Abstract
Loggerhead turtles nesting in the Mediterranean Sea exhibit remarkable genetic structuring. This paper tests the hypothesis that young loggerhead turtles from different rookeries do not distribute homogeneously among the major Mediterranean foraging grounds, due to a complex pattern of surface currents. We extracted long fragments of mitochondrial DNA from 275 stranded or bycaught juvenile turtles from six foraging grounds (Catalano-Balearic Sea, Algerian basin, Tyrrhenian Sea, Adriatic Sea, northern Ionian Sea and southern Levantine Sea). We used a Bayesian mixed-stock analysis to estimate the contributions from rookeries in the Mediterranean, the North-west Atlantic and Cape Verde to the studied foraging grounds. Differences were found in the relative contribution of juvenile turtles of Atlantic and Mediterranean origin to each foraging ground. A decreasing proportion of Atlantic juveniles was detected along the main surface current entering the Mediterranean, with a high prevalence of turtles from eastern Florida in the Algerian basin and lower numbers elsewhere. In regard to the turtles of Mediterranean origin, juveniles from Libya prevailed in central and western Mediterranean foraging grounds other than the Algerian basin. Conversely, the Adriatic Sea was characterised by a large presence of individuals from western Greece, while the southern Levantine Sea was inhabited by a heterogeneous mix of turtles from the eastern Mediterranean rookeries (Turkey, Lebanon and Israel). Overall, the distribution of juveniles may be related to surface circulation patterns in the Mediterranean and suggests that fisheries might have differential effects on each population depending on the overlap degree between foraging and fishing grounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Great migrations are often found in the animal kingdom and at very different scales (Hoare 2009). By migrating, species have adapted to increase their fitness and reproductive success for millions of years, but nowadays many anthropogenic threats affect populations at their origin, destination and along migratory corridors. Only by understanding the distribution of these migratory species and the overlap with anthropogenic threats will conservation be possible.
Sea turtles are among these highly migratory species, undertaking long-distance journeys sometimes spanning entire oceans (Bolten 2003; Plotkin 2003). One of the best-known oceanic migrators is the loggerhead turtle (Caretta caretta), distributed in all tropical and warm-temperate areas and the most abundant sea turtle in the Mediterranean Sea (Broderick et al. 2002; Casale and Margaritoulis 2010). Loggerhead turtles of different origins coexist in this area, as juveniles from western Atlantic rookeries share foraging grounds with those clutched within the Mediterranean (Laurent et al. 1993, 1998; Bowen et al. 2003; Carreras et al. 2006, 2011). Small Atlantic juveniles enter the Mediterranean Sea through the Strait of Gibraltar during their pelagic stage and remain there until they are large enough to swim against the strong and permanent eastward current of the strait (Revelles et al. 2007a; Eckert et al. 2008). During this period, juvenile turtles of Atlantic origin use the same foraging grounds as juveniles born in Mediterranean rookeries but rarely interbreed (Carreras et al. 2011), maintaining isolation between these two genetically distinct Regional Management Units (RMU; Wallace et al. 2010).
The distribution of juvenile loggerhead turtles of Atlantic and Mediterranean origin in the Mediterranean Sea has been widely studied through the use of satellite telemetry (Cardona et al. 2005; Bentivegna et al. 2007; Revelles et al. 2007b; Cardona et al. 2009; Casale et al. 2013), mark recapture techniques (Margaritoulis et al. 2003; Casale et al. 2007; Revelles et al. 2008) and genetics (Carreras et al. 2006; Maffucci et al. 2006; Casale et al. 2008; Saied et al. 2012; Garofalo et al. 2013). In the western Mediterranean Sea, juvenile turtles of Atlantic origin mainly inhabit foraging grounds off the North African coast and juvenile turtles of Mediterranean origin forage mainly along the European coasts (Carreras et al. 2006). However, little is known about the distribution and proportion of Atlantic juveniles in other areas within the Mediterranean Sea (Laurent et al. 1998; Maffucci et al. 2006; Casale et al. 2008; Piovano et al. 2011). Furthermore, nothing is known about the distribution of young turtles from the different nesting populations existing in the Mediterranean Sea (Carreras et al. 2007; Garofalo et al. 2009; Saied et al. 2012; Clusa et al. 2013).
The relative contribution of each rookery to specific foraging grounds can be studied through mixed-stock analysis (MSA; Grant et al. 1980). Previous research in the Mediterranean Sea has mostly used a ~380-bp fragment of non-coding mitochondrial DNA (mtDNA) as the genetic marker for MSA (Laurent et al. 1998; Maffucci et al. 2006; Carreras et al. 2007; Casale et al. 2008; Carreras et al. 2011; Saied et al. 2012; but see Garofalo et al. 2013). However, the limited assignment power of this marker has precluded a fine-scale assessment of the contribution of Mediterranean rookeries to the Mediterranean foraging grounds. A new set of primers has been developed (Abreu-Grobois et al. 2006), which amplifies a longer segment of the mitochondrial control region (815 bp) and hence increases the resolution of genetic structuring among the different nesting areas (Monzón-Argüello et al. 2010; Shamblin et al. 2012; Clusa et al. 2013). With this increase in the genetic resolution, origin assignment power of juveniles from Mediterranean foraging grounds is expected to improve at regional and fine-scale levels, potentially unveiling previously unknown distribution patterns.
Bycatch of juvenile turtles at their foraging grounds is one of the most significant anthropogenic threats for sea turtles in the Mediterranean Sea, with over 132,000 annual captures estimated in the area (Casale and Margaritoulis 2010; Casale 2011). The impact of fisheries bycatch depends on habitat use, type of fishing gear, fishing effort, abundance of the affected populations and origin of these populations (Wallace et al. 2008). Thus, fine-scale information on the composition of bycatch in each fishing ground is essential for a proper impact assessment of turtle bycatch in the Mediterranean Sea.
This paper analyses the origin of juvenile loggerhead turtles from seven distinct foraging grounds within the Mediterranean Sea through a mixed-stock analysis with longer fragments of mtDNA with the aim to (1) describe the distribution of juveniles of Atlantic origin within the Mediterranean Sea (regional level), (2) unveil the use of Mediterranean foraging grounds by juveniles of Mediterranean origin (fine-scale level), (3) understand the mechanisms of such distributions and (4) evaluate the impact that incidental bycatch in foraging grounds might have on nesting populations.
Materials and methods
Sample collection
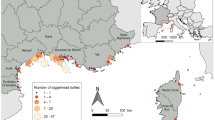
Tissue samples were taken from 275 stranded or bycaught juvenile loggerhead turtles from several developmental foraging grounds in the Mediterranean Sea between 2002 and 2012 (Table 1). Only turtles smaller than 69 cm curved carapace length (CCL) were sampled, as this is the average minimum size of nesting females in the Mediterranean (Margaritoulis et al. 2003) and turtles of Atlantic origin become adults at a much larger size (Piovano et al. 2011). Sampling was designed to ensure coverage of several juvenile foraging grounds within the major sub-basins in the region (Fig. 1): the Catalano-Balearic Sea (CAB), the Algerian basin (ALG), the Tyrrhenian Sea (TYR), the northern Adriatic Sea (NADR), the southern Adriatic Sea (SADR), the northern Ionian Sea (ION) and the southern Levantine Sea (LEV). No samples could be obtained from the southern Ionian Sea or the Aegean Sea, areas also known to be used by juvenile turtles as foraging grounds (Margaritoulis et al. 2003; Casale et al. 2013).
Foraging grounds for juvenile loggerhead turtles sampled in this study: CAB (the Catalano-Balearic Sea), ALG (the Algerian basin), TYR (the Tyrrhenian basin), NADR (the northern Adriatic Sea), SADR (the southern Adriatic Sea), ION (the northern Ionian Sea) and SLE (the southern Levantine Sea). Black lines represent surveyed coastlines
Muscle samples were collected from dead animals and stored in 95 % ethanol. Blood samples were taken from live animals and stored frozen.
Laboratory procedures
DNA from samples was extracted with the QIAamp extraction kit (QIAGEN®), following the manufacturer’s instructions. An 815-bp fragment of the mtDNA control region was amplified by polymerase chain reaction (PCR) using the primer pair LCM15382 (5′-GCTTAACCCTAAAGCATTGG-3′) and H950 (5′-GTCTCGGATTTAGGGGTTT-3′) (Abreu-Grobois et al. 2006), following the protocols described in Clusa et al. (2013). All samples were sequenced in both forward and reverse directions to confirm variable sites on both strands of DNA on an ABI 3730 automated DNA analyser at the Scientific-Technical Services at the University of Barcelona or at the Molecular Biology Service of the Stazione Zoologica Anton Dohrn.
Genetic structuring of foraging grounds
Sequences were aligned with BioEdit version 7.1.6 (Hall 1999) and compared to the 815-bp haplotypes previously described for this species compiled by the Archie Carr Center for Sea Turtle Research of the University of Florida (ACCSTR; http://accstr.ufl.edu). The resulting fragment also contains the 380-bp fragment, traditionally used in molecular studies on marine turtles (Norman et al. 1994).
Our results of the northern Ionian Sea were compiled with haplotype frequencies previously published from the same area (Garofalo et al. 2013) in order to increase sample size. Pseudoreplication between these two sample sets was not expected as all the individuals in this region were found dead in both studies. Compilation of haplotype frequencies for the other foraging grounds also analysed in Garofalo et al. (2013) was not done as individual carapace sizes fell off the considered range for juvenile loggerheads (Margaritoulis et al. 2003; Piovano et al. 2011).
Haplotype diversity (h; Nei 1987) and nucleotide diversity (π; Nei 1987) were estimated for each foraging ground using ARLEQUIN version 3.5 (Excoffier and Lischer 2010) to analyse the genetic diversity of the sampled areas. Pairwise genetic distances (F ST ) between foraging grounds were calculated with the DnaSP version 5.10 software package (Librado and Rozas 2009). The significance of genetic differentiation among these regions was assessed using Hudson’s nearest neighbour statistic (S NN) with 1,000 permutations. Statistical significance when analysing multiple pairwise comparisons was evaluated with a modified false discovery rate (FDR) (Narum 2006). Pairwise genetic distances between foraging grounds (F ST ) were plotted with a principal coordinate analysis (PCoA) inferred with GenAlEx version 6.5 (Peakall and Smouse 2012).
Stock composition
A Bayesian mixed-stock analysis (MSA) was used to assess the composition of each foraging ground as implemented in Bayes (Pella and Masuda 2001). This analysis estimates the proportion of individuals in each foraging ground coming from different rookeries. We used a baseline with a total of 23 rookeries (Supplementary Table 1) analysed in previous studies using the same primer pair (Garofalo et al. 2009; Monzón-Argüello et al. 2010; Yilmaz et al. 2011; Saied et al. 2012; Shamblin et al. 2012; Clusa et al. 2013). This baseline included haplotype frequencies from 10 Atlantic rookeries (Monzón-Argüello et al. 2010; Shamblin et al. 2012) and 13 Mediterranean rookeries (Garofalo et al. 2009; Yilmaz et al. 2011; Saied et al. 2012; Clusa et al. 2013), as loggerheads from both areas may potentially coexist in any of the Mediterranean foraging grounds considered. A ‘many-to-many’ MSA (Bolker et al. 2007) was not used in the present study because the genetic characterisation of Atlantic foraging grounds based on 815-bp mtDNA fragments is still unknown and this is needed for the ‘many-to-many’ approach.
Estimates on the size of each rookery (expressed as the mean number of nests per year; Supplementary Table 1) were included in the Bayesian approach as a weighting factor as suggested by previous studies (Bass et al. 2004). Iterated chains were only considered reliable when the Gelman–Rubin criterion was fulfilled (G-R shrink factor <1.2 for all parameters; Gelman et al. 1996). The analyses were undertaken twice: first considering two regional areas (Atlantic and Mediterranean; regional level) and second considering all rookeries as independent units (fine-scale level).
Results
Genetic structuring of foraging grounds
A total of 17 different haplotypes were found in the Mediterranean foraging grounds analysed (Table 1), all of them described in previous studies. Haplotype CC-A2.1 was the most dominant (70.9 %), followed by CC-A1.1 (10.2 %). Five haplotypes were exclusive to Atlantic rookeries (CC-A1.1, CC-A1.3, CC-A5.1, CC-A10.4 and CC-A14.1), six exclusive to Mediterranean rookeries (CC-A2.8, CC-A2.9, CC-A6.1, CC-A29.1, CC-A31.1 and CC-A32.1) and three shared between Atlantic and Mediterranean rookeries (CC-A2.1, CC-A3.1 and CC-A20.1). The remaining haplotypes (CC-A10.3, CC-A28.1 and CC-A55.1) have only been described in foraging grounds but have not been found in any rookery to date. However, their combined frequency was very low (1.1 %). Overall, haplotype and nucleotide diversities in foraging areas were highly variable (h range: 0.095–0.668; π range: 0.0001–0.0248), with the Algerian basin presenting the highest haplotype (0.668 ± 0.041) and nucleotide (0.0248 ± 0.0123) diversities (Table 1).
Highly significant genetic structuring was found among the studied foraging grounds (global F ST = 0.201, p < 0.001). Because F ST differentiation tests showed no statistical differences between the northern and southern Adriatic Sea (F ST = − 0.037, p = 0.936), these two foraging grounds were pooled as Adriatic Sea (ADR) for further analyses. The majority of pairwise statistically significant differences occurred between the Algerian basin and the central eastern side of the Mediterranean (Table 2). PCoA ordination also reflected the deepest differentiation between the Algerian basin and the rest of foraging grounds, explaining 93.89 % of the observed variation with the first two axes (Fig. 2). This analysis also separated the Catalano-Balearic Sea and the Tyrrhenian Sea from the rest, although only by the second axis, which in turn explained only 11 % of the total variation.
Principal coordinate analysis based on genetic distances (F ST ) between juvenile loggerhead turtles in Mediterranean foraging grounds. Percentage of variation explained by each coordinate included in brackets. Foraging ground acronyms as shown in Table 2
Stock composition
MSA results showed that the deep differentiation between the Algerian basin and the other foraging grounds reported above was due to the overwhelming prevalence of individuals of Atlantic origin in the Algerian basin (Fig. 3). Individuals of Atlantic origin could be detected in all the foraging grounds considered but nowhere was the Atlantic contribution as strong as in the Algerian basin (58.4 ± 11.2 %). Overall, the majority of the Atlantic contribution came from central eastern Florida and south-eastern Florida (CEF and SEF; Supplementary Table 2). All the other foraging grounds studied hosted mainly Mediterranean individuals, with the strongest Mediterranean contribution (Fig. 3) found in the northern Ionian Sea (96.4 ± 3.6 %) and the Adriatic Sea (93.6 ± 16.2 %).
Atlantic (light grey) and Mediterranean (dark grey) juvenile contributions to each Mediterranean foraging ground estimated by MSA. Standard deviation bars included. Foraging ground acronyms as shown in Table 2
Results based on unclustered rookeries (Fig. 4, Supplementary Table 2) showed that juveniles from Mediterranean rookeries were not homogenously mixed in the Mediterranean Sea, with major differences between adjoining foraging grounds. While the Adriatic Sea was inhabited by a high proportion of turtles from western Greece (57.8 ± 33.3 %), the northern Ionian Sea hosted individuals mainly from Misrata in Libya (70.4 ± 34.9 %). The Tyrrhenian Sea also hosted mainly individuals from Misrata (47.4 ± 31.3 %), but there was also relevant contribution from Calabria (14.5 ± 12.5 %). Juvenile turtles from Misrata (38.6 ± 29.1 %) and from western Greece (31.3 ± 23.7 %) had a similar abundance in the Catalano-Balearic Sea. Finally, the southern Levantine Sea showed a particularly different composition as this hosted a high proportion of individuals from the easternmost rookeries in the Mediterranean Sea: Israel, Lebanon and Turkey (Supplementary Table 2). However, their contributions were unequal and western Turkey was the source of 28.4 ± 36.6 % of its turtles in comparison with eastern Turkey or Israel and Lebanon (~10 % each).
Fine-scale rookery contributions (%) to Mediterranean foraging grounds estimated by MSA. Rookeries: ATL (Atlantic), MIS (Misrata, Libya), WGR (western Greece), WTU (western Turkey), LEV (Israel; Lebanon; Cyprus; eastern Turkey; middle Turkey; Dalaman and Dalyan, Turkey), OTHER (Sirte, Libya; Calabria, Italy; Crete, Greece). Stars show Mediterranean rookery locations
Discussion
The contribution of different nesting beaches to any particular juvenile foraging ground will depend on the size of the population nesting at each beach and the pattern of surface currents connecting these beaches with the foraging ground (Bowen and Karl 2007; Hays et al. 2010). The largest nesting aggregation of loggerhead turtles in the North Atlantic is found along the coasts of North America (Ehrhart et al. 2003) and is connected with the European coasts by the Gulf Stream (Carr 1986; Bolten et al. 1998). Furthermore, the negative water balance of the Mediterranean Sea generates a permanent eastward flow of Atlantic water at the Strait of Gibraltar (Millot and Taupier-Letage 2004), thus connecting the Mediterranean with the Gulf Stream. The Cape Verde Archipelago hosts the second largest nesting aggregation in the North Atlantic (Marco et al. 2012), but is connected with northern South America by the North Equatorial Current rather than with the Mediterranean Sea (Mansfield and Putman 2013). In this scenario, it is hardly surprising that most of the juvenile loggerhead turtles found in the foraging grounds of the eastern Atlantic and the south-western Mediterranean had a North American origin, with only a few juveniles coming from Cape Verde (Monzón-Argüello et al. 2009, 2010; Carreras et al. 2011; this study).
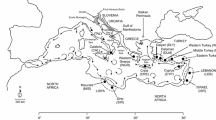
Once into the Mediterranean Sea, Atlantic water flows initially eastwards along the slope of Northern Africa (Fig. 5) and then splits in two major currents, one flowing northwards into the Tyrrhenian Sea and the other flowing eastwards along the coast of Libya to the southern Levantine Sea (Millot and Taupier-Letage 2004). Accordingly, the relative abundance of juvenile loggerhead turtles of Atlantic origin decreases downstream, from the Algerian basin to the Adriatic Sea (Carreras et al. 2006; Maffucci et al. 2006; this study). However, the contribution of Atlantic rookeries to the Algerian basin reported here is lower than that detected in previous studies (Carreras et al. 2006; Carreras et al. 2011). This is because the longer mtDNA fragment allowed the differentiation of the Libyan CC-A2.9 haplotype from the widespread CC-A2.1 haplotype, something impossible with the short fragment. Thus, some of the turtles occurring in the Algerian basin and previously considered of Atlantic origin come actually from Libya.
Conversely, the occurrence of turtles of Atlantic origin in the eastern Mediterranean is higher than previously reported. This is likely to be a consequence of analysing only turtles shorter than 69 cm CCL, as turtles of Atlantic origin migrate back to the Atlantic at an average length of 58.8 cm CCL (Revelles et al. 2007a), and hence, the proportion of turtles of Atlantic origin in any foraging ground will decline when larger turtles are considered. Casale et al. (2008), on the basis of data from Laurent et al. (1998), estimated that only 11 % of the turtles in the southern Levantine Sea had an Atlantic origin, whereas our MSA results based on long fragments indicate a much higher proportion (20 %). It should be noted that the turtles sampled by Laurent et al. (1998) ranged in size from 49.4 to 86.3 cm CCL, whereas here only turtles shorter than 69 cm have been considered. This might also explain why the proportion of turtles of Atlantic origin present in the Adriatic Sea is slightly larger than that previously estimated on the basis of a wider size range (Giovannotti et al. 2010; Yilmaz et al. 2012).
Another methodological difference is the use of population size as a weighting factor for the MSA (Bass et al. 2004), while other studies in the region did not use it (Maffucci et al. 2006). Thus, an underestimation of the contribution of juveniles from Atlantic rookeries could have also occurred in these previous studies as they did not consider the much larger number of nests per year recorded in Atlantic beaches (ca. 100,000 nests per year; SWOT 2007) compared to the Mediterranean (ca. 7,200 nests per year; Casale and Margaritoulis 2010).
The surface circulation pattern might also explain the distribution patterns of turtles from Mediterranean nesting beaches to the different sub-basins. The prevalence in the Adriatic Sea of turtles from western Greece might be explained by the pattern of water entering the Adriatic Sea having previously flowed past the coast of western Greece (Fig. 5; Millot and Taupier-Letage 2004). Likewise, the prevalence of turtles from Libyan beaches in the Ionian Sea may be linked to the mesoscale eddies present in the Ionian Sea (Robinson et al. 2001; Hamad et al. 2006; Hays et al. 2010), which might trap the hatchlings and juveniles swimming off Libya in the sub-basin and prevent dispersal across the eastern Mediterranean (Fig. 5). A proportion of juveniles from Libya might also be trapped in coastal systems and pushed by a westward current to the Algerian basin, the Catalano-Balearic Sea and the Tyrrhenian Sea, where its contribution is also relevant. This westwards dispersal perfectly fits the one suggested by Hays et al. (2010) for hatchlings drifting in the Mediterranean Sea.
Nevertheless, if the hypothesis that currents determine the observed distribution patterns of juveniles is true, a higher proportion of juvenile turtles from western Greece would be expected to occur in the northern Ionian Sea, as hatchlings swimming off western Greece encounter a water current bifurcation, with one current flowing northwards into the Adriatic Sea and another one flowing south-eastwards (Fig. 5; Hays et al. 2010). Accordingly, half of the adult turtles departing from western Greece migrate to the Ionian Sea after nesting and the other half to the Adriatic Sea (Zbinden et al. 2011; Schofield et al. 2013). In this scenario, the low estimated contribution of western Greece to the foraging grounds in the northern Ionian Sea might be caused by two non-excluding processes. On the one hand, currents flowing off western Greece fluctuate seasonally (Hays et al. 2010) and most hatchlings might emerge when northward flowing prevails, thus drifting to the Adriatic Sea. This hypothesis could be tested combining particle-tracking modelling with detailed data about the seasonality of hatchling emergence at rookeries in western Greece. Expanding this kind of studies to the remaining rookeries in the Mediterranean would improve our understanding of hatchling dispersal within the whole basin. On the other hand, a very large nesting population might exist in Libya (Laurent et al. 1999), which might result in the dilution of contributions from western Greece. Although recently published figures do not support that claim (Casale and Margaritoulis 2010), nest numbers in Libya are poorly known due to political unrest and further research in the region is urgently needed.
The turtles considered in this study ranged from 30 to 69 cm CCL and hence were capable of dispersing independently of prevailing currents within the Mediterranean, except in the Strait of Gibraltar, the Alboran Sea and the Algerian Stream (Revelles et al. 2007a). However, the results reported here revealed genetic structuring consistent with the distribution of water masses and the pattern of surface currents. There is increasing evidence that young turtles become imprinted by the habitats they visit during their developmental migration, which in turn determines the habitats where they will settle and forage as adults (Hatase et al. 2002; Hays et al. 2010; Fossette et al. 2010; Eder et al. 2012). Turtles of Mediterranean origin begin settlement at approximately 40 cm CCL (Casale et al. 2008), which suggests that the genetic structuring here reported might emerge from such a process as imprinting. This, however, might not apply to turtles of Atlantic origin, as their natal rookeries are more than 6,000 km away from the Mediterranean foraging grounds they used as juveniles. This results in a remarkable trade-off between philopatry and habitat knowledge that finally leads them to leave the Mediterranean once they are large enough to overcome the currents in the Alboran Sea and the Strait of Gibraltar and settle in the western Atlantic (Bowen et al. 2005). Accordingly, adult turtles of Atlantic origin are highly scarce in the Mediterranean Sea.
The contributions from specific rookeries to Mediterranean foraging grounds described here are important not only for a better understanding of the biology of this species but also for its conservation. Fisheries bycatch stands as one of the major anthropogenic factors threatening sea turtle populations worldwide (Lewison et al. 2004; Lewison and Crowder 2007, Wallace et al. 2008), and available evidence indicates that tens of thousands of turtles are bycaught incidentally every year around the Mediterranean Sea (Carreras et al. 2004, Lewison et al. 2004; Alessandro and Antonello 2010; Casale 2011; Álvarez de Quevedo et al. 2010, 2013). However, the impact of these high levels of bycatch is unevenly distributed among nesting areas, according to the heterogeneous admixture revealed by genetic markers in this study. For example, bycatch in the western Mediterranean might be a threat for populations nesting in North America and in Libya, but less of a threat for those nesting elsewhere. Likewise, the Tyrrhenian Sea is an important foraging area for turtles not only from Libya but also from Calabria. Thus, bycatch in the Tyrrhenian Sea may directly impact the small nesting population of Calabria. Bycatch in the Adriatic Sea might primarily affect the population nesting in western Greece, whereas bycatch in the Levantine Sea might affect primarily the populations nesting in Turkey, Lebanon and Israel. This shows that knowing the degree of overlap between fishing and foraging grounds is a key factor to protect specific populations nesting in the Mediterranean Sea.
Overall, the present study has revealed previously unknown distributions of Atlantic and Mediterranean juvenile turtles within the Mediterranean Sea at a regional and fine-scale level through the use of population genetics. We highlighted the importance of large studies comprising vast sampling areas (particularly in the case of migratory species) and the use of long fragments of mtDNA as these highly enhance genetic resolution. We have underlined MSA as a useful tool in conservation biology, and with it, we suggest that future management plans include updated genetic assessments of wild populations as a conservation method to unveil population structuring and life-stage-specific distributions.
References
Abreu-Grobois A, Horrocks J, Formia A, Dutton P, LeRoux R, Vélez-Zuazo X, Soares L, Meylan P (2006) New mtDNA dloop primers which work for a variety of marine turtle species may increase the resolution capacity of mixed stock analysis. Proceedings of the 26th annual symposium on sea turtle biology and conservation, p 179
Alessandro L, Antonello S (2010) An overview of loggerhead sea turtle (Caretta caretta) by-catch and technical mitigation measures in the Mediterranean Sea. Rev Fish Bio Fish 20:141–161
Álvarez de Quevedo I, Cardona L, De Haro A, Pubill E, Aguilar A (2010) Sources of bycatch of loggerhead sea turtles in the western Mediterranean other than drifting longlines. ICES J Mar Sci 67:677–685
Álvarez de Quevedo I, Sanfélix M, Cardona L (2013) Mortality rates in by-caught loggerhead turtle (Caretta caretta) in the Mediterranean Sea and implications for the Atlantic populations. Mar Ecol Prog Ser 489:225–234
Bass AL, Epperly SP, Braun-McNeill J (2004) Multi-year analysis of stock composition of a loggerhead turtle (Caretta caretta) foraging habitat using maximum likelihood and Bayesian methods. Conserv Genet 5:783–796
Bentivegna F, Valentino F, Falco P, Zambianchi E, Hochscheid S (2007) The relationship between loggerhead turtle (Caretta caretta) movement patterns and Mediterranean currents. Mar Biol 151:1605–1614
Bolker BM, Okuyama T, Bjorndal KA, Bolten AA (2007) Incorporating multiple mixed stocks in mixed stock analysis: ‘many-to-many’ analyses. Mol Ecol 16:685–695
Bolten AB (2003) Active swimmers-passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtle. Smithsonian Books, Washington, pp 63–78
Bolten AB, Bjorndal KA, Martins HR, Dellinger T, Biscoito MJ, Encalada SE, Bowen BW (1998) Transatlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analysis. Ecol Appl 8:1–7
Bowen BW, Karl SA (2007) Population genetics and phylogeography of sea turtles. Mol Ecol 16:4886–4907
Bowen B, Avise JC, Richardson JI, Meylan AB, Margaritoulis D, Hopkins-Murphy SR (2003) Population structure of loggerhead turtles (Caretta caretta) in the northwestern Atlantic Ocean and Mediterranean Sea. Conserv Biol 7:834–844
Bowen BW, Bass AL, Soares L, Toonen RJ (2005) Conservation implications of complex population structure: lessons from the loggerhead turtle (Caretta caretta). Mol Ecol 14:2389–2402
Broderick AC, Glen F, Godley BJ, Hays GC (2002) Estimating the number of green and loggerhead turtles nesting annually in the Mediterranean. Oryx 36:227–235
Cardona L, Revelles M, Carreras C, San Félix M, Gazo M, Aguilar A (2005) Western Mediterranean immature loggerhead turtles: habitat use in spring and summer assessed through satellite tracking and aerial surveys. Mar Biol 147:583–591
Cardona L, Revelles M, Parga ML, Tomás J, Aguilar A, Alegre F, Raga A, Ferrer X (2009) Habitat use by loggerhead sea turtles Caretta caretta off the coast of eastern Spain results in a high vulnerability to neritic fishing gear. Mar Biol 156:2621–2630
Carr A (1986) Rips, FADS, and little loggerheads. Bioscience 36:92–101
Carreras C, Cardona L, Aguilar A (2004) Incidental catch of the loggerhead turtle Caretta caretta off the Balearic Islands (western Mediterranean). Biol Conserv 117:321–329
Carreras C, Pont S, Maffucci F, Pascual M, Barceló A, Bentivegna F, Cardona L, Alegre F, SanFélix M, Fernández G, Aguilar A (2006) Genetic structuring of immature loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea reflects water circulation patterns. Mar Biol 149:1269–1279
Carreras C, Pascual M, Cardona L, Aguilar A, Margaritoulis D, Rees A, Turkozan O, Levy Y, Gasith A, Aureggi M, Khalil M (2007) The genetic structure of the loggerhead sea turtle (Caretta caretta) in the Mediterranean as revealed by nuclear and mitochondrial DNA and its conservation implications. Conserv Gen 8:761–775
Carreras C, Pascual M, Cardona L, Marco A, Bellido JJ, Castillo JJ, Tomás J, Raga JA, SanFélix M, Fernández G, Aguilar A (2011) Living together but remaining apart: Atlantic and Mediterranean loggerhead sea turtles (Caretta caretta) in shared feeding grounds. J Hered 102:666–677
Casale P (2011) Sea turtle by-catch in the Mediterranean. Fish Fish 12:299–316
Casale P, Margaritoulis D (2010) Sea turtles in the Mediterranean: distribution, threats and conservation priorities. IUCN, Gland
Casale P, Freggi D, Basso R, Vallini C, Argano R (2007) A model of area fidelity, nomadism, and distribution patterns of loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea. Mar Biol 152:1039–1049
Casale P, Freggi D, Gratton P, Argano R, Oliverio M (2008) Mitochondrial DNA reveals regional and interregional importance of the central Mediterranean African shelf for loggerhead sea turtles (Caretta caretta). Sci Mar 72: 541–548
Casale P, Freggi D, Cinà A, Rocco M (2013) Spatio-temporal distribution and migration of adult male loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea: further evidence of the importance of neritic habitats off North Africa. Mar Biol 160:703–718
Clusa M, Carreras C, Pascual M, Demetropoulos A, Margaritoulis D, Rees AF, Hamza AA, Khalil M, Aureggi M, Levy Y, Türkozan O, Marco A, Aguilar A, Cardona L (2013) Mitochondrial DNA reveals Pleistocenic colonisation of the Mediterranean by loggerhead turtles (Caretta caretta). J Exp Mar Biol Ecol 439:15–24
Eckert SA, Moore JE, Dunn DC, Sagarminaga van Buiten R, Eckert KL, Halpin PN (2008) Modelling loggerhead turtle movement in the Mediterranean: importance of body size and oceanography. Ecol Appl 18:290–308
Eder E, Ceballos A, Martins S, Pérez-García H, Marín I, Marco A, Cardona L (2012) Foraging dichotomy in loggerhead sea turtles Caretta caretta off northwestern Africa. Mar Ecol Prog Ser 470:113–122
Ehrhart LM, Bagley DA, Redfoot WE (2003) Loggerhead turtles in the Atlantic Ocean: geographic distribution, abundance, and population status. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtle. Smithsonian Books, Washington, pp 157–174
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Fossette S, Girard C, López-Mendilaharsu M, Miller P, Domingo A, Evans D, Kelle L, Plot V, Prosdocimi L, Verhage S, Gaspar P, Georges J-Y (2010) Atlantic leatherback migratory paths and temporary residence areas. PLoS One 5:e13908
Garofalo L, Mingozzi T, Micò A, Novelletto A (2009) Loggerhead turtle (Caretta caretta) matrilines in the Mediterranean: further evidence of genetic diversity and connectivity. Mar Biol 156:2085–2095
Garofalo L, Mastrogiacomo A, Casale P, Carlini R, Eleni C, Freggi D, Gelli D, Knittweis L, Mifsud C, Mingozzi T, Novarini N, Scaravelli D, Scillitani G, Oliveiro M, Novelleto A (2013) Genetic characterization of central Mediterranean stocks of the loggerhead turtle (Caretta caretta) using mitochondrial and nuclear markers, and conservation implications. Aquat Conserv. doi:10.1002/aqc.2338
Gelman A, Carlin JB, Stern HS, Rubin DB (1996) Bayesian data analysis. Chapman and Hall, New York
Giovannotti M, Franzellitti S, Nisi Cerioni P, Fabbri E, Guccione S, Vallini C, Tinti F, Caputo V (2010) Genetic characterization of loggerhead turtle (Caretta caretta) individuals stranded and caught as bycatch from the North-Central Adriatic Sea. Amphibia-Reptilia 31:127–133
Grant WS, Milner GB, Krasnowski P, Utter FM (1980) Use of biochemical genetic variants for identification of sockeye salmon (Oncorhynchus nerka) stocks in Cook Inlet, Alaska. Can J Fish Aquat Sci 37:1236–1247
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hamad N, Millot C, Taupier-Letage I (2006) The surface circulation in the eastern basin of the Mediterranean Sea. Sci Mar 70: 457–503
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281
Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G (2010) Ontogenetic development of migration: lagrangian drift trajectories suggest a new paradigm for sea turtles. J R Soc Interface 7:1319–1327
Hoare B (2009) Animal migration: remarkable journeys in the wild. University of California Press, California
Laurent L, Lescure J, Excoffier L, Bowen B, Domingo M, Hadjichristophorou M, Kornaraki L, Trabuchet G (1993) Genetic studies of relationship between Mediterranean and Atlantic populations of loggerhead turtle Caretta caretta with mitochondrial marker. Comptes Rendus de l’Académie des Sciences Séries III 316:1233–1239
Laurent L, Casale P, Bradai MN, Godley BJ, Gerosa G, Broderick AC, Schroth W, Schierwater B, Levy AM, Freggi D, Abd El-Mawla EM, Hadoud DA, Gomati HE, Domingo M, Hadjichristophorou M, Kornaraky L, Demirayak F, Gautier CH (1998) Molecular resolution of marine turtle stock composition in fishery bycatch: a case study in the Mediterranean. Mol Ecol 7:1529–1542
Laurent L, Bradai MN, Hadoud DA, El Gomati HM, Hamza A (1999) Marine turtle nesting activity assessment on Libyan coasts. Phase 3: Survey of the coast to the west of Misuratah. Marine Biology Research Centre (Tajura, Libya), MEDASSET, RAC/SPA (MAP-UNEP), TCEP (Tripoli), WWF International Mediterranean Programme (Rome)
Lewison R, Crowder LB (2007) Putting longline bycatch of sea turtles into perspective. Conserv Biol 21:79–86
Lewison R, Crowder LB, Read AJ, Freeman SA (2004) Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol 19:598–604
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Maffucci F, Kooistra WHCF, Bentivegna F (2006) Natal origin of loggerhead turtles, Caretta caretta, in the neritic habitat off the Italian coasts, Central Mediterranean. Biol Conserv 127:183–189
Mansfield KL, Putman NF (2013) Oceanic habits and habitats Caretta caretta. In: Wineken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles volume III. CRC Press, Florida, pp 189–210
Marco A, Abella E, Liria-Loza A, Martins S, López S, Jiménez-Bordón S, Medina M, Oujo C, Gaona P, Godley BJ, López-Jurado LF (2012) Abundance and exploitation of loggerhead turtles nesting in Boa Vista island, Cape Verde: the only substantial rookery in the eastern Atlantic. Anim Conserv 15:351–360
Margaritoulis D, Argano R, Baran I, Bentivegna F, Bradai MN, Camiñas JA, Casale P, De Metrio G, Demetropoulos A, Gerosa G, Godley BJ, Haddoud DA, Houghton J, Laurent L, Lazar B (2003) Loggerhead turtles in the Mediterranean Sea: present knowledge and conservation perspectives. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtle. Smithsonian Books, Washington, pp 175–198
Millot C, Taupier-Letage I (2004) Circulation in the Mediterranean Sea. The handbook of environmental chemistry. Springer, Berlin, vol I
Monzón-Argüello C, Rico C, Carreras C, Calabuig P, Marco A, López-Jurado LF (2009) Variation in spatial distribution of juvenile loggerhead turtles in the eastern Atlantic and western Mediterranean Sea. J Exp Mar Biol Ecol 373:79–86
Monzón-Argüello C, Rico C, Naro-Maciel E, Varo-Cruz N, López P, Marco A, López-Jurado LF (2010) Population structure and conservation implications for the loggerhead sea turtle of the Cape Verde Islands. Conserv Genet 11:1871–1884
Narum SR (2006) Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv Genet 7:783–787
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, Oxford
Norman JA, Moritz C, Limpus CJ (1994) Mitochondrial DNA control region polymorphisms: genetic markers for ecological studies of marine turtles. Mol Ecol 3:363–373
Peakall R, Smouse PE (2012) GENALEX 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Pella J, Masuda M (2001) Bayesian methods for analysis of stock mixtures from genetic characters. Fish Bull 99:151–167
Piovano S, Clusa M, Carreras C, Giacoma C, Pascual M, Cardona L (2011) Different growth rates between loggerhead sea turtles (Caretta caretta) of Mediterranean and Atlantic origin in the Mediterranean Sea. Mar Biol 158:2577–2587
Plotkin PT (2003) Adult migrations and habitat use. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles 2. CRC Press, Florida, pp 225–241
Revelles M, Carreras C, Cardona L, Marco A, Bentivegna F, Castillo JJ, De Martino G, Mons JL, Smith MB, Rico C, Pascual M, Aguilar A (2007a) Evidence for an asymmetric size exchange of loggerhead sea turtles between the Mediterranean and the Atlantic trough the Straits of Gibraltar. J Exp Mar Biol Ecol 349:261–271
Revelles M, Isern-Fontanet J, Cardona L, San Félix M, Carreras C, Aguilar A (2007b) Mesoscale eddies, surface circulation and the scale of habitat selection by immature loggerhead sea turtles. J Exp Mar Biol Ecol 347:41–57
Revelles M, Camiñas JA, Cardona L, Parga M, Tomás J, Aguilar A, Alegre F, Raga A, Bertolero A, Oliver G (2008) Tagging reveals limited exchange of immature loggerhead sea turtles (Caretta caretta) between regions in the western Mediterranean. Sci Mar 72:511–518
Robinson AR, Leslie WG, Theocharis A, Lascaratos A (2001) Ocean circulation currents: Mediterranean Sea Circulation. In: Turekian KK, Thorpe SA (eds) Encyclopedia of ocean sciences. Academic Press, London, pp 1689–1703
Saied A, Maffucci F, Hochscheid S, Dryag S, Swayeb B, Borra M, Oureghi A, Procaccini G, Bentivegna F (2012) Loggerhead turtles nesting in Libya: an important management unit for the Mediterranean stock. Mar Ecol Prog Ser 450:207–218
Schofield G, Dimadi A, Fossette S, Katselidis KA, Koutsoubas D, Lilley MKS, Luckman A, Pantis JD, Karagouni AD, Hays GC (2013) Satellite tracking large numbers of individuals to infer population level dispersal and core areas for the protection of an endangered species. Divers Distrib 19:834–844
Shamblin BM, Bolten AB, Bjorndal KA, Dutton PH, Nielsen JT, Abreu-Grobois FA, Reich KJ, Witherington BE, Bagley DA, Ehrhart LM, Tucker AD, Addison DS, Arenas A, Johnson C, Carthy RR, Lamont MM, Dodd MG, Gaines MS, LaCasella E, Nairn CJ (2012) Expanded mitochondrial control region sequences increase resolution of stock structure among North Atlantic loggerhead turtle rookeries. Mar Ecol Prog Ser 469:145–160
SWOT (2007) SWOT report 2: a global glimpse of loggerhead nesting. State of the World’s Sea Turtles, Arlington
Wallace BP, Heppell SS, Lewison RL, Kelez S, Crowder LB (2008) Impacts of fisheries bycatch on loggerhead turtles worldwide inferred from reproductive value analyses. J Appl Ecol 45:1076–1085
Wallace BP, DiMatteo AD, Hurley BJ, Finkbeiner EM, Bolten AB, Chaloupka MY, Hutchinson BJ, Abreu-Grobois FA, Amorocho D, Bjorndal KA, Bourjea J, Bowen BW, Briseño Dueñas R, Casale P, Choudhury BC, Costa A, Dutton P, Fallabrino A, Girard A, Girondot M, Godfrey MH, Hamann M, López-Mendilaharsu M, Marcovaldi MA, Mortimer JA, Musick JA, Nel R, Pilcher NJ, Seminoff JA, Troëng S, Witherington B, Mast RB (2010) Regional Management Units for marine turtles: a novel framework for prioritizing conservation and research across multiple scales. PLoS One 5:e15465
Yilmaz C, Turkozan O, Bardakci F (2011) Genetic structure of loggerhead turtle (Caretta caretta) populations in Turkey. Biochem Syst Ecol 39:266–276
Yilmaz C, Turkozan O, Bardakci F, White M, Kararaj E (2012) Loggerhead turtles (Caretta caretta) foraging at Drini Bay in Northern Albania: genetic characterisation reveals new haplotypes. Acta Herpetol 7:155–162
Zbinden JA, Bearhop S, Bradshaw P, Gill B, Margaritoulis D, Newton J, Godley BJ (2011) Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Mar Ecol Progr Ser 421:291–302
Acknowledgments
We are thankful to all the researchers, assistants and volunteers who collaborated in sample collection. This study was cofunded by projects CGL2009-10017 and CTM2010-22218 of the Spanish Government (CICYT) and partially funded by the EU project Protección de Praderas de Posidonia en LICs de Baleares LIFE00NAT/E/7303 and Zoo de Barcelona. The tissue samples used in this paper were provided by the BMA tissue bank managed by the Fundació Bosch i Gimpera with the support of the Fundació pel Desenvolupament Sostenible and by the Italian TARTANET network of rescue centres, with a special thanks to Marco Affronte of Fondazione Cetacea, Giovanni Furii of Legambiente Oasi di Lago Salso and Annalisa Liotta of CTS Brancaleone. Marcel Clusa was supported by the Biodiversity Research Institute (IRBio) of the University of Barcelona and Carlos Carreras by the Beatriu de Pinós programme of the Generalitat de Catalunya. All the IRBio authors are part of the research groups 2009SGR-842 and 2009SGR-636 of the Generalitat de Catalunya. JT and JAR are supported by project PROMETEO/2011/40 of the Generalitat Valenciana and project CGL2011-30413 of the Spanish Ministry of Sciences and Innovation. Maps created with Maptool (www.seaturtle.org). We thank Michele Masuda for her help with Bayes and Gregg Ashcroft for English grammar corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Reusch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clusa, M., Carreras, C., Pascual, M. et al. Fine-scale distribution of juvenile Atlantic and Mediterranean loggerhead turtles (Caretta caretta) in the Mediterranean Sea. Mar Biol 161, 509–519 (2014). https://doi.org/10.1007/s00227-013-2353-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2353-y