Abstract
The effects of air drying temperature on dietary fibre, texture and microstructure of the Cape gooseberry fruits during convective dehydration in the range of 50–90 ºC were investigated. The ratio of insoluble dietary fibre to soluble dietary fibre was higher than 7:1 for all dehydrated samples. At 50 ºC tissue structure damage was evidenced leading to the maximum water holding capacity (47.4 ± 2.8 g retained water/100 g water) and the lowest rehydration ratio (1.15 ± 0.06 g absorbed water/g d.m.). Texture analysis showed effects of drying temperatures on TPA parameters. Changes in microstructure tissue were also observed at the studied drying temperatures. Hot air drying technology leads not only to fruit preservation but also increases and adds value to Cape gooseberry, an asset to develop new functional products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cape gooseberry or golden berry (Physalis peruviana L., Solanaceae) is an herbaceous, semi-shrub, upright, and perennial plant in subtropical zones. They are golden spheres, the size of marbles, with a pleasing taste. They are protected by papery husks that act as a protecting shield against insects, birds, diseases and adverse climatic situations (Ramadan and Morsel 2003; Hassanien 2011; Puente et al. 2011). The benefits associated to Cape gooseberry are mainly due to its nutritional composition as well as to the presence of its bioactive components (Pinto et al. 2009; Puente et al. 2011; Hassanien 2011). In addition, they are an excellent source of provitamin A, vitamin C, iron, and some of the vitamin B-complex (Salazar et al. 2008). In addition, Cape gooseberry is an interesting source of dietary fibre which acts as a bulking agent, normalizing intestinal motility and preventing diverticular disease among other diseases (Garau et al. 2007; Borchani et al. 2011). Therefore, due to its high content of phytochemicals, Cape gooseberry lends itself to the production of a number of processed products, in particular, dehydrated products.
Nowadays, there is growing recognition of the potential role of functional foods in helping to reduce health risks and improve health (Pinto et al. 2009; Betoret et al. 2011). Dehydration is one of the most important unit operations used in formulation of a functional food product (Oliveira et al. 2008). It is a post-harvest technology, being the main objective the removal of water to the level at which microbial spoilage and deterioration reactions are minimised. Regardless of these benefits, dehydration causes evident changes in the microstructure of fruit tissues (Vega-Gálvez et al. 2011). Structuring of the food matrix is an important tool to improve the stability of healthier formulations and the bioavailability of bioactive compounds (Kauffmann and Palzer 2011). Thus, drying process requires microscopical analysis to address structural and microstructural changes produced at cellular level and their potential influence on firmness. These changes are related to the loss of water from the inner parts towards the surface and the surrounding air, possibly causing stiffness, spoilage, and disruption of the cell walls, or even a collapse of the cell tissues (Krokida and Maroulis 2001). Structural properties of the product depend on the type of drying, operative variables and food microstructure formed during the process (Vega-Gálvez et al. 2011). In addition, rehydration capacity which is related to water holding capacity can be considered as a measure of the injury to the material caused by drying (Krokida and Philippopoulos 2005).
Furthermore, dehydration is regarded not only as a preservation process, but also as a method to increase and add value to foods. Thus, by means of the selection of appropriate control variables (air-drying temperature, air flow rate, relative humidity, etc.) higher yield, from the operational and capital investment points of view, can be achieved, leading to a final product with high quality (Di Scala et al. 2011).
Therefore, the aim of the present investigation was to evaluate the effect of air-drying temperature on drying rates, rehydration indices, dietary fibre, texture profile and microstructure of Cape gooseberry during convective dehydration at 50, 60, 70, 80 and 90 ºC.
Materials & methods
Raw materials and drying experiments
Cape gooseberries were cultivated and purchased in the city of Olmue (V-Region, Neuquen Agricultural) Chile. The samples were selected to provide a homogeneous group, based on date of harvest, colour, size, and freshness according to visual analysis. They were refrigerated at 5 ºC until drying. The moisture content was determined by AOAC method n◦ 934.06 (AOAC 1990) employing a vacuum oven (Gallenkamp, OVL570, UK) and an analytical balance accurate to ± 0.0001 g (CHYO, Jex120, Japan).
Drying experiments, performed in triplicate, were carried out at four temperatures (50, 60, 70, 80 and 90 ºC), employing a constant air flow of 1.5 ± 0.2 m/s (perpendicular direction to sample). The Cape gooseberries were arranged in a thin layer within a stainless steel basket with a load density of 14.51 ± 0.66 kg/m2. The drying process was carried out in a convective dryer (Fig. 1) designed and built at the Department of Food Engineering of Universidad de La Serena. The weight of the sample was measured on an analytical balance (Ohaus, SP402, USA), with an accuracy of ± 0.01 g at defined time intervals, connected by a system interface (Ohaus, RS232, USA) to a personal computer (PC), which served as a monitor to record the data until constant weight (equilibrium condition) is reached.
Total dietary fibre
The total dietary fibre (TDF) was determined by a gravimetric-enzymatic method (AOAC nº 985.29) using a Total Dietary Fibre Assay Kit (TDF100A; Sigma-Aldrich, Missouri, USA). Briefly, the samples were suspended in phosphate buffer 0.08 M, pH 6.0 and digested sequentially with heat stable α-amylase at 95–100 °C, protease at 60 °C, and amyloglucosidase at 60 °C. To precipitate the soluble dietary fibre 4 volumes of 95 % ethanol, preheated at 60 °C, were added. The residue was filtered through tarred fritted glass crucibles. The crucibles containing TDF were rinsed with dilute alcohol followed by acetone and were dried overnight in an oven at 105 °C. Half of the samples were analyzed for protein content (Kjeldahl nitrogen × 6.25) and the other half was ashed in a muffle furnace at 525 °C for 5 h. Thus, TDF was measured as the difference between residues weight and protein and ash weights, and expressed in g/kg dry matter. All the analyses were made in triplicate.
Rehydration properties: Water holding capacity and rehydration ratio
Dehydrated Cape gooseberries were put to soak in distilled water at 20 °C for 14 h, using a solid to liquid ratio of 1:50. The samples were then removed, drained for 30 s, and weighed. This process was done in triplicate for each treatment. Rehydration ratio (RR) was calculated according to Eq. (1) (Di Scala et al. 2011). Water holding capacity (WHC) was determined by centrifuging the rehydrated samples at 4000xg for 10 min at 5 °C in tubes fitted with a centrally placed plastic mesh which allowed water to drain freely from the sample during centrifugation. WHC was calculated from the amount of water removed according to Eq. (2):
Where: Wrs is weight of rehydrated sample (g), Xrs is moisture content of rehydrated sample (wet matter), Wds is weight of dried sample (g), Xds is moisture content of dried sample (wet matter) and Wdl is weight of dripped liquid after centrifugation.
Instrumental texture profile analysis (TPA)
TPA was performed with a TA-XT Plus texture analyzer (Stable Micro Systems Ltd., Tokyo, Japan) on 15 units of Cape gooseberry of each treatment group. All experiments were conducted in a controlled temperature room at 20 °C. The test conditions were two consecutive cycles of 50 % compression with 5 s between cycles. Measurements were taken with cylindrical aluminium probe of 40 mm in diameter. The crosshead moved at a constant speed of 3 mm/s. From the resulting force–time curve, the following parameters were determined: hardness (N), maximum force required to compress the sample; fracturability (N), the force during the first compression at which the material fractures; cohesiveness, extent to which the sample could be deformed prior to rupture; springiness (cm), ability of sample to recover its original form after the deforming force is removed; gumminess (N/cm2), the force needed to disintegrate a semisolid sample at a steady state of swallowing (hardness × cohesiveness); chewiness (N/cm), the work needed to chew a solid sample at a steady state of swallowing (springiness × gumminess).
Cryo-SEM observations
Sample microstructure was observed by Cryo-SEM in a JEOL JSM-5410 microscope (Jeol, Tokyo, Japan). Freeze dried Cape gooseberry samples were rehydrated for 10 h at room temperature. Square rehydrated samples of more or less 4 mm × 1.5 mm × 5 mm were cryo-fixed by immersion in slush nitrogen (−210 °C), fractured, etched (at −90 °C, 10−5 Torr for 20 min), gold-coated, and viewed on the SEM cold-stage. The fractured surface was viewed directly while it was maintained at −150 °C or lower. Observations were carried out when the microscope was at 10 kV and the working distance was set at 15 mm. Two samples were studied for each treatment.
Statistical analysis
The effect of air-drying temperature on each quality parameter was estimated using Statgraphics® Plus 5 (Statistical Graphics Corp., Herndon, VA, USA). The results were analyzed by an analysis of variance (ANOVA). Differences among the media were analyzed using the least significant difference (LSD) test with a significance level of α = 0.05 and a confidence interval of 95 % (p < 0.05). In addition, the multiple range test (MRT) included in the statistical program was used to demonstrate the existence of homogeneous groups within each of the parameters.
Results and discussion
Drying kinetics
Cape gooseberry presented an initial moisture content of 83.45 ± 0.15 g/100 g sample. Figure 2 shows the variation of the experimental drying rate as a function of moisture ratio for the five working temperatures (50, 60, 70, 80 and 90 °C). As can be observed, increasing the temperature of the drying medium increased the drying potential and the moisture removal rates. Consequently, the drying time to reach similar moisture content decreased as process temperature increased. For example, the time required to achieve a moisture ratio of 0.1 at 90 °C is 180 min compared to almost 800 min needed to reach that moisture content at 50 °C (Vega-Gálvez et al. 2012). These results were comparable to those reported in previous investigations during fruits drying like apple, sour cherry, strawberry, blueberries, sweet cherry, pepino fruit and sultana grapes (Karabulut et al. 2007; Doymaz 2008; Vega-Gálvez et al. 2012; Doymaz and Ismail 2011; Uribe et al. 2009; Li et al. 2010). Cost-effectiveness and dried product quality are highly dependent on air-drying temperature (Chong and Law 2010). Therefore, by careful selection of this process variable, minimization of processing time as well as maintenance or enhancement of fruit final quality could be achieved.
Dietary fibre
The definition of dietary fibre (DF) as the sum of lignin and the plant polysaccharides that are not digested by the endogenous secretions of the human digestive tract is a physiological, not a chemical definition. It covers a wide variety of chemical substances with different physical properties. DF was classified according to its solubility, as soluble dietary fibre (SDF) and insoluble dietary fibre (IDF). As a consequence of such differences in its hydratation capabilities, each fibre type produces a different physiological response (Garcia et al. 2010). In addition, the functional properties of dietary fibre vary widely depending on sources of the fibre (Peerajit et al. 2012). Table 1 presents the values of IDF, SDF as well as TDF of fresh and dehydrated Cape gooseberry. The IDF fraction of fresh sample was higher than those reported previously for different Indian fruits (Ramulu and Rao 2003) but comparable with the correspondent values reported for mango and passion fruit (Martínez et al. 2012). Moreover, the high IDF content indicates considerable amounts of celluloses and hemicelluloses present in the fruit. A high proportion of IDF could be considered an advantage because IDF can be used by the food industry as an ingredient to increase the content of indigestible insoluble compounds. In addition, a high IDF content could have beneficial health effects related to increases in satiety and in the volume and weight of faecal mass, thus promoting improved functioning of the digestive system (Peerajit et al. 2012).
When comparing dehydrated samples with fresh ones, the results showed that the applied drying temperatures had significant effects on IDF except for 90 °C (p < 0.05). The minimum retention of IDF was observed at 50 °C (68 %), probably due to long drying times. Regarding SDF, treatments at 60, 70 and 80 °C presented significant differences with fresh samples (p < 0.05). However, among dehydrated samples significant differences were not observed. Comparable results were reported by Borchani et al. (2012) working with dehydrated date fibre concentrate.
Since IDF has a high water holding capacity and seems to relate to the intestinal regulation and SDF is involved in decreasing the rate of lipid and carbohydrate absorption, it is recommended that the ratio of IDF to SDF should be in the range of 1.0–2.3 for certain food application (Peerajit et al. 2012). The ratio IDF to SDF in fresh Cape gooseberry samples presented a value of 7:1.
Rehydration indices
Rehydration behaviour has been considered as a measure of the induced damage in the material during drying. The ability of food products to reconstitute depends primarily on the internal structure of the dried pieces and the extent to which the water-holding components (e.g., proteins and starch) have been damaged during drying (Krokida and Philippopoulos, 2005).
Figure 3 presents the behaviour of the rehydration ratio (RR) as well as the water holding capacity (WHC) for each air-drying temperature studied. The WHC decreased as the air temperature increased in the range of 50–70 °C and presented an increase with temperature in the range of 70–90 °C (p < 0.05). The maximum WHC was 47.4 ± 2.8 (g retained water/100g water) at 50 ºC, which implies that this drying temperature causes less tissue structure damage; thus, the Cape gooseberry dehydrated at this temperature retained a great amount of water. On the other hand, samples dried at 80 and 90 ºC have reduced their WHC, thereby preventing the complete rehydration of the dried product. Similar investigations reported that drying temperature is the main factor affecting the WHC (Vega-Gálvez et al. 2011; Miranda et al. 2010). In the same figure, RR was affected by the drying temperatures, since absorbed water decreased with temperature. However, RR showed no significant differences (p > 0.05). The lowest RR value was 1.15 ± 0.06 (g absorbed water/g d.m.) at 50 °C, this could be explained due to cellular structure damage resulting in modifications of osmotic properties of the cell as well as lower diffusion of water through the surface during rehydration (Kaymak-Ertekin 2002). It is generally accepted that the degree of rehydration is dependent on the degree of cellular and structural disruption. Since, rehydration of dried plant tissues is composed of three simultaneous processes: the imbibition of water into the dried material, the swelling and finally the leaching of soluble solids from the dried material, the shrinkage that takes place during dehydration prevents rehydration and produces products with lower apparent density and higher porosity (Krokida and Philippopoulos 2005).
Texture profile analysis
Textural properties of Cape gooseberry as affected by air drying temperature are shown in Table 2. The mechanical properties measured by means of TPA are very important because they relate to the food sensory properties detected by humans. Hardness of dehydrated samples showed lower values than the correspondent value for the fresh sample, showing significant differences with the original value (p < 0.05). Among dehydrated samples, a maximum decrease of 30 % with respect to the fresh sample was evidenced at 80 ºC (p < 0.05). Softening of Cape gooseberry could be associated with the breakdown of structural cell wall made of carbohydrates, e.g. insoluble pectin, xyloglucan and hemicelluloses located in the middle lamella with a concomitant increase in soluble pectin. These changes in pectic substances result in weakening of the cell walls and reduction of the cohesive forces that bind cells together (Trinchero et al. 1999). Similar behaviour during drying was also reported by other researchers working with cherry tomato (Heredia et al. 2007), pepino fruit (Di Scala et al. 2011) and blueberries (López et al. 2009).
The fracturability and adhesivenesss (work necessary to pull the compression anvil away from the sample) of dehydrated samples did not present significant differences compared to that of fresh samples (p < 0.05). Adhesiveness is more of a surface characteristic and depends on a combined effect of adhesive and cohesive forces, and others include viscosity and viscoelastic as well (Rahman and Al-farsi 2005). Gumminess, chewiness and resistance of dried samples showed significant differences with respect to fresh samples (p < 0.05). The observed results showed that the textural parameters changed in different directions indicating very complex instrumental texture of dried Cape gooseberry. Similar results, regarding the instrumental TPA attributes for dried dates were reported in previous investigations (Rahman and Al-farsi 2005).
Microstructure
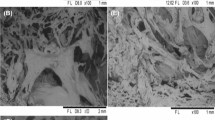
Structural changes of the Cape gooseberry were observed by Cryo-SEM analysis. Figure 3a shows the cell structure of fresh fruits and Fig. 4 b–f presents morphological and microstructure changes observed in dry–rehydrated samples. Fresh Cape gooseberry presented a tissue with a well-organized structure consisting of cells and intercellular spaces forming a spongy parechymatic tissue (Fig. 4a). Similar observations were reported in other Solanaceae fleshy and juicy fruits (Chiarini and Barbosa 2007). The cellulose of the cell wall gives stiffness and strength to the structure, whereas pectin and hemicelluloses of the middle lamella give plasticity and dictate the degree to which the cells can be pulled apart during deformation (Lewicki and Pawlack 2005). This porous structure can be used to generate added value for this fruit, incorporating physiologically active components within new functional foods that can be developed by means of matrix engineering methodology (Puente et al. 2011).
Microstructure of convective dried Cape gooseberry was affected by air-drying temperature and moisture gradients as can be observed in Fig. 4 b–f. It can be observed that after the rehydration of the samples, the cell structure presented an irregular form, with a decrease in the intracellular integrity as a consequence of the thermal processing. Hot air drying of Cape gooseberry tissue results in numerous breaks of cell walls and formation of many microcavities. In consequence, the dominating cross-sectional area of cells moves toward values smaller than those observed in raw fruits (Lewicky and Pawlack 2005). A tendency of the cell walls to separate from each other leading to contact loss has been observed, especially at high drying temperatures (e.g. 70–80–90 °C). Intercellular spaces are filled by exudate resulting from the loss of cell content as a result of cellular stress produced during the drying stage and after rehydration. In addition, it also showed a clear turgor loss, presenting degradation and causing a probable shrinkage in the contours of the cell wall (Vega-Gálvez et al. 2011).
Macroscopic changes incurred in a material undergoing drying must be related to tissue alterations on the microscopic level. These microstructural changes (shrinkage, collapse, etc.), which affect all the properties of Cape gooseberry, are related to very complex mechanisms. Consequently, it is of utmost importance in food engineering research to develop a detailed understanding of the time-dependent transient changes in all of the structural aspects of food matrices from raw material harvesting to product processing (Kauffmann and Palzer 2011). In this sense, during the last decades important advances in food science have emanated from the understanding of the food structure and its relation to properties (so-called structure–property relationships) (Aguilera 2005). Correlations of changes that occur at microscopic level with those observed macroscopically is one interesting area that still offers a lot of features to be exploited.
Conclusions
Cape gooseberry, as a dried matrix, has an interesting functional structure for product development. Fruit matrix can be engineered externally so that they may interact favourably in further heat and mass transfer processes (e.g., rehydration) and be appealing to the consumer (texture, appearance, etc.). Thus, this fruit could be considered as important source of biologically active components such as dietary fibre to assess the requirements of today’s consumers, who are much interested in the potential role of functional foods in improving health. Based on the results, drying at 60 °C could be an appropriate working temperature to achieve a final dried product with high quality aspects.
References
Aguilera JM (2005) Why food microstructure? J Food Eng 67:3–1
AOAC (1990) Official method of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC, USA
Betoret E, Betoret N, Vidal D, Fito P (2011) Functional foods development: Trends and technologies. Trends Food Sci Technol 22:498–508
Borchani C, Besbes S, Masmoudi M, Blecker C, Paquot M, Attia M (2011) Effect of drying methods on physico-chemical and antioxidant properties of date fibre concentrates. Food Chem 125:1194–1201
Borchani C, Besbes S, Masmoudi M, Ali Bouaziz M, Blecker C, Attia H (2012) Influence of Oven-Drying Temperature on Physicochemical and Functional Properties of Date Fibre Concentrates. Food and Bioprocess Technology. Food Bioprocess Technol 5(5):1541–1551.
Chiarini F, Barbosa G (2007) Anatomycal studies of different fruit types in Argentine species of Solanum Subgen. Leptostumonun (Solanaceae). An Jardín Bot Madrid 64:165–175
Chong C, Law C (2010) Drying of Exotic Fruits. In: Jangam SV, Law CL, Mujumdar AS (eds) Vegetables and Fruits. Volume 2, (ISBN - 978-981-08-7985-3, Published in Singapore, pp 1-42.
Di Scala K, Vega-Gálvez A, Uribe E, Oyanadel R, Miranda M, Vergara J, Quispe I, Lemus-Mondaca R (2011) Changes of quality characteristics of pepino fruit (Solanum muricatum Ait) during convective drying. Int J Food Sci Technol 46:746–753
Doymaz I (2008) Convective drying kinetics of strawberry. Chem Eng Proc 47:914–919
Doymaz I, Ismail O (2011) Drying characteristics of sweet cherry. Food Bioprod Proc 89:31–38
Garau MC, Simal S, Rossello C, Femenia A (2007) Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem 104:1014–1024
Garcia OE, Infante B, Rivera CJ (2010) Comparison of dietary fibre values between two varieties of cawpea (Vigna UnguiculataL Walp) of Venezuela, using chemical and enzymatic gravimetric methods. Rev Chilean Nutri 37:455–460
Hassanien MFR (2011) Physalis Peruviana: A rich Source of Bioactive Phytochemicals for functional Foods and Pharmaceutical. Food Rev Int 27(3):259–273
Heredia A, Barrera C, Andrés A (2007) Drying of cherry tomato by a combination of different dehydration techniques. Comparison of kinetics and other related properties. J Food Eng 80:111–118.
Karabulut I, Hayaloglu AA, Yildirim H (2007) Thin-layer drying characteristics of Kurut, a Turkish dried dairy by-product. Int J Food Sci Technol 42:1080–1086
Kauffmann SFM, Palzer S (2011) Food structure engineering for nutrition, health and wellness. Proc Food Sci 1:1479–1486
Kaymak-Ertekin F (2002) Drying and rehydrating kinetics of green and red peppers. J Food Sci 67(1):168–175
Krokida MK, Maroulis ZB (2001) Structural properties of dehydrated products during rehydration. Int J Food Sci Technol 36:529–538
Krokida MK, Philippopoulos C (2005) Rehydration of Dehydrated Foods. Drying Technol 23:799–830
Lewicki P, Pawlak G (2005) Effect of Drying on Microstructure of Plant Tissue. Drying Technol 21:657–683
Li L, Wang Z, Hu X, Wu J, Liao X, Chen F, Zhao G (2010) Drying effects of two air-drying shelters in a pilot test on sultana grapes. J Food Proc Eng 33(1):162–178
López J, Uribe E, Vega-Gálvez A, Miranda M, Vergara J, González E, Di Scala K (2009) Effect of Air Temperature on Drying Kinetics, Vitamin C, Antioxidant Activity, Total Phenolic Content, Non-enzymatic Browning and Firmness of Blueberries Variety O´Neil. Food Bioproc Technol 3(5):772–777
Martínez R, Torres P, Meneses M, Figueroa J, Pérez-Alvarez J, Viuda-Martos M (2012) Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem 135:1520–1526
Miranda M, Vega-Gálvez A, García P, Di Scala K, Shi J, Xue S, Uribe E (2010) Effect of Temperature on Structural Properties of Aloe vera (Aloe barbadensis Miller) Gel and Weibull Distribution for Modelling Drying Process. Food Bioprod Proc 88(2–3):138–144
Oliveira EG, Rosa GS, Moraes MA, Pinto LAA (2008) Phycocyanin content of spirulina platensis dried in spouted bed and thin layer. J Food Proc Eng 31(1):34–50
Peerajit P, Chiewchan N, Devahastin S (2012) Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem 132:1891–1898
Pinto M, Galvez Ranilla L, Apostolidis E, Lajolo FM, Genovese MI, Shetty K (2009) Evaluation of Antihyperglycemia and Antihypertension Potential of Native Peruvian Fruits Using In Vitro Models. J Med Food 12(2):278–291
Puente LA, Pinto-Muñoz CA, Castro ES, Cortés M (2011) Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res Int 44(7):1733–1740
Rahman MS, Al-farsi S (2005) Instrumental texture profile analysis (TPA) of date flesh as a function of moisture content. J Food Eng 66:505–511
Ramadan MF, Morsel J (2003) Oil Goldenberry (Physalis peruviana L.). J Agric Food Chem 51:969–974
Ramulu P, Rao PU (2003) Total, insoluble and soluble dietary fiber contents of Indian fruits. J Food Comp Anal 16:677–685
Salazar MR, Jones JW, Chaves B, Cooman A (2008) A model for the potential production and dry matter distribution of Cape gooseberry (Physalis peruviana L.). Sci Hort 115:142–148
Trinchero GD, Sozzi GO, Cerri AM, Vilella F, Fraschina AA (1999) Ripening-related changes in ethylene production, respiration rate and cell-wall enzyme activity in goldenberry (Physalis peruviana L.), a solanaceous species. Post Biol Technol 16:139–145
Uribe E, Vega-Gálvez A, Di Scala K, Oyanadel R, Saavedra J, Miranda M (2009) Characteristics of Convective Drying of Pepino Fruit (Solanum muricatum Ait.): Application Weibull Distribution. Food Bioprocess Technol 4(8):1349–1356
Vega-Gálvez A, Ah-hen K, Chacana M, Martínez-Monzó J, García-Segovia P, Lemus-Mondaca R, Di Scala K (2011) Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem 132(1):51–59
Vega-Gálvez A, Puente-Diaz L, Lemus-Mondaca R, Miranda M, Torres MJ (2012) Mathematical modelling of thin-layer drying of Cape Gooseberry (Physalis peru viana L.). J Food Proc Preserv. doi:10.1111/jfpp.12024
Acknowledgements
The authors gratefully acknowledge financial support for this investigation from the Research Department of Universidad de La Serena, Chile.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vega-Gálvez, A., Zura-Bravo, L., Lemus-Mondaca, R. et al. Influence of drying temperature on dietary fibre, rehydration properties, texture and microstructure of Cape gooseberry (Physalis peruviana L.) . J Food Sci Technol 52, 2304–2311 (2015). https://doi.org/10.1007/s13197-013-1235-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1235-0