267
Benedek GB, Pande J, Thorston GM, Clark JI (1999) Theoretical and experimental basis for the inhibition of cataract. Prog Retin Eye Res 18:391–402
Bidinost C, Matsumoto M, Chung D, Salem N, Zhang K, Stockton DW, Khoury A, Megarbane A, Bejjani BA, Traboulsi EI (2006) Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci 47(4):1274–1280
Blakely EA, Bjornstad KA, Chang PY, McNamara MP, Chang E, Aragon G, Lin SP, Lui G, Polansky JR (2000) Growth and differentiation of human lens epithelial cells in vitro on matrix. Invest Ophthalmol Vis Sci 41(12):3898–3907
Boswell BA, Overbeek PA, Musil L (2008) Essential role of BMPs in FGF-induced secondary lens fiber cell differentiation. Dev Biol 324:201–212
Brémond-Gignac D, Bitoun P, Reis LM, Copin H, Murray JC, Semina EV (2010) Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol Vis 16:1705–1711
Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, Liu J, Hu L, Zhao G, Hayden MR, Kong X (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31(3):276–278
Burlacu A (2006) Can 5-azacytidine convert the adult stem cells into cardiomyocytes? A brief overview. Arch Physiol Biochem 112(4–5):260–264
Cai J, Li W, Su H, Qin D, Yang J, Zhu F, Xu J, He W, Guo X, Labuda K, Peterbauer A, Wolbank S, Zhong M, Li Z, Wu W, So KF, Redl H, Zeng L, Esteban MA, Pei D (2010) Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem 285(15):11227–11234
Chamberlain CG, McAvoy JW (1989) Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1(2):125–134
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280
Chow RL, Lang RA (2001) Early eye development in vertebrates. Annu Rev Cell Dev Biol 17(255–296)
Churchill A, Graw J (2011) Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci 366(1568):1234–1249
Cvekl A, Duncan MK (2007) Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res 26(6):555–597
Cvekl A, Mitton KP (2010) Epigenetic regulatory mechanisms in vertebrate eye development and disease. Heredity 105(1):135–151
Cvekl A, Piatigorsky J (1996) Lens development and crystallin gene expression: many roles for Pax-6. Bioessays 18(8):621–630
Danysh BP, Duncan MK (2009) The lens capsule. Exp Eye Res 88:151–164
Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr 133(7):2485S–2493S
Donner AL, Lachke SA, Maas RL (2006) Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol 17(6):676–685
Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA (2008) Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev 34(3):206–222
Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA (2001) Fgf receptor signaling plays a role in lens induction. Development 128(22):4425–4438
Fujimoto M, Oshima K, Shinkawa T, Wang BB, Inouye S, Hayashida N, Takii R, Nakai A (2008) Analysis of HSF4 binding regions reveals its necessity for gene regulation during development and heat shock response in mouse lenses. J Biol Chem 283(44):29961–29970
Furuta Y, Hogan BL (1998) BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12:3764–3775
Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, Lafferty J, McGinn JT, Brodman M, Fuster V, Hajjar RJ, Polgar K (2010) Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cloning Stem Cells 12:1–10
Garcia CM, Huang J, Madakashira BP, Liu Y, Rajagopal R, Dattilo L, Robinson ML, Beebe DC (2011) The function of FGF signaling in the lens placode. Dev Biol 351(1):176–185
Gehring WJ, Ikeo K (1999) Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet 15(9):371–377
Gerhart J, Pfautz J, Neely C, Elder J, DuPrey K, Menko AS, Knudsen K, George-Weinstein M (2009) Noggin producing, MyoD-positive cells are crucial for eye development. Dev Biol 336(1):30–41
Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y, Lax I, Schlessinger J, Shibuya M, Lang RA (2004) Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci USA 101(49):17144–17149
Gögel S, Gubernator M, Minger SL (2011) Progress and prospects: stem cells and neurological diseases. Gene Ther 18(1):1–6
Göttlicher M (2004) Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol 83(Suppl):91–92
Grainger RM (1992) Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet 8(10):349–355
Graw J (2009) Genetics of crystallins: cataract and beyond. Exp Eye Res 88(2):173–189
Griep AE (2006) Cell cycle regulation in the developing lens. Semin Cell Dev Biol 17(6):686–697
Gunhaga L (2011) The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philos Trans R Soc Lond B Biol Sci 366(1568):1193–1203
Heiba IM, Elston RC, Klein BE, Klein R (1995) Evidence for a major gene for cortical cataract. Invest Ophthalmol Vis Sci 36:227–235
Hejtmancik JF (2008) Congenital cataracts and their molecular genetics. Semin Cell Dev Biol 19(2):134–149
Hejtmancik JF, Kantorow M (2004) Molecular genetics of age-related cataract. Exp Eye Res 79(1):3–9
Hemmat S, Lieberman DM, Most SP (2010) An introduction to stem cell biology. Facial Plast Surg 26(5):343–349
Hever AM, Williamson KA, van Heyningen V (2006) Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin Genet 69(6):459–470
Hirano M, Yamamoto A, Yoshimura N, Tokunaga T, Motohashi T, Ishizaki K, Yoshida H, Okazaki K, Yamazaki H, Hayashi S, Kunisada T (2003) Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Dev Dyn 228:664–671
Ibaraki N, Lin LR, Reddy VN (1995) Effects of growth factors on proliferation and differentiation in human lens epithelial cells in early subculture. Invest Ophthalmol Vis Sci 36(11):2304–2312
Ibaraki N, Lin LR, Reddy VN (1996) A study of growth factor receptors in human lens epithelial cells and their relationship to fiber differentiation. Exp Eye Res 63(6):683–692
Iyengar SK, Klein BE, Klein R, Jun G, Schick JH, Millard C, Liptak R, Russo K, Lee KE, Elston RC (2004) Identification of a major locus for age-related cortical cataract on chromosome 6p12-q12 in the Beaver Dam Eye Study. Proc Natl Acad Sci USA 101:14485–14490
Jonasova K, Kozmik Z (2008) Eye evolution: lens and cornea as an upgrade of animal visual system. Semin Cell Dev Biol 19(2):71–81
Jones PA (1985) Altering gene expression with 5-azacytidine. Cell 40(3):485–486
Keller G (2005) Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 19:1129–1155
Kidd GL, Reddan JR, Russell P (1994) Differentiation and angiogenic growth factor message in two mammalian lens epithelial cell lines. Differentiation 56(1–2):67–74
Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS (2009) Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4:472–476
Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hübner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Schöler HR (2009) Oct4-induced pluripotency in adult neural stem cells. Cell 136:411–419
Klein BE, Klein R, Lee KE (2002) Incidence of age-related cataract over a 10-year interval: the Beaver Dam Eye Study. Ophthalmology 109:2052–2057
Klein BE, Klein R, Lee KE, Moore EL, Danforth L (2001) Risk of incident age-related eye diseases in people with an affected sibling: the Beaver Dam Eye Study. Am J Epidemiol 154:207–211
Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z (2007) Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 45(4):157–168
Kunisato A, Wakatsuki M, Shinba H, Ota T, Ishida I, Nagao K (2011) Direct generation of induced pluripotent stem cells from human nonmobilized blood. Stem Cells Dev 20(1):159–168
Kupfer C (1987) Public health ophthalmology. Br J Ophthalmol 71:116–117
Lang RA (2004) Pathways regulating lens induction in the mouse. Int J Dev Biol 48(8–9):783–791
Le TT, Conley KW, Brown NL (2009) Jagged 1 is necessary for normal mouse lens formation. Dev Biol 328:118–126
Lewis KE, Drossopoulou G, Paton IR, Morrice DR, Robertson KE, Burt DW, Ingham PW, Tickle C (1999) Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development 126(11):2397–2407
Liu W, Lagutin OV, Mende M, Streit A, Oliver G (2006) Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J 25:5383–5395
Lovicu FJ, McAvoy JW (2005) Growth factor regulation of lens development. Dev Biol 280(1):1–14
Lovicu FJ, McAvoy JW, de Iongh RU (2011) Understanding the role of growth factors in embryonic development: insights from the lens. Philos Trans R Soc Lond B Biol Sci 366(1568):1204–1218
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9(22):2808–2820
Marchetti V, Krohne TU, Friedlander DF, Friedlander M (2010) Stemming vision loss with stem cells. J Clin Invest 120(9):3012–3021
Martinez G, de Iongh RU (2010) The lens epithelium in ocular health and disease. Int J Biochem Cell Biol 42(12):1945–1963
Matsuo T, Tsutsui Y, Matsuo N (1998) Transdifferentiation of chick embryonic retinal pigment epithelial cells to lentoid structure in suspension culture. Acta Med Okayama 52(3):125–130
Medina-Martinez O, Jamrich M (2007) Foxe view of lens development and disease. Development 134(8):1455–1463
Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP (1997) Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 243(1–2):527–536
Michael R, Bron AJ (2011) The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci 366(1568):1278–1292
Mochii M, Ono T, Matsubara Y, Eguchi G (1998) Spontaneous transdifferentiation of quail pigmented epithelial cells is accompanied by a mutation in the Mitf gene. Dev Biol 196:145–159
Mohapatra S, Coppola D, Riker AI, Pledger WJ (2007) Roscovitine inhibits differentiation and invasion in a three-dimensional skin reconstruction model of metastatic melanoma. Mol Cancer Res 5(2):145–151
Monneret C (2005) Histone deacetylase inhibitors. Eur J Med Chem 40(1):1–13
Nagineni CN, Bhat SP (1992) Lens fiber cell differentiation and expression of crystallins in co-cultures of human fetal lens epithelial cells and fibroblasts. Exp Eye Res 54(2):193–200
O'Connor MD, McAvoy JW (2007) In vitro generation of functional lens-like structures with relevance to age-related nuclear cataract. Invest Ophthalmol Vis Sci 48(3):1245–1252
Ooto S, Haruta M, Honda Y, Kawasaki H, Sasai Y, Takahashi M (2003) Induction of the differentiation of lentoid from primate embryonic stem cells. Invest Ophthalmol Vis Sci 44:2689–2693
Pajak B, Orzechowski A, Gajkowska B (2007) Molecular basis of sodium butyrate-dependent proapoptotic activity in cancer cells. Adv Med Sci 52:83–88
Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X (2006) Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133(24):4933–4944
Pandit T, Jidigam VK, Gunhaga L (2011) BMP-induced L-Maf regulates subsequent BMP-independent differentiation of primary lens fibre cells. Dev Dyn 240(8):1917–1928
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146
Piatigorsky J (1981) Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19(3):134–153
Placantonakis DG, Tomishima MJ, Lafaille F, Desbordes SC, Jia F, Socci ND, Viale A, Harrison N, Tabar V, Studer L (2009) BAC transgenesis in human embryonic stem cells as a novel toll to define the human neuronal lineage. Stem Cells 27:521–532
Plimack ER, Kantarjian HM, Issa JP (2007) Decitabine and its role in the treatment of hematopoietic malignancies. Leuk Lymphoma 48(8):1472–1481
Qiu X, Yang J, Liu T, Jiang Y, Le Q, Lu Y (2012) Efficient generation of lens progenitor cells from cataract patient-specific induced pluripotent stem cells. PLoS One 7(3):e32612
Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC (2009) The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol 335(2):305–316
Reddy VN, Lin LR, Arita T, Zigler JS Jr, Huang QL (1988) Crystallins and their synthesis in human lens epithelial cells in tissue culture. Exp Eye Res 47(3):465–478
Robinson ML (2006) An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol 17(6):726–740
Rosato RR, Almenara JA, Maggio SC, Atadja P, Craig R, Vrana J, Dent P, Grant S (2005) Potentiation of the lethality of the histone deacetylase inhibitor LAQ824 by the cyclin-dependent kinase inhibitor roscovitine in human leukemia cells. Mol Cancer Ther 4(11):1772–1785
Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL (2008) Notch signaling regulates growth and differentiation in mammalian lens. Dev Biol 321(1):111–122
Rubin LL, Haston KM (2011) Stem cell biology and drug discovery. BMC Biol 9:42
Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Si I, Yamamoto TS, Ueno N, Noda M (2001) Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 293(5527):111–115
Saravanamuthu SS, Le TT, Gao CY, Cojocaru RI, Pandiyan P, Liu CQ, Zhang J, Zelenka PS, Brown NL (2012) Conditional ablation of the Notch2 receptor in the ocular lens. Dev Biol 362(2):219–229
Schnekenburger M, Grandjenette C, Ghelfi J, Karius T, Foliguet B, Dicato M, Diederich M (2011) Sustained exposure to the DNA demethylating agent, 2′-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem Pharmacol 81(3):364–378
Seki T, Yuasa S, Fukuda K (2011) Derivation of induced pluripotent stem cells from human peripheral circulating T cells. Curr Protoc Stem Cell Biol 18:4A3.1–4A3.9
Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC (1998) A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet 19(2):167–170
Shiels A, Hejtmancik JF (2007) Genetic origins of cataract. Arch Ophthalmol 125:165–173
Shiels A, Bennett TM, Hejtmancik JF (2010) Cat-Map: putting cataract on the map. Mol Vis 16:2007–2015
Sjödal M, Edlund T, Gunhaga L (2007) Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell 13(1):141–149
Smaoui N, Beltaief O, BenHamed S, M'Rad R, Maazoul F, Ouertani A, Chaabouni H, Hejtmancik JF (2004) A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci 45(8):2716–2721
Smith AN, Miller LA, Song N, Taketo MM, Lang RA (2005) The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol 285(2):477–489
Smith AN, Radice G, Lang RA (2010) Which FGF ligands are involved in lens induction? Dev Biol 337(2):195–198
Sperduto RD, Siegel D (1980) Senile lens and senile macular changes in a population-based sample. Am J Ophthalmol 90:86–91
Stadtfeld M, Maherali N, Breault DT, Hochedlinger K (2008) Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2(3):230–240
Streit A (2004) Early development of the cranial sensory nervous system: from a common field tom individual placodes. Dev Biol 276:1–15
Swindell EC, Liu C, Shah R, Smith AN, Lang RA, Jamrich M (2008) Eye formation in the absence of retina. Dev Biol 322(1):56–64
Takahashi M, Haruta M (2006) Derivation and characterization of lentoid bodies and retinal pigment epithelial cells from monkey embryonic stem cells in vitro. Methods Mol Biol 330:417–429
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Tomishima MJ, Hadjantonakis AK, Gong S, Studer L (2007) Production of green fluorescent protein transgenic embryonic stem cells using the GENSAT bacterial artificial chromosome library. Stem Cells 25(1):39–45
Ueno M, Matsumura M, Watanabe K, Nakamura T, Osakada F, Takahashi M, Kawasaki H, Kinoshita S, Sasai Y (2006) Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc Natl Acad Sci USA 103(25):9554–9559
Wagner LM, Takemoto DJ (2001) Protein kinase C alpha and gamma in N/N 1003A rabbit lens epithelial cell differentiation. Mol Vis 7:57–62
Walker JL, Wolff IM, Zhang L, Menko AS (2007) Activation of SRC kinases signals induction of posterior capsule opacification. Invest Ophthalmol Vis Sci 48(5):2214–2223
Walker JL, Zhang L, Menko AS (2002) Transition between proliferation and differentiation for lens epithelial cells is regulated by Src family kinases. Dev Dyn 224(4):361–372
Wandl S, Wesierska-Gadek J (2009) Is olomoucine, a weak CDK2 inhibitor, able to induce apoptosis in cancer cells? Ann N Y Acad Sci 1171:242–249
Wawersik SP, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R (1999) BMP7 acts in murine lens placode development. Dev Biol 207:176–188
Wederell ED, de Iongh RU (2006) Extracellular matrix and integrin signaling in lens development and cataract. Semin Cell Dev Biol 17(6):759–776
Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458:766–770
Wu G, Glickstein S, Liu W, Fujita T, Li W, Yang Q, Duvoisin R, Wan Y (2007) The anaphase-promoting complex coordinates initiation of lens differentiation. Mol Biol Cell 18(3):1018–1029
Yabut O, Bernstein HS (2011) The promise of human embryonic stem cells in aging-associated diseases. Aging (Albany, NY) 3(5):494–508
Yamanaka S (2009) A fresh look at iPS cells. Cell 137:13–17
Yang C, Yang Y, Brennan L, Bouhassira EE, Kantorow M, Cvekl A (2010) Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J 24(9):3274–3283
Yang Y, Cvekl A (2007) Large Maf transcription factors: cousins of AP-1 proteins and important regulators of cellular differentiation. Einstein J Biol Med 23(1):2–11
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324:797–801
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML (2008) Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol 318(2):276–288
Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang F, Luo YN, Yin GW, Li Y, Wang J, Li LS, Yao YQ, Wang XH (2010) Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation 80(2–3):123–129
Acknowledgements
We thank Dr. Louise Wolf for critical suggestions. We are grateful to Dr. Eric Bouhassira and ES cell core facilities at the Ruth L. and Davis S. Gottesman Institute for Stem Cell Research and Regenerative Medicine of the Albert Einstein College of Medicine for their continuous support. Grant support to AC: R01 EY102200, EY014237 and R21 EY020621. The Department of Ophthalmology and Visual Sciences is supported by an unrestricted grant from Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cvekl, A., Yang, Y., Jing, Y., Xie, Q. (2013). Lens Differentiation from Embryonic Stem (ES) and Induced Pluripotent Stem (iPS) Cells. In: Tsang, S. (eds) Stem Cell Biology and Regenerative Medicine in Ophthalmology. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-5493-9_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5493-9_4
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-5492-2
Online ISBN: 978-1-4614-5493-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)