Abstract
This research aimed to investigate drying characteristics and quality properties of quince subjected to freeze and convective drying at air temperatures of 45, 55, 65 and 75 °C. To determine the best drying model for those techniques, nine thin-layer mathematical models were fitted to the experimental results. The statistical analyses revealed that the Two Term, Midilli et al., Diffusion Approach and Two Term Exponential models were better than the other tested models. The results of the color analyses showed that the drying treatments had an effect on the fresh quince samples, and the color of the freeze dried samples was closest to the color values of the fresh samples. The highest rehydration ratio (2.78) was recorded for the freeze dried samples, and the lowest ratio (2.25) was observed in the samples subjected to convective drying at 75 °C. Scanning electron microscopy showed that the freeze dried quince samples were only slightly different from the fresh samples and that the disruption of the structure of the quince samples significantly increased as the convective drying temperature increased. Consequently, freeze drying can be an appropriate method for obtaining good-quality fruit samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Quince (Cydonia oblonga Miller), a member of the Rosaceae family, is mostly produced in Uzbekistan, China and Turkey [1]. These countries produce 326,520 t of quince, which is 50% of the total production [2]. Quince is a beneficial product due to its nutritional and medicinal properties. It is composed of 83.8% water and 15.3% carbohydrates. Other components include proteins (0.4%) and fats (0.1%). Previous studies on quince have reported its beneficial health properties [1, 3, 4]. Quince has high levels of phenolic compounds and dietary antioxidants [3]. Therefore, it has health benefits in terms of strong antioxidant and free-radical scavenging activity. It contains high levels of polysaccharides; therefore, it is a potential source of pectin and dietary fibers [5].
Various methods have been adopted to lower the moisture contents in organic and inorganic materials, and different treatments are used on an industrial scale. One of the suitable methods for storing quince and preventing spoilage is drying the product. As is the case for other fruits, quince is perishable; however, drying is a good alternative for the preservation of quince. It decreases the water activity of the material, which lowers the microbiological activity to a level that does not allow deterioration. Dried quince fruits are used in jams, marmalades, jellies, and puddings, and they can also be included in other food products [6]. Convective drying is a common method in the food industry for producing dried fruit. The most important disadvantages of convective drying are this technique being neither a low energy-intensive process nor an energy efficient process. Dehydration by sublimation of water from a frozen product is the basis of freeze drying. As there is no liquid water in the structure of the product and a low temperature is required for freeze drying, deterioration and microbiological activity are mostly inhibited or retarded, resulting in a final product of high quality [7]. Recently, several agricultural products and byproducts have been dried with convective and freeze dry techniques to compare the changes in physical and phytochemical properties caused by the two methods. Guine and Barroca [8] studied the effects of freeze and convective drying procedures on the color and textural attributes of green bell peppers and pumpkin. The physical parameters, volatiles profile and sensory qualities of cabbage pieces have been evaluated following convective and freeze drying processes by Rajkumar et al. [9]. Rajkumar et al. [9] also evaluated the changes to the physical parameters and aromatic profile of carrot and compared the freeze and convective drying. Citrus ‘Hallabong’ powders were prepared using freeze and convective drying methods, and their physicochemical and flow properties were measured by Lee et al. [10]. Although there have been numerous studies comparing freeze and convective drying methods, notably, relatively little published data are available in the literature for drying quince. This study aims to examine the effects of different processing techniques (freeze and convective drying) on the drying kinetics, to fit the experimental results to nine different thin-layer mathematical models, to study the rehydration ratio, to determine the color differences between dried and fresh fruits and to investigate the effects of the drying techniques on the quince microstructure by scanning electron microscopy (SEM).
2 Materials and methods
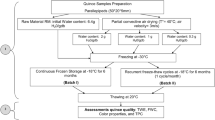
2.1 Sample preparation and drying procedure
The fresh quince samples used in the analyses were bought from a local market in Bursa, a province of Turkey, and they were stored at 4 ± 0.5 °C before the experiments. Prior to drying, the quince samples were first sliced into cubes (0.7 cm3) using a dicer (Börner, Belgium) and dried in this form [11]. The initial moisture level of these samples was calculated to be 8.43 (g water/g dry matter) on a dry basis (d.b.) by use of a forced air convection oven (ED115 Binder, Germany) operating at 105 °C for 24 h.
Two different drying methods were utilized in this study, a convective method and a freeze drying method. Convective drying (45, 55, 65 and 75 °C) was carried out using a modified convective oven (Whirlpool, Italy). A digital balance (Shimadzu UX-6200H, Japan) with a precision of ±0.01 g was placed under the convective oven to determine the mass changes [12]. Freeze drying was carried out using a laboratory-type freeze dryer (Alpha 1–2 LD Plus, Germany) at a constant pressure of 52 Pa and a processing temperature of −50 °C in the drying chamber. The amount of moisture lost throughout the drying process was determined using a digital balance (Radwag, Poland) with an accuracy of ±0.01 g. The measurements were carried out in three repetitions.
2.2 Mathematical modeling of the drying data
The experimental results of quince were fitted with 9 commonly used drying equations (listed in Table 1). The moisture ratio (MR) and drying rate (DR) are determined as follows:
In the formulas above, Mt is the instantaneous moisture content (d.b.) at a given time, Mo is the initial moisture content (d.b.) at the beginning, Me is the equilibrium moisture content (d.b.), Mt + dt is the moisture content at t + dt and t is the drying time (min). The values of Me are relatively smaller than Mt or Mo; hence, the error involved in the simplification is negligible. As a result, the moisture ratio formula was simplified in line with the previous studies to the equation given below [16]:
2.3 Color measurement
The color parameters of the quince samples were measured with a colorimeter (MSEZ-4500 L, HunterLab, Reston, USA). The color values were defined as lightness (L*), redness/greenness (a*) and yellowness/blueness (b*). Calibration of the color meter was carried out using a standard black and white plate before measuring the color values. A glass sample holder was placed close to the nose cone of the device, and the measurements of L*, a* and b* were carried out. The color measurements were carried out on the surface of the quince samples. The mean value of ten measurements was taken as the color value. Additionally, the calculations of the chroma (C) and hue angle (α) were carried out according to the following formulas [22]:
2.4 Rehydration ratio
The rehydration ratio (R) is one of the most important parameters determining the quality of a dried product, and it was calculated using Eq. 5 in which M0 and M2 are the sample weights (g) before and after rehydration, respectively.
The rehydration ratios were determined by immersing 3 ± 0.01 g of the dried sample in a glass beaker filled with 400 mL of distilled water at 20 °C (±1 °C) [23]. The samples were drained at 20-min intervals, blotted with tissue paper, and the weights of the samples were measured using a digital balance (Radwag, Radom, Poland) with an accuracy of ±0.01 g.
2.5 Microstructure
A scanning electron microscope (EVO 40, Germany) was used to examine the effect of different drying methods on the microstructures of the quince samples. Particles extracted from the dried samples were vertically cut along the center of the pith and appropriately placed in aluminum discs. The samples were then coated with gold-palladium with a thickness of 40–50 nm under a low vacuum (20 kV) using a coating device (SCD-005, Baltec, Wetzlar, Germany), and then micrographs of the samples were taken [24].
2.6 Statistical analysis
The research was carried out by randomized factorial plot design type of experiment. The analyses were performed in three repetitions. The statistical analysis of the data was conducted using JMP (Version 7.0, USA) and MATLAB (MathWorks Inc., Natick, MA) software. Calculation of the mean differences and application of the LSD (least significant difference) test to measure significance was conducted at a significance level of 5%. The thin-layer model best identifying the drying kinetics of the quince samples is the model with the highest coefficient of determination (R2) and the lowest root mean square error (RMSE) and reduced chi-squared (χ2) values as follows [20]:
where, MRpre, i is the predicted moisture ratio, MRexp, i is the experimental moisture ratio, N is the number of constants in the drying model and n is the number of observations.
2.7 Uncertainty analysis
The uncertainty analysis is needed to prove the accuracy of the experiments. Uncertainty of any measurement can be expressed using the general expression given below [25]:
where \( {\left(\frac{\partial F}{\partial {y}_1}{w}_1\right)}^2 \) is the uncertainty in the result, w1, w2, …,wn are the uncertainty in the independent variables; y1, y2,…,yn, are the independent variables and F is the function of the independent variables. In drying experiments of quinces, the temperatures, pressures, and weight losses were measured with appropriate instruments.
3 Results and discussion
3.1 Drying kinetics of quince
The drying curves of the quince fruits under convective and freeze drying conditions are given in Fig. 1. It was found that the drying times of the quince cubes at 45, 55, 65 and 75 °C by convective drying were 120, 90, 70 and 50 min, respectively. With freeze drying, the drying process took 7 h until the desired moisture content was reached. For convection drying, as the air temperature was increased from 45 to 75 °C, the drying time decreased by 58.33%. Thus, the increase in temperature significantly shortened drying time. Aral and Beşe [24] have stated that the increase in temperature enhances the vapor pressure in the fruit, and this causes the moisture from the inside of the fruit to be removed faster than the moisture at the surface. On the other hand, convective drying is a slow process and drying occurs mostly by moisture diffusion. The factor effecting the diffusion is concentration difference. The relative humidity decreases by temperature increase. This results in an increase in the concentration difference and drying fastens. The results obtained in this study were in line some previous studies on the drying of various food products including tomato [19], onion slices [26] and cape gooseberry [27]. In the freeze drying process, the moisture in the fruit was primarily removed in the early stages of the drying process, and the removal rate decreased in the later stages. Siriamornpun et al. [12] reported that in the freeze drying of marigold, the petals were frozen at −40 °C under vacuum drying for 48 h using a freeze dryer; thereafter, the petals had 7.6% moisture. A convective dryer was utilized for convective drying at 60 °C for 4 h; the resulting dried marigold had a moisture content of 7.4%. Additionally, Gaware et al. [28] came to a similar conclusion about the drying times with convective and freeze drying procedures for tomatoes. Caliskan and Dirim [13] reported that depending on the parameters, including humidity of the drying medium, temperature, and air velocity, the drying times for freeze drying are substantially longer than those required for convective drying.
The drying rates versus the moisture content data under the different drying conditions are presented in Fig. 2. The results indicated that higher drying rates were obtained with higher temperatures; thus the air temperature has an important effect on the drying rate of quinces. The drying rate has been reported to increase with an increase in the air temperature [14]. Furthermore, convective drying results in large increases in drying rates compared to freeze drying.
3.2 Modeling of the drying curves
The statistical results of the 9 thin-layer mathematical drying models are shown in Table 2. In all cases, the R2 values were higher than 0.97, and the RMSE and χ2 values were lower than 0.0566 and 31.0022 × 10−4, respectively, indicating good fits. These results show that the drying methods used in the present study were sufficient to describe the drying behavior of quince. Additionally, the best statistical values were obtained with Two Term (freeze drying), Midilli et al. (45 °C), Diffusion Approach (65 °C) and Two Term Exponential (55 and 75 °C) fits. For all drying conditions, the R2, RMSE and χ2 values of the Two Term, Midilli et al., Diffusion Approach and Two Term Exponential models ranged from 0.9781 to 0.9997, 0.0065 to 0.0565 and 0.3241 × 10−4 to 31.0021 × 10−4; 0.9980 to 0.9996, 0.0066 to 0.0158 and 0.3718 × 10−4 to 2.3496 × 10−4; 0.9891 to 0.9988, 0.0112 to 0.0374 and 0.9545 × 10−4 to 11.1368 × 10−4; and 0.9964 to 0.9995, 0.0071 to 0.0208 and 0.2827 × 10−4 to 4.9740 × 10−4, respectively.
The changes in the predicted and experimental moisture ratios using the best models for various drying times for freeze drying and for selected temperatures for dried quince are shown in Fig. 3. Obviously, the results obtained from the Two Term, Midilli et al., Diffusion Approach and Two Term Exponential models are quite close to the experimental values. Based on these results, the Two Term, Midilli et al., Diffusion Approach and Two Term Exponential models could adequately describe the drying curves of quince under the selected experimental conditions. The results of this study are in agreement with earlier results reported for the drying of ginger [29] using the Two Term model, tomato [16] using the Midilli et al. model, castor oil seeds [20] using the diffusion approach model and kiwi slices [18] using the Two Term Exponential model.
3.3 Color changes
Color is an important indicator for visually estimating the quality of dried quince as color can even indicate the contents of bioactive and thermally sensitive components [30].
L* value
The difference in the lightness values of the dried quince cubes compared to the values of the fresh samples are given in Fig. 4a. It was found that the L* values of the dried samples decreased significantly from 79.70 to 60.78 by the end of the drying process carried out at 75 °C. The highest L* value was found for the freeze dried method (71.87), and the L* values of the other samples were closer to that of the fresh sample. The colors of the samples dried at 75 °C were significantly darker than those of the freeze dried samples. It was determined that the increase in the temperature decreased the L* values of the samples.
a* value
As seen Fig. 4b, the redness value of the fresh sample increased significantly in all the drying methods at all parameters (P < 0.05). The a* values of the samples ranged from 7.91 to 10.39 for convective drying. The a* values of the freeze dried samples were the closest to those of the fresh samples.
b* value
The yellowness values of the fresh and dried quince samples are shown in Fig. 4c. It was found that the b* values of all samples decreased significantly from 37.75 to 31.31 by the end of the drying process carried out at 75 °C (P < 0.05). The highest b* value was found for the freeze dried sample (37.75). The samples processed at 75 °C had the lowest b* value (31.31).
C and α values
The CIE Lab color coordinates and the chroma and hue angle color attributes are commonly used to evaluate the optical properties of fruits and vegetables [31]. Chroma and hue angle are also very important color parameters. The chroma and hue angle values determined for the quince samples are given in Figs. 4d and e. It was determined that there was a significant increase in the hue angle values of the dried quince cubes compared to those of the fresh samples (P < 0.05). The α value of the freeze dried sample (81.99°) was close to that of the fresh sample (86.27°). The α values of all the dried samples were lower than that of the fresh sample. This shows that browning was higher in the dried samples. The C values of the fresh samples decreases in all dried samples, similar to the trend in the α values. The decreases observed for the C and α values can be associated with the degradation of carotenoids due to heating [8]. Higher chroma values in the freeze dried quince cubes and lower chroma values in the convective dried samples support the high L* values of the freeze dried samples and low L* values of the convective dried samples. The higher chroma values are indicators of the color intensity of the dried samples perceived by consumers [32]. It was determined that in the convective drying samples, the yellowness values of the samples decreased with increasing processing time and temperature, and the samples gained a brownish color during the drying process. This result is also in line with the decrease observed in the L* and b* values. However, it was found that there was an increase in the a* values for all drying temperatures. It was thought that the increase in a* and the decreases in L* and b* can be attributed to the enzymatic activity, especially non-enzymatic browning reactions, in addition to the decomposition of some pigments including carotenoids and chlorophyll [33]. Similar results have been reported by Izli and Isik [34] in their study on the convective drying of corn. The best color values were found in the freeze dried samples. Color changes in the quince samples caused by heating can be attributed to pigment degradation, especially the degradation of carotenoids, and the formation of brown pigments by enzymatic and non-enzymatic reactions (Maillard reaction) [35]. Similar experiments have been reported by Izli [17] with dates and by Topuz et al. [22] with paprika.
3.4 Rehydration ratio
Rehydration is a complex reaction mechanism, and the product characteristics and processing conditions influence rehydration. The rehydration of dried products with cellular structures is quite complex because the drying applications can affect the rehydration process due to changes in the chemical and physical properties of the material during the removal of water [36]. The rehydration curves obtained for the freeze dried samples and the convective dried samples at the four tested temperatures are seen in Fig. 5. Among the used methods, the highest and lowest rehydration ratio was 2.78 min with freeze drying and 2.25 with convective drying (75 °C), respectively. It was determined that at 45 °C, the final absorption rate was higher than at the other temperatures tested in the convective drying processes. This could be due to tissue collapse and shrinkage caused by higher air temperatures, resulting in a decrease in rehydration capacity [37]. Similar results have been observed by Beigi [38] with wormwood (Artemisia absinthium L. leaves). The rehydration ratio of convective dried quince cubes was found to be significantly lower than that of freeze dried quince cubes (P < 0.05). Caliskan and Dirim [13] reported a similar conclusion for the final moisture content when comparing the rehydration of freeze and convective dried pumpkin slices. Additionally, Hernando et al. [39] observed the same trend in the drying of Boletus edulis mushrooms. This result is regarded as normal because convective drying usually disrupts the structure of the food cells due to the high level of shrinkage. This shrinking reduces the hydrophilicity of the sample. Therefore, freeze drying yields better results in terms of a porous cell structure, which results in the presence of more capillary paths in the quince cubes. Yi et al. [40] have stated that these paths might promote water infiltration during immersion, causing a quicker rehydration rate and higher rehydration capacity for the freeze dried product.
3.5 Microstructural analysis
Figure 6 shows the micrographs of quince. It is obvious from the photograph that the freeze dried quince possess a homogeneous honeycomb structure. This indicates that freeze drying has a minimal effect on the cell structure. In freeze drying, water removal takes place by sublimation from frozen substances while under vacuum. This explains why freeze dried samples decrease in density and their cell structure remains relatively intact. As a result of the convective drying process, the cell walls break and microcavities form in the structure of quince. The SEM images show that the level of porosity decreases at higher drying temperatures. Water evaporation is slower at lower temperatures, and the moisture gradient is small between the center and surface of the fruit, leading to lower internal stress. In addition, the required exposure time is longer for low temperatures, and this can lead to the merging or collapsing of cell walls. Such processes eventually lead to significant changes in the structure of the fruit cells. Similar differences between convective and freeze dried samples have been observed by Huang et al. [41] and An et al. [42] with quince.
3.6 Uncertainty analyses for the measurement
The uncertainty values for experimental parameters are shown in Table 3. The results of the uncertainty obtained for the quince drying analysis in this study were well below the acceptable limit of 5%. This maximum value is proposed by Kayacan et al. [25] and Salehi et al. [43] and for dying of bee pollen and button mushroom, respectively.
4 Conclusions
In this study, the drying kinetics, color, microstructural properties and rehydration behavior of convective and freeze dried and fresh quince cubes were determined. In addition, the experimental moisture loss results were fitted to 9 different thin-layer drying models. The total drying times were found to be 130, 90, 70 and 50 min for 45, 55, 65 and 75 °C, respectively, for convective drying; and for 7 h for freeze drying. The results indicated that increasing the air temperature caused shorter drying times. In contrast to the convective drying process, the freeze drying process provided the best product color results, which were closest to the L* and b* values of the fresh sample. Among the thin-layer drying models tested, the Two Term, Midilli et al., Diffusion Approach and Two Term Exponential models provided the best representations of the experimental results. The rehydration ratio values of the freeze dried cubes were found to be higher than those of the convective dried cubes, and the rehydration properties improved when the quince samples were dried at lower hot air temperatures. Additionally, the SEM images showed that the size of the pores in the freeze dried quince samples were similar to those of the cellular microstructure of fresh quince, and higher drying temperatures caused deformation in the microstructure of the quince samples. In conclusion, freeze drying is an important application for improving the quality of dried quince, including improving its microstructure, color, and rehydration ratio.
References
Pacifico S, Gallicchio M, Fiorentino A, Fischer A, Meyer U, Stintzing FC (2012) Antioxidant properties and cytotoxic effects on human cancer cell lines of aqueous fermented and lipophilic quince (Cydonia oblonga Mill.) preparations. Food Chem Toxicol 50(11):4130–4135. https://doi.org/10.1016/j.fct.2012.07.061
FAO (2015) Food and Agriculture Organization. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor. Accesed 29 November 2017
Wojdyło A, Teleszko M, Oszmiański J (2014) Antioxidant property and storage stability of quince juice phenolic compounds. Food Chem 152:261–270. https://doi.org/10.1016/j.foodchem.2013.11.124
Adhami S, Rahimi A, Hatamipour MS (2013) Freeze drying of quince (Cydonia oblonga): Modelling of drying kinetics and characteristics. Korean J Chem Eng 30(6):1201–1206. https://doi.org/10.1007/s11814-013-0038-3
Rop O, Balik J, Reznicek V, Jurikova T, Skardova P, Salas P, Sochor J, Mlcek J, Kramarova D (2011) Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech J Food Sci 29:65–73. https://doi.org/10.17221/212/2009-CJFS
Noshad M, Mohebbi M, Shahidi F, Mortazavi SA (2012) Multi-objective optimization of osmotic–ultrasonic pretreatments and hot-air drying of quince using response surface methodology. Food Bioprocess Technol 25(6):2098–2110. https://doi.org/10.1007/s11947-011-0577-8
Rawson A, Tiwari BK, Tuohy MG, O’Donnell CP, Brunton N (2011) Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason Sonochem 18(5):1172–1179. https://doi.org/10.1016/j.ultsonch.2011.03.009
Guine RPF, Barroca MJ (2012) Effect of drying treatments on texture and color of vegetables (pumpkin and green pepper). Food Bioprod Process 90:58–63. https://doi.org/10.1016/j.fbp.2011.01.003
Rajkumar G, Shanmugam S, Galvao MDS, Leite Neta MTS, Dutra Sandes RD, Mujumdar AS, Narain N (2017) Comparative evaluation of physical properties and aroma profile of carrot slices subjected to hot air and freeze drying. Dry Technol 35(6):699–708. https://doi.org/10.1080/07373937.2016.1206925
Lee CW, Oh HJ, Han SH, Lim SB (2012) Effects of hot air and freeze drying methods on physicochemical properties of citrus ‘hallabong’ powders. Food Sci Biotechnol 21(6):1633–1639. https://doi.org/10.1007/s10068-012-0217-8
Oikonomopoulou VP, Krokida MK (2012) Structural properties of dried potatoes, mushrooms, and strawberries as a function of freeze-drying pressure. Dry Technol 30(4):351–361. https://doi.org/10.1080/07373937.2011.639475
Siriamornpun S, Kaisoon O, Meeso N (2012) Changes in colour, antioxidant activities and carotenoids (lycopene, β-carotene, lutein) of marigold flower (Tagetes erecta L.) resulting from different drying processes. J Funct Foods 4(4):757–766. https://doi.org/10.1016/j.jff.2012.05.002
Caliskan G, Dirim SN (2017) Drying characteristics of pumpkin (Cucurbita moschata) slices in convective and freeze dryer. Heat Mass Transf 53(6):2129–2141. https://doi.org/10.1007/s00231-017-1967-x
Tzempelikos DA, Vouros AP, Bardakas AV, Filios AE, Margaris DP (2015) Experimental study on convective drying of quince slices and evaluation of thin-layer drying models. Eng Agric Environ Food 8(3):169–177. https://doi.org/10.1016/j.eaef.2014.12.002
Doymaz İ, Demir H, Yildirim A (2015). Drying of quince slices: effect of pretreatments on drying and rehydration characteristics. Chem Eng Commun 202(10):1271–1279. https://doi.org/10.1080/00986445.2014.921619
Sadin R, Chegini GR, Sadin H (2014) The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat Mass Transf 50(4):501–507. https://doi.org/10.1007/s00231-013-1255-3
İzli G (2017) Total phenolics, antioxidant capacity, colour and drying characteristics of date fruit dried with different methods. Food Sci Technol 37(1):139–147. https://doi.org/10.1590/1678-457x.14516
Ergün K, Çalışkan G, Dirim SN (2016) Determination of the drying and rehydration kinetics of freeze dried kiwi (Actinidia deliciosa) slices. Heat Mass Transf 52(12):2697–2705. https://doi.org/10.1007/s00231-016-1773-x
Belghith A, Azzouz S, ElCafsi A (2016) Desorption isotherms and mathematical modeling of thin layer drying kinetics of tomato. Heat Mass Transf 52(3):407–419. https://doi.org/10.1007/s00231-015-1560-0
Perea-Flores MJ, Garibay-Febles V, Chanona-Perez JJ, Calderon-Dominguez G, Mendez-Mendez JV, Palacios-González E, Gutierrez-Lopez GF (2012) Mathematical modelling of castor oil seeds (Ricinus communis) drying kinetics in fluidized bed at high temperatures. Ind Crop Prod 38:64–71. https://doi.org/10.1016/j.indcrop.2012.01.008
Mohamadi M, Pourfallah M, Nahardani Z, Meshkani M (2015) Mathematical modeling and optimization of drying kinetic of quince (Cydonia olonga) fruit slices. J Food Technol Nutr 12(1):49–58
Topuz A, Feng H, Kushad M (2009) The effect of drying method and storage on color characteristics of paprika. LWT-Food Sci Technol 42(10):1667–1673. https://doi.org/10.1016/j.lwt.2009.05.014
Doymaz İ (2012) Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energ Convers Manage 56:199–205. https://doi.org/10.1016/j.enconman.2011.11.027
Aral S, Beşe AV (2016) Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chem 210:577–584. https://doi.org/10.1016/j.foodchem.2016.04.128
Kayacan S, Sagdic O, Doymaz I (2018) Effects of hot-air and vacuum drying on drying kinetics, bioactive compounds and color of bee pollen. J Food Meas Charact 12(2):1274–1283. https://doi.org/10.1007/s11694-018-9741-4
Lee JH, Kim HJ (2008) Drying kinetics of onion slices in a hot-air dryer. Prev Nutr Food Sci 13(3):225–230. https://doi.org/10.3746/jfn.2008.13.3.225
Vega-Gálvez A, Zura-Bravo L, Lemus-Mondaca R, Martinez-Monzó J, Quispe-Fuentes I, Puente L, Di Scala K (2015) Influence of drying temperature on dietary fibre, rehydration properties, texture and microstructure of Cape gooseberry (Physalis peruviana L.). J Food Sci Technol 52(4):2304–2311. https://doi.org/10.1007/s13197-013-1235-0
Gaware TJ, Sutar N, Thorat BN (2010) Drying of tomato using different methods: comparison of dehydration and rehydration kinetics. Dry Technol 28(5):651–658. https://doi.org/10.1080/07373931003788759
Parlak N (2015) Fluidized bed drying characteristics and modeling of ginger (Zingiber officinale) slices. Heat Mass Transf 51(8):1085–1095. https://doi.org/10.1007/s00231-014-1480-4
Ban ZJ, Wei WW, Yang XZ, Feng JH, Guan JF, Li L (2015) Combination of heat treatment and chitosan coating toimprove postharvest quality of wolfberry (Lycium barbarum). Int J Food Sci Technol 50:1019–1025. https://doi.org/10.1111/ijfs.12734
Garcia-Martinez E, Igual M, Martin-Esparza ME, Martinez-Navarrete N (2013) Assessment of the bioactive compounds, color, and mechanical properties of apricots as affected by drying treatment. Food Bioprocess Technol 6:3247–3255. https://doi.org/10.1007/s11947-012-0988-1
Pathare PB, Opara UL, Al-Said FAJ (2013) Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol 6(1):36–60. https://doi.org/10.1007/s11947-012-0867-9
Fang S, Wang Z, Hu X, Chen F, Zhao G, Liao X, Wu J, Zhang YAN (2011) Energy requirement and quality aspects of Chinese jujube (Zizyphus jujuba Miller) in hot air drying followed by microwave drying. J Food Process Eng 34(2):491–510. https://doi.org/10.1111/j.1745-4530.2009.00372.x
Izli N, Isık E (2013) Batch drying characteristics of dent corn (Zea mays var. indentata Sturt.). J Food Agric Environ 11(1):259–263
Kammoun Bejar A, Kechaou N, Boudhrioua Mihoubi N (2011) Effect of microwave treatment on physical and functional properties of orange (Citrus sinensis) peel and leaves. J Food Process Technol 2(02):109–116. https://doi.org/10.4172/2157-7110.1000109
Seremet L, Botez E, Nistor OV, Andronoiu DG, Mocanu GD (2016) Effect of different drying methods on moisture ratio and rehydration of pumpkin slices. Food Chem 195:104–109. https://doi.org/10.1016/j.foodchem.2015.03.125
Vega-Gálvez ALMS, Lemus-Mondaca R, Bilbao-Sáinz C, Fito P, Andrés A (2008) Effect of air drying temperature on the quality of rehydrated dried red bell pepper (var. Lamuyo). J Food Eng 85(1):42–50. https://doi.org/10.1016/j.jfoodeng.2007.06.032
Beigi M (2017) Thin layer drying of wormwood (Artemisia absinthium L.) leaves: dehydration characteristics, rehydration capacity and energy consumption. Heat Mass Transf 53(8):2711–2718. https://doi.org/10.1007/s00231-017-2018-3
Hernando I, Sanjuán N, Pérez-Munuera I, Mulet A (2008) Rehydration of freeze-dried and convective dried Boletus edulis mushrooms: effect on some quality parameters. J Food Sci 73(8):356–362. https://doi.org/10.1111/j.1750-3841.2008.00913.x
Yi J, Zhou L, Bi J, Chen Q, Liu X, Wu X (2016) Impacts of pre-drying methods on physicochemical characteristics, color, texture, volume ratio, microstructure and rehydration of explosion puffing dried pear chips. J Food Process Preserv 40(5):863–873. https://doi.org/10.1111/jfpp.12664
Huang TC, Chung CC, Wang HY, Law CL, Chen HH (2011) Formation of 6-shogaol of ginger oil under different drying conditions. Dry Technol 29(16):1884–1889. https://doi.org/10.1080/07373937.2011.589554
An K, Zhao D, Wang Z, Wu J, Xu Y, Xiao G (2016) Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem 197:1292–1300. https://doi.org/10.1016/j.foodchem.2015.11.033
Salehi F, Kashaninejad M, Jafarianlari A (2017) Drying kinetics and characteristics of combined infrared-vacuum drying of button mushroom slices. Heat Mass Transf 53(5):1751–1759. https://doi.org/10.1007/s00231-016-1931-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izli, N., Polat, A. Freeze and convective drying of quince (Cydonia oblonga Miller.): Effects on drying kinetics and quality attributes. Heat Mass Transfer 55, 1317–1326 (2019). https://doi.org/10.1007/s00231-018-2516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-018-2516-y