Abstract
High levels of antibiotic consumption are driving levels of bacterial resistance that threaten public health. Nonetheless, antibiotics still provide highly effective treatments for common diseases with important implications for human health. The challenge for public education is to achieve a meaningful reduction in unnecessary antibiotic use without adversely affecting the management of bacterial infections. This paper focuses on the lessons learned from national campaigns in countries (Belgium and France) with high antibiotic use. Evaluation of these national campaigns showed the importance of television advertising as a powerful medium to change attitudes and perhaps also behaviour with regard to antibiotics. Moreover, in both countries, strong evidence suggested reduced antibiotic prescribing. However, adverse effects associated with a reduction in antibiotic prescribing were not monitored. We conclude that carefully designed mass education campaigns could improve antibiotic use nationally and should be considered in countries with high antibiotic use. However, these campaigns should employ techniques of social marketing and use appropriate outcome measures. The benefits and risks of such campaigns have been less well established in countries where antibiotic use is already low or declining.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are priority drugs and bacterial resistance is a major public health issue [1]. Antibiotic consumption is a key driver of resistance, although the relationships are complex. In Europe, antibiotic consumption varies from approximately 10 defined daily doses (DDD) per 1,000 inhabitants and day in the Netherlands to 32 DDD per 1,000 inhabitants and day in France [2]. In comparison with northern European countries, southern and eastern countries tend to have greater seasonal variation in antibiotic consumption (indicating unnecessary usage for viral respiratory infections) together with higher overall usage [2]. Campaigns and interventions are aiming at optimizing antibiotic use. A campaign targets the population level using mass-distribution media, which may include posters, leaflets, newspapers, television, radio and the Internet [3]. It is not the same as a community intervention, which attempts to achieve changes on a community level using a model that encourages community-wide participation of persons with various roles and relationships [4]. National campaigns to improve antibiotic use have been conducted in Australia, Belgium, France, and the UK. In the USA, the Centres for Disease Control and Prevention has funded campaigns in 28 states and is expanding the program incrementally (http://www.cdc.gov/getsmart/). In contrast, developing countries such as Chile and Korea have concentrated on the regulation of antibiotic sales and dispensing [5, 6].
This paper presents and evaluates data, presented at a workshop in Brussels (23–24 September 2004), indicating that antibiotic use can be influenced at the national level by mass campaigns. It focuses on the lessons learned from two national campaigns conducted in Europe and on the issues that public health policy makers should consider when deciding whether a national campaign is a good use of scarce resources.
Overview of the available evidence of impact
There are few data concerning the impact of national campaigns on antibiotic prescribing and resistance. In this overview, we will describe the available evidence from Belgium and France on the impact of a multifaceted, sustained, antibiotic campaign.
Belgian data
In 1998, Belgium had the second highest rate of community antibiotic consumption in Europe (data from the European Surveillance of Antimicrobial Consumption downloadable from http://www.ua.ac.be/ESAC). Nationwide campaigns promoting rational use of antibiotics in the community, organised by the Belgian Antibiotic Policy Co-ordination Committee, were launched in November 2000. Three successive, 3-months long, annual campaigns targeted both the public and prescribers during the winter (November to February). The three main, complementary messages were: ‘Use antibiotics less frequently but better’, ‘Save antibiotics, they may save your life’, and ‘Talk to your doctor, talk to your pharmacist’. The public was targeted using television, radio, posters, brochures and information folders. General practitioners (GPs), paediatricians, pneumonologists, ear nose and throat specialists, and community pharmacists received personal letters accompanied by campaign materials for patients.
The impact of the first two campaigns has been evaluated through (1) pre-and post-campaign face-to face interviews of the public (n=1,014 in 2000 and n=1,015 in 2001), with respondents representing the national population in terms of age, socio-economic category, region and habitat, and (2) post-campaign surveys of the general practitioners (n=400 in 2001 and 2002). The post-campaign survey carried out 1–2.5 months after the end of the first Belgian campaign, showed that 46% of the public remembered the campaign: 79% through television spots, 17% through newspaper advertisements and 14% through radio spots. The first campaign also had a high visibility for the GP’s (100%), was judged positively, and shifted opinion in favour of using antibiotics more sparingly (public, 75%; GP’s, 63%). Expectation for antibiotics significantly (p<0.05) decreased for acute bronchitis, flu, sore-throat, common cold and diarrhoea (data not shown). To assess the effect of the campaigns on antibiotic sales, Bauraind et al. [7] applied a statistical time-series analysis, controlling for the influence of the seasonal variation of influenza-like illnesses in 2000–2001 and 2001–2002, as compared with the previous years. The corrected reduction in DDDs was 6.5% (p<0.05) and 3.4% (non-significant) after the first and second campaigns, respectively [7]. The lack of a further reduction in 2002–2003 may be explained by an increase in the licensed and recommended dosage of amoxicillin-clavulanate, and by the introduction of the ‘respiratory’ fluoroquinolones. Reimbursement data on antibiotic use in the period 1997–2003, expressed in DDDs and antibiotic packs (as a surrogate of prescriptions), indicate that community antibiotic usage has indeed decreased since 2000 (first launch of the annual national campaigns), and this reduction was most pronounced for packs (from 3.8 to 2.9 packs per 1,000 inhabitants per day, a 25% reduction).
The decrease of total overall exposure of antibiotics expressed in DDD was less pronounced (6%), because during these years, the content of an average pack increased (both by increase of strength and of pack size) from 6.9 to 7.8 DDD per pack.
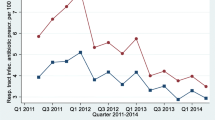
Annual data assessed by epidemiological seasons of respiratory tract infections (from July to June), rather than by calendar year, seem to reflect observed trends more accurately (Fig. 1).
French data
Levels of antibiotic use and bacterial resistance in France are among the highest in Europe [8]. The long-term French national campaign (“Keep Antibiotics Working”), started in 2002, was designed to reduce unjustified antibiotic use, and to improve the quality of care, in both the community and hospital settings.
The primary goal of the community campaign was to reduce antibiotic use in children (aged 0–6 years) and for the treatment of upper and lower RTIs [9]. The campaign was mainly driven by the National Health Insurance System, with the key message “Les antibiotiques, c’est pas automatique”. The public were targeted by written materials and television advertisements. Interventions aimed at GPs included academic detailing, peer-to-peer visits, and the promotion of streptococcal rapid diagnostic tests for sore throat. In hospitals, the campaign favoured stricter antibiotic policies implemented by Antibiotic Policy Committees, surveys of antibiotic use, the identification of anti-infective specialists, and the promotion of a multidisciplinary approach involving clinicians, microbiologists and pharmacists. The campaign was introduced by the publication of “Plan national pour préserver l’efficacité des antibiotiques” in 2001 [10] of and reinforced by the Consensus Conference statement on hospital antibiotic policies by the French Society of Infectious Diseases in 2002. Preliminary results comparing the period of October–March showed a 13% reduction in total antibiotic use between 2001–2002 and 2004–2005. A relevant decrease was observed in children (Fig. 2), with a 19% decrease in children aged under 15 years. Overall, the greatest 3-year reduction rates were seen for penicillins (25%), macrolides (20%) and cephalosporins (13%) (Fig. 2). In addition, sequential public opinion polls showed changes in knowledge about antibiotics and their role in general medical practice.
Figure 3 compares annual antibiotic consumption in Belgium and France with the lower, but static, rates in England. These data suggest the effectiveness of national campaigns in countries with high antibiotic use. However, there is little evidence that campaigns are effective in countries where antibiotic use is lower and/or already decreasing. A national Australian campaign was relatively ineffective in a context of relatively low and declining baseline levels of antibiotic use [11]. National campaigns have not been evaluated in countries with relatively low antibiotic usage. However, in Sweden, the STRAMA initiative—comprising a decentralised network of interventions and media awareness activities—appears to have significantly reduced antibiotic use, especially among children [12]. In these countries, it may be more appropriate for health policy makers to focus solely on the quality of antibiotic use (e.g. the correct indication, dosage, and duration of treatment) without regard to its quantity.
Finally, other confounding factors may explain the reduction in antibiotic prescribing, such as reductions in respiratory tract infections presenting to GPs [13], reduction in the incidence of respiratory tract infections, a shift in the distribution of respiratory tract infections for which antibiotics are justified, and reductions in number of GP consultations.
Discussion
Campaigns to reduce antibiotic prescribing should be based on evidence concerning social marketing and the impact of mass media on healthcare use [3]. The design of national campaigns requires a multidisciplinary approach that recognises the strengths of each discipline.
Evaluation
Understanding the impact of information on knowledge and attitudes toward antibiotics is a necessary first step in any campaign, but is no substitute for measurement of the impact on antibiotic consumption. As indicated by a recent Cochrane review [3], interrupted time series analysis is a quasi-experimental approach most often used in evaluation studies of mass media intervention [14]. It was not possible to use this method for antibiotic campaigns, because the number of yearly data points is limited (3 years before the intervention) and because methodological adaptations to adjust for data showing seasonal variation have not yet been fully developed.
This method was used in all 17 mass media interventions included in a Cochrane review [3]. However, 6 studies did not perform any statistical analysis, and 7 used inappropriate statistical tests (i.e. not taking into account the effect of temporal trends). In the original reports, the authors concluded that 16 interventions had been successful, whereas the Cochrane reviewer’s re-analysis of the data showed that only 7 (41%) of the 17 interventions has a statistically significant impact on outcome. In the evaluation of future campaigns bias can be minimised by the use of rules presented on the Cochrane Effective Practice and Organisation of Care (EPOC) website (http://www.epoc.uottawa.ca).
Target populations
Target populations should be determined according to national or regional antibiotic consumption. High levels of unnecessary use for viral infections occur in children. However, the likely impact on total antibiotic usage should be assessed before a campaign targeting children is undertaken. For example, unpublished data from Tayside Scotland in 2000 show a U-shaped use of antibiotics by age. The highest proportion of users was among those aged <5 years (63%, CI 62–64%) declining to 32% (CI 32–33%) at age 45–49 and increasing again to 47% (CI 46–48%) at age 85–89. However, people aged 45–49 account for 6% of total use, compared with 8% for children under 5 years and 4% for people aged 85–89 years (P.G. Davey, unpublished data). A campaign targeted specifically at high users may therefore have a relatively minor impact on total antibiotic use. This does not mean that such a campaign is not worthwhile, but it is important to have reasonable expectations of the potential results. The educational needs of the population should also be determined, e.g. according to age and socio-economic status. If the intention is to reduce antibiotic use in specific groups, then a community intervention is likely to be more appropriate than a campaign [4].
Messages and media
Campaign messages should be devised with the support of experts in behavioural science or social marketing rather than epidemiologists or microbiologists alone. When devising messages, campaign developers should consider the barriers to change within the target population, as determined through appropriate research. Messages should address both the ‘how’ and ‘why’ of behaviour change. However, the optimal approach to public messages is controversial and is likely to differ by region and country. Threat messages have often been used, with the ‘personal’ risks associated with resistance considered more effective than the ‘ecological’ impact. However, social marketing would suggest that positive messages are more likely to result in behaviour change.
Many campaigns have emphasised the distinction between viral and bacterial RTIs. However, this might be confusing to some groups and it implies that all bacterial infections must be treated with antibiotics. A better approach may be to foster an understanding among the public that antibiotics are not always required and that health professionals have knowledge and skills that enable them to judge when the benefits of antibiotics exceed the risks.
Campaigns have typically used a variety of printed, electronic and broadcast media to reach target populations. Evidence from Belgium and other countries underscores the importance of television advertising.
Campaigns must be sustained if they are to be effective. A good example of a sustained, large-scale campaign based on social marketing is the multinational ‘Bob’ campaign designed to reduce alcohol-related traffic accidents (http://www.bob.be). The campaign uses a positive, personified approach to encourage people to act as designated non-drinking drivers. This campaign has run annually in Belgium since its introduction in 1995 and has been adapted for use in other European countries.
Outcomes of decreased consumption
The use of the surrogate endpoint of antibiotic consumption is necessary because correlating antibiotic usage and resistance remains a challenge. Preliminary French data from the AUBEPPIN study, a regional community intervention trial assessing the potential impact of optimising antibiotic use on pneumococcal resistance, are promising [15]. In a more recent and comprehensive paper, Guillemot et al. [16] showed that intensive educational strategies aimed at optimizing antibiotic use can significantly reduce the rate of penicillin G-nonsusceptible Streptococcus pneumoniae colonisation in areas with high resistance rates. Conjugate pneumococcal vaccine was not available in France in 2002 and was not used at the time of this study.
Full evaluation of the impact of antibiotic education campaigns will require the integration of antibiotic consumption data with demographic/clinical data and resistance surveillance data at the individual patient level. This should allow assessment of appropriate antibiotic use, rather than total use as at present. This is technically possible and potential ethical/legal barriers with regard to patient confidentiality can be overcome using appropriate procedures [17, 18]. Prescribing panels (e.g. produced by IMS Health) are widely used by industry, and their results concerning specific disease-type or age-type prescribing patterns appear to correlate with data from other sources. Other potential endpoints that could be used to assess campaigns include patient consulting behaviour [13].
Of central importance is the potential for unintended adverse effects associated with a reduction in antibiotic prescribing. These considerations relate not only to antibiotic prescribing patterns, but also to the organisation of healthcare systems and access to physicians. There is some evidence that complications of bacterial RTIs, such as mastoiditis and quinsy, may have increased in countries with low levels of antibiotic use [19–21], although data are lacking. It is possible that adverse effects may also occur owing to the widespread usage of anti-inflammatory drugs in the context of decreased antibiotic use. Therefore, it is vital that the incidence of complications and other outcomes is monitored in countries where national campaigns to reduce antibiotic use are undertaken.
Cost/saving ratio
Each Belgian campaign cost about € (euro) 385,000. Based on the decreases in antibiotic sales attributable to the campaigns [7], we calculated the corresponding savings to the Social Security. These gave us estimated values of €2,370,407 (CI 828,121–3,912,692) and €1,912,464 (CI 55,726–3,769,202), for the first and the second campaign, respectively, yielding an overall saving/cost ratio of 5.54. Thus, the immediate reduction of antibiotic consumption largely offset the direct costs of the Belgian campaigns. However, cost-saving should not be conceived as a primary goal for such campaigns because it may deter the public, the GPs, and the pharmacists to give full support to these actions. One-third of the responding Belgian GPs, indeed, perceived the campaigns as simply a mean to reduce Social Security costs.
Following the model of the Bob campaign in Belgium, a national campaign would require about €0.5 million per year, sustained for 10 years. The key question is, should a government that intends to invest €5 million on reducing antimicrobial resistance in the community be advised to spend it all on a public information campaign?
Conclusions
Only two European countries, Belgium and France, have undertaken and evaluated nationwide campaigns to improve antibiotic use. Campaigns in both countries appeared to have been successful in reducing existing high rates of antibiotic consumption. Our conclusions are based on ecological studies, which lack control of confounding. More and carefully evaluated national public campaigns should be undertaken and sustained in countries and communities with high antibiotic use and high seasonal variation in this high use. Moreover, the benefits and risks of such campaigns have been less well established in countries where antibiotic use is already low and/or declining. Finally, the best means of addressing antibiotic resistance in developing countries are unclear.
Future campaigns should take advantage of experience gained to date, for example regarding social marketing, cultural adaptation and population targeting. They should include appropriate, prospective evaluation methods based on an anticipation of likely outcomes. The incidence of adverse clinical outcomes should be monitored in order to maintain optimal standards of patient care.
Competing interests
Herman Goossens has been reimbursed by Bayer (the manufacturer of ciprofloxacin and moxifloxacin), AstraZeneca (the manufacturer of meropenem) and Bristol-Myers Squibb (the manufacturer of cefepime) for attending conferences, and has been paid a speaker’s fee. He has conducted research on resistance to macrolides among streptococci for Aventis (the manufacturer of telithromycin), and Abbott (the manufacturer of clarithromycin). He has also received educational grants from Bristol-Myers Squibb for the Elzenveld Workshops and is a member of the Infectious Disease Advisory Board sponsored by GlaxoSmithKline (the manufacturer of amoxicillin-clavulanate, cefuroxime, and ceftazidime).
Didier Guillemot: none declared.
Matus Ferech: none declared.
Benoit Schlemmer has been reimbursed by Bayer, Aventis, Pfizer (the manufacturer of azithromycin and linezolid), GlaxoSmithKline, Merck Sharp and Dohme (the manufacturer of ertapenem), and Lilly (the manufacturer of drotrecogin-alpha) for attending conferences, and has been paid a speaker’s fee by Abbott, Aventis, Merck Sharp and Dohme, and Pfizer. He has been involved as an investigator in a trial on sepsis treatment funded by Lilly. He has been paid consulting fees by Abbott and GlaxoSmithKline and for organising education by Aventis and Pfizer.
Michiel Costers: none declared.
Marije van Breda: none declared.
Lee J. Baker has been employed by Aventis for medical writing work and for organising symposia and academic colloquia.
Otto Cars: none declared.
Peter G. Davey has been reimbursed by Aventis, Bayer, Pfizer and Merck Sharp and Dohme for attending conferences, and has been paid a speaker’s fee. He has also received consulting fees from the same companies. He has conducted research in intra-abdominal sepsis for Merck Sharp and Dohme.
Contributions
Herman Goossens organised the Workshop on Educational campaigns held in Brussels (23–24 September 2004), contributed to all revisions, was responsible for the final draft, and is guarantor. Michiel Costers and Marije van Breda participated in the organisation of the Workshop, and contributed to the first draft. Herman Goossens and Matus Ferech provided original data on antibiotic use in Belgium. Matus Ferech also designed the ESAC data protocol and analysed collected data. Lee Baker and Peter Davey wrote a first draft of the paper based on presentations at the Brussels meeting and contributed to all subsequent revisions of the text. Peter Davey provided original data on antibiotic use over time in England and on antibiotic use by age from Tayside Scotland. Didier Guillemot and Benoit Schlemmer contributed to all draft revisions and provided original data on antibiotic use in France. Otto Cars contributed to the first draft. All authors contributed to the final draft and approved it.
References
Nordberg P, Monnet DL, Cars O (2004) Antibacterial resistance. Background document for the WHO project: Priority medicines for Europe and the World-a public health approach to innovation. World Health Organization, Geneva. (Available at http://mednet3.who.int/prioritymeds, accessed 23 Nov 2005)
Goossens H, Ferech M, Stichele RV, Elseviers M, the ESAC Project Group (2005) Outpatient antibiotic use in Europe and association with resistance. Lancet 365:579–587
Grilli R, Freemantle N, Minozzi S, Domenighetti G, Finer D (1997) Mass media interventions: effects on health services utilisation (Cochrane Review). The Cochrane Library 2001 (Issue 1). Update Software, Oxford
Person B, Cotton D (1996) A model of community mobilization for the prevention of HIV in women and infants. Prevention of HIV in women and infants demonstration projects. Public Health Rep 111(suppl 1):89–98
Bavestrello L, Cabello A (2004) Impact of regulatory measures on antibiotic sales in Chile. 2nd international conference on improving use of medicines, 30 March-2 April 2004, Chiang Mai, Thailand. http://www.icium.org/icium2004 (accessed 8 Dec 2004)
Park S (2004) Decreased inappropriate antibiotic use following a Korean National Policy to prohibit medication dispensing by physicians. 2nd international conference on improving use of medicines, 30 March-2 April 2004, Chiang Mai, Thailand. http://www.icium.org/icium2004 (accessed 23 Nov 2005)
Bauraind I, Lopez-Lozano JM, Beyaert A, Marchal JL, Seys B, Yane F et al (2004) Association between antibiotic sales and public campaigns for their appropriate use. JAMA 292:2468–2470
Aubry Damon H, Carlet J, Courvalin P, Desenclos JC, Drucker J, Guillemot D et al (2000) Bacterial resistance to antibiotics in France: a public health priority. Euro Surveill 5:135–138
Sommet A, Sermet C, Boelle PY, Tafflet M, Bernede C, Guillemot D (2004) No significant decrease in antibiotic use from 1992 to 2000, in the French community. J Antimicrob Chemother 54:524–528
Plan national pour préserver l’efficacité des antibiotiques (2001) Paris; Ministère de la santé et des solidarités (Available at http://www.sante.gouv.fr, accessed 23 Nov 2005)
Mackson J (2004) Implementation and outcomes of a 5-year intervention program to improve use of antibiotics in respiratory tract infections in primary care. 2nd international conference on improving use of medicines, 30 March-2 April 2004, Chiang Mai, Thailand. http://www.icium.org/icium2004 (accessed 23 Nov 2005)
Cars O, Stålsby Lundborg C, Mölstad S (2004) Improving antibiotic use through a nationwide decentralized project-a nine-year experience. 2nd international conference on improving use of medicines, 30 March-2 April 2004, Chiang Mai, Thailand. http://www.icium.org/icium, 2004 (accessed 23 Nov 2005)
Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M (2004) Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the general practice research database. J Pub Health 26:268–274
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D (2002) Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharmacol Therapeut 27:299–309
Guillemot D, Henriet L, Lecoeur H, Weber P, Carbon C (2001) Optimization of antibiotic use rapidly decreases penicillin resistant Streptococcus pneumoniae (PRSP) carriage: The AUBEPPIN Study (Abstract 1527). In: Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 16–19 Dec, 2001, Chicago, Ill. American Society for Microbiology, Washington, DC, p 264
Guillemot D, Varon E, Bernede C, Weber P, Henriet L, Simon S et al (2005) Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin Infect Dis 41:930–938
Donnan PT, Wei L, Steinke DT, Phillips G, Clark R, Noone A (2004) Presence of bacteriuria caused by trimethoprim resistant bacteria in patients prescribed antibiotics: multilevel model with practice and individual patient data. BMJ 328:1295–1300
Wilson P (2004) Commentary: legal issues of data anonymisation in research. BMJ 328:1300–1301
Van Zuijlen DA, Schilder AG, Van Balen FA, Hoes AW (2001) National differences in incidence of acute mastoiditis: relationship to prescribing patterns of antibiotics for acute otitis media. Pediatr Infect Dis J 20:140–144
Little P, Watson L, Morgan S, Williamson I (2002) Antibiotic prescribing and admissions with major suppurative complications of respiratory tract infections: a data linkage study. Br J Gen Pract 52:187–190, 193
Price DB, Honeybourne D, Little P, Mayon-White RT, Read RC, Thomas M et al (2004) Community-acquired pneumonia mortality: a potential link to antibiotic prescribing trends in general practice. Resp Med 98:17–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goossens, H., Guillemot, D., Ferech, M. et al. National campaigns to improve antibiotic use. Eur J Clin Pharmacol 62, 373–379 (2006). https://doi.org/10.1007/s00228-005-0094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0094-7