Abstract
Purpose
To evaluate paediatric antibiotic prescription patterns in Italy in an extra-hospital setting at the national, regional, and Local Health Unit (LHU) level.
Methods
Data sources were regional prescription databases. Eight Italian regions participated in the study providing data for the year 2008, with two exceptions (where the data for 2009 and 2006 were provided instead). A total of 4,828,569 children were included: 58% of the Italian population under 15 years old. Antibiotic prevalence rates, prescription rates and prescriptions distribution by class were evaluated at the regional and LHU levels. The correlation among mean latitude, Human Development Index (HDI), hospitalisation rate, satisfaction index for the National Health Service, number of paediatricians per 1,000 resident children and prevalence rate was evaluated by regions.
Results
The estimated pooled average prevalence rate was 50.5% (95%CI 45.7–55.3). Between-regions prevalence rates ranged from 42.6% to 62.1% and at the LHU level they ranged from 35.6% to 68.5%. There was a trend indicating that in southern regions antibiotics are more frequently prescribed than in the northern and central regions (Cochrane–Armitage test Z = −187.5 p < 0.0001). Overall, penicillin covered 53.1% of antibiotic prescriptions, with differences between regions ranging from 39.2% to 62.5%. A direct correlation was found between the prevalence rate and HDI (p = 0.031), while an inverse correlation was found with paediatricians per 1,000 resident children (p = 0.038).
Conclusions
We found that relevant differences exist between the northern and the southern part of the country, and the heterogeneity among LHUs is higher. The greater use of antibiotics in the southern regions is related to lower HDI and does not seems to be justified by the higher prevalence of infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics are the most frequently prescribed drugs in children [1]. It is well known that antibiotics are too often prescribed to children for treatment of paediatric diseases such as upper respiratory tract infections that are, in the majority of cases, easily self-managed [2]. It has been estimated that almost half of antibiotic prescriptions given to children by a primary care physician are unnecessary [3]. Acute otitis media is the most commonly diagnosed disease in children and it is often self-limiting [4]. The application of clinical practice guidelines on the correct use of antibiotics in acute otitis could avoid antibiotic therapy in three quarters of cases [1]. Antibiotic over-prescription is a very complex problem also in children and several reasons for this phenomenon can be ascribed to paediatricians. The most important of these factors are diagnostic uncertainty and perceived parental expectations of antibiotic prescriptions [5]. The existing healthcare system and patient- or parent-related sociocultural and economic determinants are also responsible for geographic differences in prescription profiles [6].
Quantitative and qualitative differences in antibiotic prescriptions were found between and within countries. Data collected by the European Surveillance of Antimicrobial Consumption (ESAC) revealed that in Europe antibiotic consumption, expressed as defined daily dose per 1,000 inhabitants (DID), ranged from 9.2 DID in the Russian Federation to 34.7 DID in Greece [7]. Italy was the third country in descending order of DID. The distribution of antibiotic prescriptions by classes also differed among countries [7].
Differences between geographic contexts within the same country were observed in studies performed in Germany [8], Switzerland [9], United Kingdom [10] and Israel [11].
According to data reported by the National Drug Utilisation Monitoring Centre, which provides reimbursed pharmaceutical expenditure monitoring at the national and regional levels, antibiotic drug consumption between regions ranged from 16.1 to 39.9 DID in Italy [12].
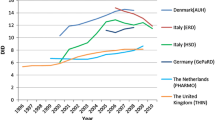
In a similar manner, quantitative and qualitative differences were also found in antibiotic prescriptions in the paediatric population. An Italian child is, on average, at a 4-fold higher risk of being exposed to antibacterial drugs than a British child and a 3-fold higher risk than a Dutch child [1, 6].
Cephalosporins, a second-line treatment in most paediatric infections, are widely prescribed in Italy (39% of paediatric antibiotic prescriptions) while in the Netherlands and Denmark this class represents less than one percent of total antibiotic paediatric prescriptions [1, 6]. Another Italian anomaly is represented by the high prescription rate of parenteral antibiotics in outpatient children, especially in the southern regions [1, 6].
Scant data are available concerning the differences in antibiotic prescriptions to children and adolescents between regions. Several drug utilisation studies evaluated antibiotic drug prescriptions to outpatient Italian children and adolescents, but most of these studies cannot be compared because of differences in age range, observation period or admission criteria [5, 13–22]. Therefore, in order to evaluate paediatric prescription differences in Italy, a multiregional study was carried out, using a shared protocol for data extraction and analysis.
Materials and methods
The Italian National Health System
Italian healthcare is provided free or at a nominal charge through a network of LHUs. Every Italian resident is registered with a family (paediatric or general) practitioner. Children are assigned to a paediatrician until they are 6 years old; afterwards, the parents can choose to register a child with a general practitioner. A national formulary is available, in which drugs are categorised into two classes: class A includes essential drugs that patients do not have to pay for and class C contains drugs not covered by the National Health System (NHS). Most antibiotics are free of charge.
Data source
Data sources were regional databases routinely updated for administrative and reimbursement reasons. The databases stored all community (i.e. outside the hospital) prescriptions reimbursed by the NHS. Aggregated and anonymous data were provided. Prescribed drugs were classified according to the Anatomic Therapeutic Chemical (ATC) classification system. Antibiotics were defined as all drugs belonging to the J01 ATC subgroup.
Regions that provided data to the National Drug Utilisation Monitoring Centre and/or published reports concerning drug prescriptions were identified [12, 23–26]. Persons in charge of regional pharmaceutical services were contacted and were asked to provide data regarding drugs prescribed to the paediatric outpatient population (≤14 years old) during 2008. If data concerning 2008 were not available, the last available data were requested.
Eight regions participated in the study: Veneto, Lombardy, Piedmont, Emilia Romagna, Umbria, Abruzzo, Lazio and Puglia. All regions provided data for the year 2008, with the exception of Lazio (2009) and Abruzzo (2006). For the Lazio region details concerning antibiotic classes and active substances were not available.
Measures
The following measures were used in order to describe the antibiotic prescription profile:
-
Prevalence rate, expressed as number of individuals who received at least one antibiotic prescription in a year, divided by the number of residents (children ≤ 14 years old). This was used to estimate the percentage of the population exposed to antibiotics, representing the chance of an individual receiving a prescription.
-
Prescription rate, expressed as number of prescriptions per treated child (child receiving at least one antibiotic in a year). This was used as a measure of the consumption.
These indicators were calculated at the national, regional and LHU levels.
The percentage of overall antibiotic prescriptions that were for amoxicillin was evaluated as the primary proxy of rational drug use since it is considered the first choice treatment for the most common paediatric infections by the main national and international guidelines [2, 4, 27–30]. The meta-analytic weighted average and 95% CIs of the prevalence rate of antibiotic drug prescriptions were estimated using a random effect model [31].
Prescription distribution, as the percentage of prescriptions per antibiotic class, was evaluated at the regional and LHU levels considering four main antibiotic classes: penicillins, cephalosporins, macrolides, and other antibiotics. The ten most frequently prescribed active substances were compared at the regional levels using the percentage of treated children.
The coefficient of variation (CV), defined as the ratio of the standard deviation to the mean, was calculated for prevalence and prescription rates to estimate the variability between and within regions. Kendall’s W was calculated between ranks associated with the most active substances in each region. Kendall’s W was also calculated comparing ranks of each region with ranks associated with the average mean values of active substances (measured by percentage of children treated).
A choropleth map of available prevalence rates data for LHUs available was created using the software Arcmap version 10. The prevalence values were categorised into three classes calculated on the basis of the mean ± one standard deviation (SD). According to NUTS (Nomenclature of Territorial Units for Statistics), the Italian regions participating in our study were grouped into three geographical areas: northern (Lombardy, Piedmont, Veneto and Emilia Romagna), central (Umbria and Lazio), and southern (Abruzzo and Puglia). A Cochrane–Armitage trend test was used to assess the presence of a trend in prevalence rates in the northern, central and southern regions.
The correlation between antibiotic prevalence rates and prescription rates and between prevalence rates and the ratio of amoxicillin and penicillin (expressed as percentage of antibiotic prescriptions) were calculated between- and within-regions using non-parametric rank correlation tests (Spearman’s tests). Spearman’s rank correlation test and Pearson’s correlation test were used to investigate the relationship between prevalence rates and the following determinants (between regions) [32, 33]:
-
Mean latitude.
-
HDI (Human Development Index), a standard complex index combining: life expectancy at birth, knowledge and education, and standard of living, adequately weighted [34].
-
Satisfaction index for the NHS.
-
Hospitalisation rate. This was estimated taking into consideration the Italian Ministry of Health’s data on hospital discharge forms for the 2008 and was expressed as the number of admissions per 1,000 inhabitants (less than 15 years old).
-
Number of paediatricians per 1,000 resident children.
A stepwise linear multiple regression analysis was performed considering regional prevalence rates as the dependent variable group and the aforementioned five determinants as the independent variable groups (fixing α = 0.15). The adjusted R-squared selection method was also used to verify the model. Co-linearity between determinants was excluded. Statistical analysis was performed using SAS software, version 9.1 (SAS, Cary, NC, USA).
Results
Quantitative differences
A total of 4,828,569 children were included in this evaluation, which represents 58% of the Italian population 0–14 years old (Table 1).
There were differences in prevalence rates between regions. These ranged from 42.6% registered in Lazio to 62.1% in Puglia. The estimated pooled average prevalence rate, adjusted and weighted by population size, was 50.5% (95%CI 45.7–55.3). There were no relevant differences in prevalence rate by sex in all the regions analysed. On average, each treated child received 2.31 prescriptions of antibiotic (from 2.05 in Veneto to 2.71 in Puglia). There was a rank correlation between the prevalence and the prescription rates at the regional level (rs = 0.96; p = 0.003).
At LHU level, the prevalence rates ranged from 35.6% to 68.5% (Fig. 1), while prescription rates ranged from 1.75 to 3.18.
Northern regions had a mean prevalence rate of 46.5% (95% CI 46.4–46.6%), central regions had a mean prevalence of 44.1% (95% CI 44.0–44.2%), and southern regions a mean prevalence rate of 61.1% (95% CI 61.0–61.2%). There was a geographic trend between the three macro-areas indicating that in southern regions prevalence is higher than in the rest of the country (Cochrane–Armitage trend test Z = −187.5 p < 0.0001). Emilia Romagna was the region with the lowest within-region prevalence CV (0.03), Lazio and Veneto those with the highest one (0.13).
Determinants of prescriptions
Spearman’s correlation tests (between-regions analyses) of prevalence rate toward possible determinants of prescription did not find significant correlations. Pearson’s correlation test found a significant correlation between prevalence rate and HDI (rs = −0.75; p = 0.03).
The multiple regression analysis found an inverse relationship between prevalence rate and HDI and a positive relationship was found towards paediatricians per 1,000 resident children (aged 0–14). The overall R-square coefficient of 0.836 indicated that the model accounted for 83.6% of the variability of prevalence rates for the regions available. HDI and number of paediatricians per 1,000 resident children (aged 0–14) respectively accounted for 56.5% (partial R-squared = 0.565; p =0.031) and 26.5% (partial R-square d= 0.265; p = 0.038) of the variability of prevalence rates in the study population. No significant correlation was found between prevalence rate and the other determinants.
Qualitative differences
Prescribing patterns of different antibiotic classes in the regions included are summarised in Fig. 2.
Overall, penicillins made up 53.1% of antibiotic prescriptions, with differences between regions ranging from 39.2% in Abruzzo to 62.5% in Lombardy. At the LHU level the overall percentage of prescriptions that were for penicillin ranged from 29.2% to 71.6%. Macrolides and cephalosporins represented, on average, 21.8% and 23.1% of overall prescriptions respectively. For cephalosporins the region with the lowest percentage of prescriptions was Lombardy with 18.6% and the highest percentage was registered in Puglia with 30.4%. Macrolides ranged between 17.4% (Lombardy) and 28.8% (Abruzzo).
Most frequently prescribed active substances
Amoxicillin-clavulanate was the most commonly prescribed antibiotic: overall, almost one in two children treated with antibiotics received at least one package of this drug in a year. A total of 13 different active substances were retrieved among the 10 most frequently prescribed in the seven regions under consideration (Table 2). In all, 8 active substances were common in the first 10 ranks among all the regions selected.
The ten most frequently prescribed active substances accounted for more than 90% of the prescriptions in all regions, ranging between 92.8% in Abruzzo and 97.4% in Veneto. Kendall’s W calculated between regions resulted in W = 0.88 and p < 0.0001, indicating a good concordance of ranks. No active substance (of the eight common between the regions) had the same rank in all regions. Amoxicillin-clavulanate was the most frequently prescribed antibiotic in 6 out of the 7 regions under consideration regions (not in Veneto) and it was the drug with the lowest between-regions CV (0.14). The percentage of children treated with this drug ranged from 39.1 to 58.0%.
Amoxicillin, was the second most frequently prescribed antibiotic in most regions, it was the first drug in Veneto, but was only the fifth in Abruzzo and Puglia. There was a wide variability in the use of amoxicillin among the regions under consideration: the percentage of treated children ranged from 15.8% (Puglia) to 40.3% (Veneto), with a between-regions CV of 0.30. Amoxicillin covered 8.7% of the antibiotic prescriptions in Puglia and 28.1% in Veneto, with an average value of 19.0% (Table 1). At the LHU level the amoxicillin percentage of prescriptions ranged from 7.1% (Puglia) to 48.0% (Emilia Romagna).
Among macrolides, clarithromycin was the most frequently prescribed in all regions and the percentage of treated children ranged from 16.8% (Emilia Romagna) to 26.2% (Puglia), with a between-regions CV of 0.16. At least five cephalosporins were among the ten most frequently prescribed active substances in all regions; the most frequently prescribed were cefaclor and cefixime. Ceftriaxone, the most used parenteral cephalosporin, figured among the ten most frequently used antibiotics in most regions (with the exception of Veneto and Umbria) and the percentage of children treated ranged from 1.5% (Lombardy) to 5.2% (Puglia), with a between-regions CV of 0.60. The drug with the highest between-regions CV was ceftriaxone, followed by cefixime (0.42) and cefpodoxime (0.37).
Discussion
To the best of our knowledge this is the first large study evaluating between- and within-region differences in antibiotic prescriptions in a paediatric population. Almost 5 million children were included: 58% of the 0- to 14-year-old Italian population. In this study we found that antibiotics are widely prescribed in paediatric outpatients in Italy with both quantitative and qualitative marked territorial differences. The overall prevalence at the national level was 50.5%, but this estimate is not representative of all the geographic contexts. In fact, there was a 2-fold difference between the LHUs with the highest and the lowest prevalence rates. Within-region prevalence rate data showed heterogeneity in all regions.
A strong correlation between prevalence and prescription rates was found. In those areas characterised by higher prevalence rates more children were exposed to a greater number of antibiotic prescriptions. A greater use of second-choice treatments was found in areas characterised by high prevalence and prescription rates.
The HDI accounted for a large part of the variability in prevalence rates, indicating that those regions with higher life expectancy, literacy, education and standard of living are more likely to have lower prevalence rates. Social and economic factors are already known determinants of child health inequalities. HDI by region represents a summary of the healthcare inequalities on a territorial basis, highlighting a north–south trend, with a more developed north [35].
There was a direct correlation between prevalence rate and the number of paediatricians per 1,000 resident children: those areas with more paediatricians were more likely to be high prevalence rate areas (i.e. southern regions). Nevertheless, the chance of receiving an antibiotic prescription was not directly related to the number of children each paediatrician cared for. Other studies in the literature have found conflicting evidence concerning the relationship between time constraints and paediatric prescriptions [36]. On the contrary, our findings suggest that the “busiest” paediatricians are not the ones that prescribe more antibiotics; other factors may play a role [5, 18].
Qualitative differences and rational drug use
Since no details were available concerning the disease for which antibiotics were prescribed, it was not possible to evaluate the appropriateness of drug prescriptions. In fact, to correctly assess if a particular disease is treated appropriately, a disease-oriented indicator is needed [37].
Nevertheless, the assumption that amoxicillin, and penicillins in general [38], should be the more frequently prescribed antibiotic is supported by the evidence, since this drug is considered the first-choice treatment for the most common infectious diseases in children [2, 4, 27–30]. Large differences were observed in the percentage of amoxicillin or penicillin prescriptions. Although no statistically significant correlation was observed between percentage of prescribed amoxicillin or penicillin and prevalence rates, a greater percentage of prescribed amoxicillin was found in the northern regions. On the contrary, the southern regions with higher prevalence rates (Puglia and Abruzzo) were the ones with the highest percentage of prescriptions for second-choice treatments, and the consequent lower use of penicillins and amoxicillin. In this area amoxicillin covered no more than 10% of antibiotic prescriptions. Despite the fact that there is no adequate monitoring of antibiotic resistance between the different regions in outpatient children [39], it is unlikely that a possible heterogeneity in resistance could be responsible for such large differences in the prescriptions of amoxicillin between north and south.
American Academy of Pediatrics [4] and NICE [2] guidelines continue to support amoxicillin, alone, as the first-choice treatment for acute otitis media and for pharyngitis with a bacterial aetiology. A source of concern could be represented by amoxicillin being only fifth in the list of the most frequently prescribed active substances in the southern regions and being exceeded by macrolides. Macrolide exposure is recognised as the most important risk factor for the development of resistance to this drug class [22]. Further, the high between-regions CV found for amoxicillin reflects a lack of agreement among paediatricians in different geographic areas.
Amoxicillin-clavulanate was ranked as first in most regions (excluding Veneto, where it was second). The drug’s rank and low between-regions CV indicated that most of the Italian paediatricians considered it to be the first-choice drug for paediatric infectious diseases.
Prescription data on ceftriaxone continue to confirm the trend observed in previous studies in the literature: it is prescribed too often in the outpatient setting, especially in the southern part of the country [1, 6]. Ceftriaxone prescriptions to outpatients are allowed in Italy, although restricted to severe infections; however, many paediatricians inappropriately prescribe the drug to children who are non-compliant with the first-line oral antibiotic therapy (i.e. vomiting) [1]. Ceftriaxone is the third-generation cephalosporin that caused the highest number of serious adverse reactions in Italian children in the 2001–2008 period [40]. In 10% of cases the drug was prescribed for pharyngo-tonsillitis [40]. This is an issue of concern and clinicians should be aware of it and prescribe this important therapeutic agent only when recommended treatments have failed. Its use should be restricted to the hospital setting.
The high level of use of other oral cephalosporins, such as cefaclor, also appears unjustified, since these are not recommended as first-choice therapy for otitis media and pharyngo-tonsillitis, the two most common paediatric infectious diseases [2, 4]. Differences in antibiotic prescriptions at the local level may be influenced by the availability of local therapeutic protocols or guidelines.
The Emilia Romagna region adopted regional guidelines on the treatment of pharyngo-tonsillitis and acute otitis media in 2007 [29, 30]. The adoption of regional guidelines was preceded by other regional projects involving paediatricians and parents, with the objective of identifying principal determinants of antibiotic prescription in children in the region. The fact that Emilia Romagna was one of the regions with the highest percentage of amoxicillin prescription (25.9%) may be due to the adoption of guidelines. In some of this region’s LHUs amoxicillin represented almost 50% of the antibiotics prescribed.
Educational and training programmes aimed at paediatricians may improve the appropriateness of drug prescriptions, as observed in other local and regional Italian settings [5, 19]. High-prevalence European countries (such as Belgium and France) have initiated national regulatory and educational programmes promoting the rational use of antibiotics in the community. These campaigns have been repeated for several years with good results in terms of better adherence to guidelines and an overall decrease in consumption [41].
The implementation of national, shared guidelines on the management of infectious diseases, especially in children, has been important in some countries (especially in France), but this is not enough to ensure a correct use of antibiotics, since adherence by physicians is not so common [42, 43]. It was observed that the senior physicians (specialists) who had the highest quantity of services were those less likely to correspond to guideline recommendations and that amoxicillin therapy is the less compliant with the guidelines, mostly because of under-dosing or therapy of inadequate duration [44].
In some cases previous studies have demonstrated that, even if using approaches that are known to influence physician behaviour, it is sometimes very difficult to change prescribing habits [42]. Nevertheless, it has been proved that children who are not treated according to guideline recommendations have a higher chance of receiving another subsequent prescription in the next few days, thus increasing the possibility of resistance [42]. Guidelines are excellent in terms of evidence and quality of the studies reviewed, but often they are not focused on specific behaviours to adopt in specific cases, and the part concerning the recommendation is not concise enough, often resulting in the recommendation getting lost [43].
The WHO and the Italian Medicines Agency have recently promoted informative campaigns on antimicrobial resistance, and, while this may be useful for the general population’s knowledge of this major issue, it is doubtful whether such large and generalised campaigns can effectively improve paediatric antimicrobial therapy in primary healthcare, taking into consideration the major territorial differences that have been demonstrated. The paediatrician’s prescribing attitude is probably responsible for a good part of the variability observed in antibiotic prescription [5, 18]. A local, focused approach is therefore recommended.
A recent meta-analysis on interventions involving physicians to improve treatment in children with upper respiratory tract infections showed that an evidence-based decision support system or clinical algorithms improved antibiotic prescribing behaviour, reducing antibiotic prescriptions [45]. It was also demonstrated that the use of rapid antigen test detection in clinical practice determines a significant decrease in antibiotic prescriptions in children [46].
Variability in antibiotic use was also detected by other studies within other countries. Such a phenomenon requires additional international studies to correctly evaluate if there are common determinants. In order to promote a more homogeneous and rational use of drugs in children within countries, the adoption of a European formulary of paediatric medicines should be considered [47].
Strengths and limitations
Regions included in this study are representative of different economic, socio-demographic and geographic settings. The study included data on a very large paediatric population with the same age ranges in the different regions, and that involved common observation periods. Nevertheless, there were some limitations. Geographic distribution of data was unbalanced towards the northern regions. Only 15% of the overall population was resident in the southern area of the country (Abruzzo or Puglia) and this could lead to an underestimation of the national prevalence and prescription rate, since it has been shown that southern regions are likely to have higher prevalence rates. Furthermore, information on the disease was lacking, but this is an intrinsic limitation of all pharmacoepidemiological studies using prescription databases.
Conclusions
In conclusion, we found that relevant differences exist between the northern and the southern parts of the country, and the heterogeneity is higher at the LHU level. The greater use of antibiotics in the southern regions is related to lower HDI and does not seems to be justified by higher prevalence of infectious diseases. There is a lower use of amoxicillin and a higher use of second-line treatments in the south of the country. Greater amoxicillin use is observed in the few LHUs that entertained educational and training programmes for paediatricians’ attitudes to antibiotic prescription, which is probably the most important modifiable variable and should be the primary target of interventions. Therefore, in order to decrease prescriptions and improve appropriateness, continuous, active teaching and auditing in primary care focused at the territorial level is recommended. Moreover, unjustified NHS expenditure differences, which are not covered in this study, exist, even within the same geographical settings, and these are strictly connected with over-prescription. An intervention is therefore particularly needed in these areas.
References
Rossignoli A, Clavenna A, Bonati M (2007) Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000–2005. Eur J Clin Pharmacol 63:1099–1106
Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. NICE clinical guideline—Centre for Clinical Practice at NICE http://guidance.nice.org.uk/CG69/Guidance Accessed 27 June 2011.
Pichichero ME (2002) Dynamics of antibiotic prescribing for children. JAMA 287:3133–3135
American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media (2004) Diagnosis and management of acute otitis media. Pediatrics 113:1451–1465
Moro ML, Marchi M, Gagliotti C (2009) Why do paediatricians prescribe antibiotics? Results of an Italian regional project. BMC Pediatr 6:9–69
Clavenna A, Bonati M (2011) Differences in antibiotic prescribing in paediatric outpatients. Arch Dis Child 96:590–595
Muller A, Coenen S, Monnet DL, Goossens H (2007) European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe, 1998–2005. Euro Surveill 12:E071011.1, http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3284 Accessed 27 June 2011
Kern WV, de With K, Nink K, Steib-Bauert M, Schröder H (2006) Regional variation in outpatient antibiotic prescribing in Germany. Infection 34:269–273
Filippini M, Masiero G, Moschetti K (2006) Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy 78:77–92
Davey P, Ferech M, Ansari F, Muller A, Goossens H (2008) Outpatient antibiotic use in the four administrations of the UK: cross-sectional and longitudinal analysis. J Antimicrob Chemother 62:1441–1447
Nitzan O, Low M, Lavi I, Hammerman A, Klang S, Raz R (2010) Variability in outpatient antimicrobial consumption in Israel. Infection 38:12–18
Rocchi F, Addis A, Martini N (2004) Current national initiatives about drug policies and cost control in Europe: the Italy example. J Ambul Care Manage 27:127–131
Cucinotta G, Mazzaglia G, Toscano MA, Arcoraci V, Tempera G, Salmeri M, Rosignoli M, Bottaro G, Boccazzi A, Nicoletti G, Caputi AP (2002) Exploring the variability in antibiotic prescribing profiles among paediatricians from two different areas of Italy. Pharmacol Res 45:369–374
Clavenna A, Sequi M, Bonati M (2010) Differences in the drug prescriptions to children by Italian paediatricians and general practitioners. Eur J Clin Pharmacol 66:519–524
Boccazzi A, Noviello S, Tonelli P, Coi P, Esposito S, Carnelli V (2002) The decision-making process in antibacterial treatment of pediatric upper respiratory infections: a national prospective office-based observational study. Inf J Infect Dis 6:103–107
Cazzato T, Pandolfini C, Campi R, Bonati M (2001) Drug prescribing in out-patient children in Southern Italy. Eur J Clin Pharmacol 57:611–616
Cantarutti L, Sturkenboom M, Bordin A, Bussi R, Cozzani S, Del Torso S, Giancola G, Girotto G, Grillone G, Katende M, Murgia V, Pasinato A, Passarella A, Ruffato B, Saretta L, Semenzato L, Spanavello W, Toffol G, Soncin G, Costa L, Bettio M, Mannino S, Giaquinto C (2001) Le infezioni respiratorie acute in pediatria: uno studio prospettico. Medico e Bambino pagine elettroniche ; http://www.medicoebambino.com/?id=RI0102_10.html Accessed 27 June 2011
Ciofi degli Atti ML, Massari M, Bella A, Boccia D, Filia A, Salmaso S (2006) Clinical, social and relational determinants of paediatric ambulatory drug prescriptions due to respiratory tract infections in Italy. Eur J Clin Pharmacol 62:1055–1064
Mauri L, Narducci M, Nova A, Zanetto F (2010) Infezioni respiratorie, self help e prescrizione antibiotica nella pratica ambulatoriale. Medico e Bambino 29:569–574
Resi D, Milandri M, Moro ML (2003) Antibiotic prescriptions in children. J Antimicrob Chemother 52:282–286
Gagliotti C, Morsillo F, Resi D, Milandri M, Moro ML (2006) Antibiotic treatments for children ages 0–23 months in a Northern Italy region: a cohort study. Infection 34:155–157
Clavenna A, Sequi M, Bortolotti A, Merlino L, Fortino I, Bonati M (2009) Determinants of the drug utilization profile in the paediatric population in Italy’s Lombardy Region. Br J Clin Pharmacol 67:565–571
Pan A, Buttazzi R, Moro ML (2009) Uso di antibiotici sistemici e resistenze antibiotiche nella popolazione pediatrica dell’Emilia-Romagna, Rapporto ; http://asr.regione.emilia-romagna.it/wcm/asr/aree_di_programma/rischioinfettivo/gr_ric/pr_antibres/stpr_sorve_usoantib/lr_uso_antibped/pubblicazioni/antibiotici_pediatria_2009/link/antibiotici_in_pediatria_2009.pdf Accessed 27 June 2011
Da Cas R, Desiderio V, Lombardozzi L, Orzella L, Pasquale L, Raschetti R. Prescrizione farmaceutica nella Regione Lazio. Analisi dei dati relativi al 2009. Rapporto ISTISAN, 11, 2011, http://www.asplazio.it/asp_online/att_territoriale/files/farmaceutica/pubblicazioni/Rapporto_2009.pdf Accessed 11 July 2011
Da Cas R, Ruggeri P, Rossi M, Bucaneve G, Di Loreto P, Gamboni B, Venegoni M, Traversa G (2008) Prescrizione farmaceutica in Umbria. Analisi dei dati relativi al. http://www.sanita.regione.umbria.it/MediaCenter/API/Risorse/StreamRisorsa.aspx?guid=B8DEA320-0CAE-40F5-923E-CD7F3FD55ECA Accessed 27 June 2011
Melena S, Zappacosta I, Romero M (2004) Progetto Monitoraggio Spesa Farmaceutica e Valutazione Appropriatezza Prescrittiva; Osservatorio sulla prescrizione farmaceutica in Abruzzo, secondo rapporto; http://www.farmaci.abruzzo.it/documenti/osservatorio_farmaci/report_prescrizioni/report_territoriali/Rapporto2004.pdf Accessed 27 June 2011
Chiappini E, Regoli M, Bonsignori F, Sollai S, Parretti A, Galli L, de Martino M (2011) Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther 33:48–58
Faringotonsillite in età pediatrica. Regional guidelines—Regional Healthcare System, Emilia Romagna region http://asr.regione.emilia-romagna.it/wcm/asr/collana_dossier/doss153.htm Accessed 27 September 2011
Otite media acuta in età pediatrica. Regional guidelines—Regional Healthcare System, Emilia Romagna region http://asr.regione.emilia-romagna.it/wcm/asr/collana_dossier/doss154/link/doss154.pdf Accessed 27 September 2011
Treatment Guidelines for Upper Respiratory Tract Infections, Centers for Disease Control and Prevention http://www.cdc.gov/getsmart/campaign-materials/treatment-guidelines.html Accessed 27 June 2011
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA (1995) A random-effects regression model for meta-analysis. Stat Med 14:395–411
ISTAT (Italian National Institute for Statistics) www.istat.it Accessed 16 September 2011
Rapporto annuale sull’attività di ricovero ospedaliero, 2008 Italian Ministry of Health http://www.salute.gov.it/imgs/C_17_pubblicazioni_1253_allegato.pdf Accessed 27 June 2011
United Nations Development Programme http://hdr.undp.org/en/statistics/hdi/ Accessed 16 September 2011
Bonati M, Campi R (2005) What can we do to improve child health in Southern Italy? PLoS Med 2:e250
Hare ME, Gaur AH, Somes GW, Arnold SR, Shorr RI (2006) Does it really take longer not to prescribe antibiotics for viral respiratory tract infections in children? Ambul Pediatr 6:152–156
Andersen M (2006) Is it possible to measure prescribing quality using only prescription data? Basic Clin Pharmacol Toxicol 98:314–319
Urrsuno RF, Balosa CM, de la Pisa BP (2010) Developing indicators of prescribing quality for primary care paediatricians. J Pharm Health Serv Res 1:167–173
AR-ISS, sorveglianza dell’antibiotico-resistenza in Italia. Rapporto del triennio 2006–2008. Italian National Healthcare Institute. http://www.iss.it/binary/publ/cont/10_37.pdf
Italian Medicines Agency, Ceftriaxone e reazioni avverse nei bambini, Bollettino d’Informazione sui Farmaci, XV N.4 2008, http://www.agenziafarmaco.gov.it/sites/default/files/bif0804176_ceftriaxone.pdf Accessed 16 September 2011
Goossens H, Guillemot D, Ferech M, Schlemmer B, Costers M, van Breda M, Baker LJ, Cars O, Davey PG (2006) National campaigns to improve antibiotic use. Eur J Clin Pharmacol 62:373–379
Bauchner H, Marchant CD, Bisbee A, Heeren T, Wang B, McCabe M, Pelton S, Boston-Based Pediatric Research Group (2006) Effectiveness of centers for disease control and prevention recommendations for outcomes of acute otitis media. Pediatrics 117:1009–1017
Michie S, Johnson M (2004) Changing clinical behaviour by making guidelines specific. Br Med J 328:343–345
Chu CH, Wang MC, Lin LY, Shiao AS (2011) Physicians are not adherent to clinical practice guidelines for acute otitis media. Int J Pediatr Otorhinolaryngol 75:955–959
Boonacker CWB, Hoes AW, Dikhoff M-J, Schilder AG, Rovers MM (2010) Interventions in health care professionals to improve treatment in children with upper respiratory tract infections. Int J Pediatr Otorhinolaryngol 74:1113–1121
Llor C, Cots JM, Lopez-Valcarcel BG, Alcántara Jde D, García G, Arranz J, Monedero MJ, Ortega J, Pineda V, Guerra G, Gómez M, Hernández S, Paredes J, Cid M, Pérez C (2011) Effect of two interventions on reducing antibiotic prescription in pharyngitis in primary care. J Antimicrob Chemother 66:210–215
Bonati M, Pandolfini C (2004) Is it time for a European formulary of paediatric medicines? Arch Dis Child 89:890–891
Acknowledgements
We wish to thank the “Antibiotic Collaborative Group” for their helpful collaboration. We are grateful to Chiara Pandolfini for language editing.
Financial Disclosure
The authors have indicated that they have no personal financial relationships relevant to this article to disclose.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Antibiotic Collaborative Group:
Italian National Institute of Health: Roberto Da Cas, Giuseppe Traversa
Lombardy Region: Angela Bortolotti, Ida Fortino, Luca Merlino
Veneto Region: Roberta Joppi, Luca Trentin
Piedmont Region: Iolanda Davletbaiev, Cristiana Pellegri
Emilia Romagna Region: Rossella Buttazzi, Carlo Gagliotti, Maria Luisa Moro, Angelo Pan
Umbria Region: Mariangela Rossi
Lazio Region: Francesco Chini, Marina Davoli, Letizia Orzella
Abruzzo Region: Antonio D’Ettorre, Stefania Melena, Marilena Romero
Puglia Region: Ambrogio Aquilino, Francesco Bux, Vito Lepore
Rights and permissions
About this article
Cite this article
Piovani, D., Clavenna, A., Cartabia, M. et al. The regional profile of antibiotic prescriptions in Italian outpatient children. Eur J Clin Pharmacol 68, 997–1005 (2012). https://doi.org/10.1007/s00228-011-1204-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1204-3