Abstract
Black-browed albatrosses (Thalassarche melanophris) disperse over the Argentinean Continental Shelf and neighboring waters during their non-breeding season. It is one of the most frequent seabirds attending fishing vessels and also the most common Procellariform in the bycatch of longliners and trawlers in the area. Understanding the use of fishery discards by this species is an important issue when assessing the potential effect of strategic discard management in decreasing the abundance, interactions, and mitigating mortality. In the present study, we analyzed carbon and nitrogen stable isotope compositions in the blood of Black-browed albatrosses to assess the relative contribution of discards from different fisheries to the diet of this species in winter. Samples were obtained in winter 2011 from fishing vessels operating between 41–43°S and 57–59°W. No sex differences in δ13C and δ15N were observed. Results indicate that during their non-breeding season, isotopic signatures of Black-browed albatrosses are closer to discards and offal generated by fisheries and in particular by trawlers. The large fishing effort of trawl fisheries in Argentina highlights the urgency of an exhaustive analysis to find practical and effective ways to reduce the number of seabirds attending trawlers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishery discards (including nontarget species and undersized fish) and offal constitute a predictable and abundant source of food to seabirds and other top predators attending fishing vessels. In addition, surface and shallow diving predators such as albatrosses and petrels can benefit by bottom prey facilitated during fishing operations (Furness 2003; Votier et al. 2004). Despite fishery discards and offal can significantly contribute to the diet of seabirds, little is known in many fisheries about the relative availability of discards and its contribution to seabird populations (Navarro et al. 2010; Votier et al. 2010, 2013). Previous studies have shown that the occurrence and composition of fishery discards affects the number of seabirds attending trawl vessels and collisions with fishing gear, hence increasing the risk of incidental mortality (Crofts 2006; González-Zevallos and Yorio 2006; Sullivan et al. 2006; Watkins et al. 2008; Abraham et al. 2009; Favero et al. 2011). In fact, strategic discard management has been referred as one way to reduce interactions between seabirds and the fishing gear, both in trawl and longline fisheries (ACAP (Agreement on the Conservation of Albatrosses and Petrels) 2011; Pierre et al. 2012). In this regard, some countries are about to implement regulations concerning discards in fisheries, and so, numerous concerns about potential favorable or detrimental effects are in discussion (Bicknell et al. 2013). Consequently, understanding the use of fishery discards by a given seabird species may not only help to identify to which extent such species is associated with certain fishery but also to assess the potential effect that discard management may have in decreasing the abundance and interactions events (e.g., incidental mortality) of this seabird.
It is broadly accepted that fisheries have contributed—through incidental mortality—to the observed decreases in seabird populations, particularly albatrosses’ populations (Arnold et al. 2006; Birdlife International 2012; Croxall et al. 2012). During the non-breeding period, albatrosses, as most pelagic seabird species, are not central place foragers and disperse widely at sea. Research on these species during this part of the annual cycle becomes difficult and, as a result, most of their ecological aspects during non-breeding season remain unknown or not well understood (Cherel et al. 2012). As a matter of fact, little is known about the trophic and spatial interaction between seabirds and fisheries in coastal waters and the high seas during non-breeding season. Only few years ago, Bugoni et al. (2010) evaluated, through the analysis of carbon and nitrogen stable isotopes, the contribution of discards from pelagic longline fisheries to the diet of several species of a Procellariform community at the South Atlantic Ocean. Naturally occurring stable isotopes (15N and 13C) are extensively used to study different aspects of birds’ ecology such as diet, foraging ecology, and migratory pathways, among others. Carbon stable isotope signatures reflect consumer’s foraging habitat (Cherel and Hobson 2007; Moreno et al. 2011; Stowasser et al. 2012), while nitrogen stable isotope signatures are widely used to reflect the consumers trophic position (Inger and Bearhop 2008). Given that the isotopic turnover rate differs between tissues, when selecting among tissues, the diet of an animal can be inferred for a range of different timescales (Inger and Bearhop 2008). Consequently, the use of stable isotope analysis is a powerful tool given that it integrates diet information during a certain extent of time, rather than the snapshot provided by direct feeding observations or the analysis of stomach contents. Other approaches to study the albatross’ non-breeding ecology have been already conducted through stable isotope analysis on feathers, allowing inference of spatial distribution, habitat and trophic segregation, and niche divergence between and within Procellariform species (Cherel and Hobson 2007; Jaeger et al. 2009, 2010a, b; Phillips et al. 2009, 2011; Weiss et al. 2009; Ceia et al. 2012, Cherel et al. 2012). However, the ample temporal window that feather tissues reflect has limited the interpretations and conclusions of these previous studies at least regarding diet. In this study, whole blood was chosen as it integrates dietary information over three to 4 weeks before sampling and so has the potential to resolve nutritional variations at a shorter scale (Bearhop et al. 2006; Cherel et al. 2007).
The Argentinean Continental Shelf and neighboring waters is a highly productive area where important numbers and biomass of top predators converge with large fisheries (Croxall and Wood 2002; Acha et al. 2004; Favero and Silva Rodriguez 2005), some of them posing a threat to seabirds (Favero et al. 2003; Gandini and Frere 2006; Gómez Laich et al. 2006; González-Zevallos and Yorio 2006; Sullivan et al. 2006; Gómez Laich and Favero 2007; Seco Pon et al. 2007; Favero et al. 2011, 2013). Among the most affected seabird species, the Black-browed albatross Thalassarche melanophris (BBA) is of particular concern. This species has undergone a substantial decline in its populations in the South Atlantic leading to the current IUCN endangered conservation status (IUCN 2012). This status is a result of several anthropogenic threats as fisheries, which are major cause of population declines through bycatch mortalities (Gales 1998). BBA is one of the most important species in the bycatch of a number of fisheries (up to 57 % of total bycatch) in the region (Neves and Olmos 1998; Favero et al. 2003, 2011, 2013 Gómez Laich et al. 2006; Baker et al. 2007; Seco Pon et al. 2007; Bugoni et al. 2008a; Watkins et al. 2008; Jiménez et al. 2009, 2010). Most of the studies analyzing the impact of fisheries on BBA populations were focused on longline vessels (bycatch rate 0.010 birds per 1,000 hooks, Favero et al. 2013). However, that ca. 45 % of the total bycatch may be associated with the trawling fleet (Baker et al. 2007). In the southern ocean, there is partial evidence that the impact of trawl fisheries can be as important, in magnitude, as that of longliners (González-Zevallos and Yorio 2006; Sullivan et al. 2006). Recent studies report estimates of 0.012 BBA killed per hour trawl. Extrapolation of these values results in an annual mortality of seabirds in the order of several hundreds to even over 1,000 albatrosses (Favero et al. 2011).
Individuals of this species breeding in Malvinas (Falklands) Islands migrate north during the austral winter reaching waters off northern Argentina, Uruguay, and Southern Brazil (Grémillet et al. 2000; Copello et al. 2013). Most of the previous studies on BBAs’ diet have been conducted during the breeding season, showing a diet comprised by fish, cephalopods, and crustaceans. Some authors highlighted the importance and the occurrence of demersal fish, likely coming from demersal fisheries in the diet of these birds (Prince 1980; Thompson 1992; Reid et al. 1996; Cherel et al. 2002; Arata and Xavier 2003). However, little is known on the diet of BBAs during the non-breeding season. Studies using bird carcasses, and/or bird specimens killed by fisheries (Colabuono and Vooren 2007), may show biased or partial results since (1) carcasses may contain over-digested items and/or samples in bad condition that difficult the identification of prey, and (2) data from birds killed by fisheries correspond to individuals effectively attending vessels and so stomach contents could be strongly composed by prey coming from such source. A recent study conducted in Brazilian waters using stable isotopes has revealed a high contribution of discards from the pelagic longline fishery to the diet of Procellariform birds, including the BBA, attending fishing vessels (Bugoni et al. 2010). Despite this background, much work still needs to be done in order to understand the role of multiple fisheries operating on the Argentinean Continental Shelf in their contribution of fisheries discards to BBAs. In the present study, we analyzed carbon and nitrogen stable isotopes in blood samples of adult BBA during the time off breeding and potential food sources in order to (1) evaluate the relative contribution to the trophic spectrum of discards coming from fisheries operating in its main wintering area and (2) determine the existence of sex-related differences in the contribution of discards that could be linked to differences in the distribution of individuals and differential risks of mortality in fisheries.
Methods
Study area
The Argentinean Continental Shelf is a ground for large fisheries, including trawlers, longliners, and jiggers (PNA-Aves 2010). The longline fishery in Argentina has experienced an important reduction during the last decade or so both in the number of vessels and hooks set, and is nowadays composed of four vessels using either autoline (Mustad), Spanish or “cachaloteras” system, and targeting Patagonian toothfish (Dissostichus eleginoides, c. 85 % of the catch) and Kingclip (Genypterus blacodes, <10 % of the catch) (Favero et al. 2013, http://www.minagri.gob.ar/site/pesca/pesca_maritima/02-desembarques/index.php hereinafter MINAGRI).The total catch during 2011 was 2,029 metric tonnes, representing 0.3 % of the total catch in Argentina. In contrast to this reduced fishing effort, other fishery such as the ice-trawl contains c.135 operative vessels fishing along the continental shelf between 37° and 48°S (PNA-Aves 2010; Copello unpubl. data). The primary target for this fleet is the Argentine hake Merluccius hubbsi (c. 70 % of the catch), followed by Hoki Macruronus magellanicus, Kingclip G. blacodes, and the Argentine shortfin squid Illex argentinus, among others (Favero et al. 2011). The total catch during 2011 was 253,201 metric tonnes, representing the 34 % of the total catch of entire Argentine fishery (MINAGRI). This fleet occasionally targets pelagic species like Argentine anchovy (Engraulis anchoita) and Chub Mackerel (Scomber japonicus), and in 2011, this fleet accounted for the 51 and 67 % of the catch for those species, respectively (MINAGRI).
The freezer-trawler fleet contains fewer operative vessels compared to the ice-trawl fleet though a large fishing effort. It operates south to the 42°S, although the fishing effort is concentrated between 45° and 48°S. Most of this fleet targets Argentine hake (c. 80 % of the catch) (Bertolotti et al. 2001) and the total catch in 2011 was 187,570 metric tonnes, representing 25 % of the total catch in Argentina (MINAGRI). In this fishery, the main component of discards (undersized hake) and offal (hake head, tail, and guts) could be assumed to have similar isotopic signatures than ice trawlers. However, given the very low level of overlapping with BBAs during the temporal window analyzed (see Copello et al. 2013; Copello unpubl. data), this fleet was considered negligible in terms of supplying any source of food to albatrosses.
The shrimp-trawler fleet operates mostly in Golfo San Jorge from mid-February to November. It is constituted by 70–80 vessels that target the Argentine Red Shrimp (Pleoticus muelleri). In this fishery, the Argentine hake constitutes de bulk of discards estimated to be about 40,000 metric tonnes during 2007 (PNA-Aves 2010; González-Zevallos and Yorio 2011; Góngora et al. 2012). Other species discarded are the Longtail southern cod (Patagonotothen ramsayii), Argentine shortfin squid (I. argentinus), Butterfish (Stromateus brasiliensis), and Kingclip (G. blacodes), but these have been considered as rare in a recent study (González-Zevallos and Yorio 2011).
The jigging fleet contains some 88 operative vessels that target the Argentine shortfin squid I. argentinus (more that 70 % of the total catch for this species). During the austral autumn season, it concentrates south to 44°S moving north toward the winter when concentrates north to 44°S (PNA-Aves 2010). Total catch during 2011 was 58,984 metric tonnes representing 8 % of total catch in Argentina (MINAGRI). We did not consider this fleet given its southern distribution and lack of overlapping during the studied period (Copello unpubl. data).
Sample collection
Blood samples were obtained from adult BBAs captured in June 2011 from commercial fishing vessels operating between 41–43°S and 57–59°W. Birds close to the vessel were captured with an adapted dip net. Approximately 0.3 ml of blood was obtained from the tarsal vein, transferred to a vial with 1.5 ml of absolute ethanol (Biopack®, Argentina), and stored at room temperature until analysis. Ethanol has being regularly used as preserver given that it has shown to have negligible effect on the stable carbon and nitrogen isotope values in a variety of tissues (Hobson et al. 1997; Kelly et al. 2006; but see Bugoni et al. 2008b). Birds were marked to avoid recapture.
Representative samples of main fish and invertebrate species discarded, including non-commercial species and undersized fish and squid, were collected onboard trawlers and longliners during autumn and winter months 2011 (Table 1). Long-term onboard observations on BBA foraging behaviors attending fishing vessels allowed us to identify the Argentine hake, the Longtail southern cod, the Butterfish, and the Argentine shortfin squid as both the most commonly discarded species in the demersal trawlers as well as the most consumed items (Seco Pon unpubl. data).
Samples of pelagic species like Argentine Anchovy (E. anchoita) and Argentine shortfin squid were obtained from landings of trawlers targeting pelagic species. Samples of Patagonian toothfish and baits (Illex sp. and Sardinella sp.) were obtained from demersal longline vessels. A portion of white muscle from the lateral region of fish and the mantle of squids was sampled. Given that satellite tracking data from our own database showed that BBAs non-breeding distribution was not only restricted to Argentina but also extended to waters off Uruguay and Southern Brazil (Copello et al. 2013), the inventory of potential food sources was completed with isotopic data of fish species targeted by the pelagic longline fleet that operates in these waters (Bugoni et al. 2010). We assumed that these isotopic values were representative of fish targeted by the pelagic longline in waters off Uruguay (Jiménez et al. 2010).
Stable isotope analysis and molecular sexing
In this study, we used whole blood though we expected to obtain information of BBA diet during the first period of the non-breeding season. All samples were dried in an oven at 60 °C and grounded using a hand mortar (Hobson et al. 1997; Cherel et al. 2007). In order to reduce carbon variability because of lipid variation between tissues, we conducted lipid extraction from potential prey sample using successive rinses in a 2:1 chloroform–methanol solution (Bligh and Dyer 1959). Stable isotope signatures were determined by mass spectrometry at the Stable Isotope Facility at UC Davis. Results are presented in the usual δ notation relative to Vienna Pee Dee Belemnite and atmospheric N2 (Air) for δ13C and δ15N, respectively. Based on the internal standards (G-11 nylon, G-13 bovine liver, G-17 USGS-41 glutamic acid, and G-9 glutamic acid), the analytical precision (±1 SD) was estimated as <0.2 ‰ and for both δ13C and δ15N.
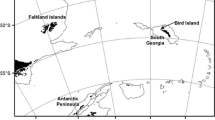
For comparative purposes, we plotted our BBA data against the literature values for BBA blood samples obtained at other southern areas during the non-breeding season (Bugoni et al. 2010) along with approximate isotopic boundaries of major oceanographic zones in the Southern Ocean and South Atlantic as identified by Phillips et al. (2009). Previous studies suggest that, although variability is similar between blood and feathers, whole blood is predictably depleted in C13 and N15 (Cherel et al. 2005; Quillfeldt et al. 2008). So, to visually facilitate this comparison, prior to plotting data, we adjusted blood stable isotope values using the mean difference between feathers and whole blood derived from previous studies (δ13C: 1.3 ‰ and δ15N: 1.1 ‰, see Quillfeldt et al. 2008)
Individuals were genetically sex-typed from blood subsamples, after DNA extraction and PCR amplification of CHD genes using primers 2550F (Fridolfsson and Ellegren 1999) and 2757R (Griffiths unpublished, see Bugoni et al. 2011). Samples of BBA killed in fisheries and sex-determined by necropsy were used as positive controls.
Statistical analysis
Isotopic signatures in blood of BBA females and males were compared using Student’s t test (Zar 1999). Means are provided ±standard deviations unless otherwise stated. To quantitatively assess the importance of different prey as food sources, we used the SIAR Bayesian stable isotope mixing model in R environment (Parnell et al. 2010). This model provides quantitative indices of food item contribution to a consumers’ diet accounting for known variability in sources, fractionation, and other unquantified variability within the model. Moreover, SIAR outputs represent true probability density functions, rather than a range of feasible solutions as it was that case of earlier mixing models (Parnell et al. 2010). We performed a mixing model assuming four functional prey clusters: demersal fish assumed to be facilitated during trawl operations and/or components of trawl discards (including M. hubbsi, P. ramsayii, and S. brasiliensis), pelagic fish and invertebrates (E. anchoita and I. argentinus), demersal longline offal (D. eleginoides and baits), and pelagic longline offal (sharks and tunas, Bugoni et al. 2010). In order to perform this analysis, the isotopic signature for food sources must be rearranged by appropriate discrimination factors, procedure that has been reported as the weakest link in the application of stable isotope mixing models and so currently matter of discussions. The use of inadequate values will result in inaccurate interpretations (Caut et al. 2008; Bond and Diamond 2011). The use of species- and tissue-specific discrimination values with constrained uncertainty has been recommended (Bond and Diamond 2011; Galván et al. 2012), but in the absence of them, the most parsimonious choice suggested is the use of generic discrimination values with high uncertainty and to input prior information from the literature and field observations (Caut et al. 2009; Galván et al. 2012).There are no diet–blood discrimination factors available for BBA; though two models were developed as potential scenarios using different published pairs of discrimination factors: proxy values in avian blood recently reviewed (model A: δ13C: 0.6 ± 0.5 ‰ δ15N: 2 ± 0.5 ‰, Caut et al. 2009) and used in recent studies with other seabirds (Quillfeldt et al. 2011); and proxy values developed for one seabird species (model B: δ13C: 1.1 ± 0.5 ‰ δ15N: 2.8 ± 0.5 ‰, Bearhop et al. 2002) and further adopted and used (Cherel et al. 2005; Bugoni et al. 2010; Votier et al. 2010; Ceia et al. 2012). Models were run with 1,000,000 iterations, with an initial discard of 40,000 resulting in 65,000 posterior draws. In both cases, the predator isotopic values were contained within the mixing space after application of discrimination corrections.
Adult sex ratio deviation from 1:1 expected proportion was tested by χ 2 with Yates’ correction for continuity because of one degree of freedom. Given that BBAs are sexually monomorphic in plumage and sex differences are visually impossible when conducting the captures, we decline the occurrence of methodological bias and assume that the sex ratio observed is representative of the population attending fishing vessels (Bugoni et al. 2011).
Results
Thirty-eight adult BBAs were captured and sampled on board vessels. Adult sex ratio was close to 1:1, with 18 males and 20 females captured (chi-square test, χ 2 Yates = 0.026, P = 0.87). Mean δ13C and δ15N values together with C/N mass ratios for BBAs are shown in Table 1. Carbon isotope values on BBA ranged from −16.2 to −17.6 ‰ while nitrogen values ranged from 14.4 to 18.3 ‰ (Fig. 1). None of the isotopic signatures showed significant difference between sexes (Student’s t test, T 36 = 0.66, P = 0.50 and Student’s t test, T 36 = 1.67, P = 0.10 for carbon and nitrogen, respectively). However, nitrogen signatures showed more variability than carbon signatures, with samples from females showing higher variability than males, although not reaching statistical levels (Levene test, F (1,36) = 3.46, P = 0.07).
Stable isotope signatures (δ13C and δ15N) from whole blood samples of adult female (gray circles) and male (open circles) Black-browed albatrosses (BBA) in relation to potential food sources (black triangles, mean ± SE). Stable isotope signatures of albatrosses are shown without correction for dietary isotopic fractionation
Isotope signatures and mixing model analysis
Mean δ13C and δ15N values together with C/N mass ratios for the food source categories are shown in Table 1. Values of δ13C of potential food sources ranged from −21.6 to −15.9 ‰, while values of δ15N of potential food resources ranged from 10.7 to 17.4 ‰. Both bottom and pelagic longline discards showed much lower mean δ15N than other categories, reflecting a lower trophic position (Fig. 1; Table 1). Significant differences were found in the δ13C and δ15N signatures of species considered as potential prey for BBAs (Table 1; δ13C: ANOVA, F (9,36) = 8.91, P < 0.001; δ15N: ANOVA, F (9,36) = 17.38, P < 0.001).
The distributions resulting from the SIAR isotope mixing models showed two potential scenarios. In Model A, demersal fish was the main food source (42 %, 95 % CI 24–63 %) followed by pelagic fish (28 %, CI 6–48 %), demersal longline offal (23 %, CI 19–29 %), and pelagic longline offal (6 %, CI 0–15 %) (Fig. 2). The mixing model predicted that demersal fish was more important in the diet of females, while males showed a relative larger contribution of resources from both demersal and pelagic longline fisheries (Table 2). Model B showed the demersal longline as the main food source with a more constraint range of contributions (39 %, CI 35–44 %) followed by demersal fish (30 %, CI 12–48 %), pelagic fish (20 %, CI 0–38 %), and pelagic longline (10 %, C 0–20 %) (Fig. 2). Although much overlap in credibility intervals existed, females showed similar contribution of both demersal longline offal and demersal fish discarded from trawlers, while males showed larger contribution of demersal longline species followed by demersal fish (Table 2). Differences between models were observed about the inclusion of zero as a possible contribution for some prey. In both models, demersal fish and demersal longline offal had more precise contributions and did not include zero as a possible solution. In Model A, pelagic fish had a broader range of contributions and pelagic longline offal included zero with high probability of occurrence, while in Model B pelagic fish did not only show a broader range of contributions but also together with pelagic longline source included zero with high probability of occurrence (Fig. 2; Table 2).
Density plots from the SIAR stable isotope mixing models for the contributions of prey types to the diet of Black-browed albatrosses. Model A was developed using 0.6 ± 0.5 and 2 ± 0.5 ‰ as discrimination factors for δ13C and δ15N, respectively (Caut et al. 2009). Model B was developed using 1.1 ± 0.5 and 2.8 ± 0.5 ‰ as discrimination factors for δ13C and δ15N, respectively (Bearhop et al. 2002)
Discussion
Most of the studies addressing non-breeding migratory movements and/or foraging strategies in oceanic birds through stable isotope analysis have used feathers as the sampled tissue (Cherel et al. 2000, 2012; Hedd and Montevecchi 2006; Phillips et al. 2009; Ramos et al. 2009; Jaeger et al. 2010a, b; Quillfeldt et al. 2008, 2010a, b; Raya Rey et al. 2012). Up to our knowledge, this is the second study using isotopic signatures in blood to analyze trophic ecology of Procellariforms, particularly BBAs, during the non-breeding season in the South Atlantic Ocean (see Bugoni et al. 2010).
Since only birds attending vessels were sampled, there is a possibility of sampling bias toward individuals that specialized in feeding on discards, with a subrepresentation of those making more use of natural resources. However, observations of marked birds suggest that individual birds only remain for short periods attending the same vessel (Bugoni et al. 2010). BBAs are known to cover vast areas in short period of time (Copello et al. 2013) and therefore have potential access to a wide range of natural prey and food source coming from anthropogenic activities. Moreover, blood samples analyzed in this study represent an integration of diet of a number of weeks (Bearhop et al. 2006; Cherel et al. 2007), hence combining several feeding events and increasing the likelihood of a more representative picture of the trophic spectrum of the species.
The isotopic signatures of BBA in the present study were in line with those from a previous study in waters off Brazil (δC13: −17.2 ± 0.6 and δN15: 16.3 ± 0.9, see Bugoni et al. 2010) during the non-breeding season. The similarity among signatures with birds (mostly subadults) captured off Brazil suggests that during their non-breeding season, BBAs of different age classes forage at similar functional trophic level. Recent studies have defined rough isotopic boundaries of major oceanographic zones based on the latitudinal variation of δ13C isotopic signatures of marine organisms in southern oceans and isotopic values from feather and blood of tracked birds (Quillfeldt et al. 2005; Cherel and Hobson 2007; Phillips et al. 2009; Jaeger et al. 2010a). The comparison of these “isoscapes” with our isotopic signatures shows that BBAs captured in Argentina foraged during the non-breeding season at subtropical and continental shelf likewise those individuals from Brazil (Fig. 3). However, these conclusions are open to consideration given potential variability in baseline signatures, this is a possible alternative destination for wintering BBA (Phillips et al. 2009). This result is in agreement with the distribution of BBAs during the same non-breeding season (Copello et al. 2013) and also close to signatures of White-chinned petrels (Procellaria aequinoctialis) known to winter at the Patagonian Continental Shelf and shelf slope (Phillips et al. 2006, 2009). Our results were also in line with recent reports of BBAs breeding in South Georgia where isotopic signatures of feathers showed a similar pattern in the use of “isoscapes” during the non-breeding season in the Benguela current system, the most common wintering area for individuals breeding in that sub-Antarctic archipelago (Phillips et al. 2011). However, the isotopic signatures for BBAs reported in this study were higher than Procellariform species, like Macronectes giganteus, M. halli, Diomedea exulans, T. chrysostoma, breeding in Antarctic, and sub-Antarctic sites (Phillips et al. 2011; Raya Rey et al. 2012). The relatively high C and N signatures might be an indicative of a more complex structure of trophic webs occurring at the continental shelf compared to those in more southerly latitudes (Forero et al. 2004, 2005; Eder et al. 2010). In the same way, values of demersal (components of trawler discards), pelagic fish and fish from pelagic longliners showed carbon signatures typical of fish from continental shelf waters (Forero et al. 2004; Bugoni et al. 2010). Contrarily, discards from demersal longliners showed a lower mean δ13C typical of southern latitudes, and values of the Patagonian Toothfish D. eleginoides were close to those from the literature (Cherel et al. 2008; Ceia et al. 2012) (Table 1).
Stable isotope signatures from whole blood of individual Black-browed albatrosses (BBA) and approximate isotopic boundaries of major oceanographic zones (gray fields) in the Southern Oceans and South Atlantic as defined by Phillips et al. (2009). Isotope signatures were adjusted using the mean difference between feather and whole blood derived from previous studies (δ13C: 1.3 ‰ and δ15N: 1.1 ‰, Quillfeldt et al. 2008). Mean (±SD) values for BBA from Brazil were used for comparison (Bugoni et al. 2010)
Most of the previous studies on diet of BBAs have been conducted during the breeding season, showing a diet comprised by fish, cephalopods, and crustaceans. The importance and the occurrence of fishery discards in the diet of these birds has been also highlighted by some authors (Prince 1980; Thompson 1992; Reid et al. 1996; Cherel et al. 2002; Arata and Xavier 2003). On the contrary, little is known on the diet of BBAs during the non-breeding season and most of this studies likely show biased results given that only bird carcasses and/or bird specimens killed by fisheries were used (Colabuono and Vooren 2007). Our mixing models suggest a high input of demersal fish species (i.e., M. hubbsi, P. ramsayii, S. brasiliensis, D. eleginoides, and baits from demersal longliners) in the diet of BBAs (Fig. 2). Even though multiple fisheries targeting a range of species operate in the study area, demersal fish considered in this study constitute the main discarded species. The first three above-mentioned species are common and abundant in the discards of Argentinean ice (and to some extent freezer and shrimp) trawlers (Aubone et al. 2004; Dato et al. 2006; González-Zevallos and Yorio 2011; Seco Pon unpubl. data). Patagonian Toothfish D. eleginoides is the main species targeted by demersal longliners and discarded as offal (i.e., head, tail, and guts), and to a lesser extent discarded in freezer trawlers. Given that these albatrosses are essentially surface-seizers and shallow divers, it is very unlikely that the demersal fish as the above mentioned could be obtained in large quantities in the absence of any facilitation processes (Prince and Morgan 1987; Bugoni et al. 2010). As it was previously stated, the overlapping between ice trawlers and BBAs strongly suggests that the contribution of demersal fish to the diet of these birds during winter comes from the ice-trawl fleet. However, given the similitude between ice and freezer trawlers in the target species and by-products of the fishing operation, we understand that freezer trawlers should be regarded in the same way for conservation and management purposes, at least during moments of the annual cycle when BBAs are distributed further south in the vicinity of breeding grounds (Granadeiro et al. 2011; Catry et al. 2013). Available information shows that the trawler fleet from Uruguay has the Argentine hake as one of main target species (99.8 % of total landing for this species, DINARA 2010) and this species is also discarded by shrimp-trawl fleet operating further south at the Argentinean Continental Shelf, hence suggesting that BBAs could be making use of discards from these fleets as well. The contribution of demersal species (both from trawlers and longliners) in the diet of BBAs was followed in both models by pelagic fish and squid (i.e., E. anchoita and I. argentinus) (Fig. 2). Even though these species are the components of the discard in trawlers, they could be as well “naturally” captured by the albatrosses.
Foraging strategies may differ between individuals and these variations may respond to several variables like sex, age, morphology, and individual preferences (Ceia et al. 2012). Such differences can be consistent over time and result in different exposure to threats like fisheries, hence affecting population dynamics by altering, for example, the sex ratio and/or age composition in a given population (Croxall and Wood 2002; Phillips et al. 2004, 2009). The c. 1:1 sex ratio of adult BBAs attending trawlers in this study contrasted with information from Brazilian waters where a clear bias toward the females was reported both in birds attending and caught in pelagic longline vessels (Bugoni et al. 2011). In Argentinean waters, the observed sex ratio of birds attending ice trawlers was in line with those of birds incidentally killed in the demersal longline fleet in waters from Patagonia (Gandini and Frere 2006; Seco Pon et al. 2007). Similar carbon and nitrogen isotopic signatures in BBA blood from both sexes were observed. This was in line with studies on other breeding and non-breeding Procellariforms, where no sex differences were found when feathers (see review in Phillips et al. 2011) or blood (Bugoni et al. 2010) were analyzed. Sex differences in stable isotope signatures have been reported in other albatross species like the Wandering albatross (D. exulans) and the Gray-headed albatross (T. chrysostoma) (Jaeger et al. 2009; Phillips et al. 2009). Our results indicate that in general terms, both females and males forage at similar trophic levels and in similar water masses. However, the stable isotope mixing model analysis evidenced (even though much overlap in credibility intervals existed) males showing a higher contribution of fish species frequently discarded by both demersal and pelagic longliners.
From the two pictures provided by different models performed and considering the background information on (1) BBA non-breeding distribution over the continental shelf, (2) BBA overlapping with different fleets, and (2) the large asymmetry in fishing effort between demersal trawl and longline fisheries, we understand that Model A offers a more realistic picture of what are the main anthropogenic sources of food for BBA in winter. In other words, it is some four longliners currently in operation (see Favero et al. 2013 for the evolution of the longline fishing effort in the decade) with little overlapping (Copello unpubl. data) against hundreds of demersal trawlers with high overlap (Favero et al. 2011; Consejo Federal Pesquero 2010). Recent studies have reported that the BBA is the seabird accounting for most of observed interactions and mortalities in Argentinean trawlers (0.012 BBA killed/h trawl, see Favero et al. 2011 and references therein). Our results indicate that during their non-breeding season, BBA relies on discards and offal generated by fisheries and in particular trawlers. The extant prevalence of trawl fisheries in Argentina and its large fishing effort highlights the urgency of exhaustive analysis on ways to reduce the number of seabirds attending these fisheries and mitigate incidental mortality in them.
References
Abraham ER, Pierre JP, Middleton DAJ, Cleal J, Walker NA, Waugh SM (2009) Effectiveness of fish waste management strategies in reducing seabird attendance at a trawl vessel. Fish Res 95:210–219
ACAP (Agreement on the Conservation of Albatrosses and Petrels) (2011) Report of the Sixth Meeting of the Advisory Committee. Annex 16: Trawl mitigation summary advice. Summary advice statement for reducing impact of pelagic and demersal trawl gear on seabirds. http://www.acap.aq/index.php/en/advisory-committee/cat_view/128-english/15-advisory-committee/330-ac6/366-ac6-report. Accessed 25 Feb 2013
Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J (2004) Marine fronts at the continental shelves of austral South America physical and ecological processes. J Mar Syst 44:83–105
Arata J, Xavier JC (2003) The diet of black-browed albatrosses at the Diego Ramirez Islands, Chile. Polar Biol 26:638–647
Arnold JM, Brault S, Croxall JP (2006) Albatross populations in peril? A population trajectory for black-browed albatrosses at South Georgia. Ecol Appl 16:419–432
Aubone A, Bezzi SI, Cañete G, Castrucci R, Dato C, Irusta G, Madirolas A, Pérez M, Renzi M, Santos B, Simonazzi M, Villarino MF et al (2004) Evaluacion y sugerencias de manejo del recurso merluza (Merluccius hubbsi). La situacion hasta 1999. In Sanchez RP, Bezzi SI (eds) El mar Argentino y sus recursos pesqueros Tomo4 Los peces marinos de interes pesquero. Caracterizacion biologica y evaluacion del estado de explotacion. INIDEP, Mar del Plata, pp 207–235
Baker GB, Double MC, Gales R, Tuck GN, Abbott CL, Ryan PG, Petersen SL, Robertson CJR, Alderman R (2007) A global assessment of the impact of fisheries-related mortality on Shy and White-capped albatrosses: conservation implications. Biol Conserv 137:319–333
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164
Bertolotti MI, Hernández DR, Pagani AN, Castañeda MF (2001) Estratificación y estimación de los rendimientos de la flota de buques procesadores congeladores arrastreros. In: Bertolotti MI, Verazay GA, Akselman R (eds) El mar argentino y sus recursos pesqueros. Evolución de la flota pesquera, artes de pesca y dispositivos selectivos. INIDEP, Mar del Plata, Argentina, pp 71–88
Bicknell AWJ, Oro D, Camphuysen KCJ, Votier SC (2013) Potential consequences of discard reform for seabirds communities. J Appl Ecol. doi:10.1111/1365-2664.12072
BirdLife International (2012). Save the albatross. http://www.birdlife.org/seabirds/save-the-albatross.html. Accessed 12 Dec 2012
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bond A, Diamond A (2011) Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecol Appl 21:1017–1023
Bugoni L, Mancini PL, Monteiros DS, Nascimento L, Neves T (2008a) Seabird bycatch in the Brazilian pelagic longline fishery and a review of capture rates in the southwestern Atlantic Ocean. Endang Species Res 5:137–147
Bugoni L, Mcgill RAR, Furness RW (2008b) Effects of preservation methods on stable isotope signatures in bird tissues. Rapid Commun Mass Spectrom 22:2457–2462
Bugoni L, McGill RAR, Furness RW (2010) The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J Exp Mar Biol Ecol 391:190–200
Bugoni L, Griffiths K, Furness RW (2011) Sex-biased incidental mortality of albatrosses and petrels in longline fisheries: differential distributions at sea or differential access to baits mediated by sexual size dimorphism? J Ornithol 152:261–268
Catry P, Lemos RT, Brickle P, Phillips RA, Matias R, Granadeiro JP (2013) Predicting the distribution of a threatened albatross: the importance of competition, fisheries and annual variability. Prog Oceanogr 110:1–10
Caut S, Angulo E, Courchamp F (2008) Caution on isotopic model use for analyses of consumer diet. Can J Zool 86:438–445
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Ceia FR, Phillips RA, Ramos JA, Cherel Y, Vieira RP, Richard P, Xavier JC (2012) Short- and long-term consistency in the foraging niche of wandering albatrosses. Mar Biol 159:1581–1591
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Cherel Y, Weimerskirch H, Trouvé C (2000) Food and feeding ecology of the neritic-slope forager black-browed albatross and its relationships with commercial fisheries in Kerguelen waters. Mar Ecol Prog Ser 207:183–199
Cherel Y, Weimerskirch H, Trouvé C (2002) Dietary evidence for spatial foraging segregation in sympatric albatrosses (Diomedea spp.) rearing chicks at Iles Nuageuses, Kerguelen. Mar Biol 141:1117–1129. doi:10.1007/s00227-002-0907-5
Cherel Y, Hobson KA, Hassani S (2005) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Cherel Y, Hobson KA, Guinet C (2007) Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. J Anim Ecol 76:826–836
Cherel Y, Ducatez S, Fontaine C, Richard P, Guinet C (2008) Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands. Mar Ecol Prog Ser 370:239–247
Cherel Y, Jaeger A, Alderman R, Jaquemet S, Richard P, Wanless RM, Phillips RA, Thompson DR (2012) A comprehensive isotopic investigation of habitat preferences in nonbreeding albatrosses from the Southern Ocean. Ecography 36:277–286. doi:10.1111/j.1600-0587.2012.07466.x
Colabuono FI, Vooren CM (2007) Diet of Black-browed Thalassarche melanophrys and Atlantic Yellow-nosed T. clororhynchos albatrosses and White-chinned Procellaria aequinoctialis and spectacled P. conspicilliata peterels off southern Brazil. Mar Ornithol 35:9–20
Consejo Federal Pesquero (2010) Resolution CFP 15-10 (Annex):Plan de Acción Nacional para reducir la interacción de aves con pesquerías en la República Argentina. www.minagri.gob.ar/site/pesca/pescamaritima/01=plan%20 de%20accion%20nacional/03-PAN-AVES/index.php. Accessed 28 Feb 2013
Copello S, Seco Pon JP, Favero M (2013) Use of marine space by Black-browed albatrosses during the non-breeding season in the Southwest Atlantic Ocean. Estuar Coast Shelf Sci 123:34–38
Crofts S (2006) Seabird interactions in the Falkland Islands Loligo trawl Fishery 2005/2006. Falklands Conservation, Stanley
Croxall JP, Wood AG (2002) The importance of the Patagonian Shelf for top predator species breeding at South Georgia. Aquat Conserv Mar Freshw Ecosyst 12:101–118
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Int 22:1–34
Dato C, Bambill G, Cañete G, Villarino ML, Aubone A et al (2006) Estimación cuantitativa del descarte en la pesquería de merluza realizado por la flota comercial argentina. INIDEP Documento Científico No 6: 31–38, Mar del Plata
DINARA (Uruguay Dirección Nacional de Recursos Acuáticos) (2010). Boletín Estadístico Pesquero 2009. Ministry of Livestock, Agriculture and Fisheries (MGAP)—Dirección Nacional de Recursos Acuáticos, Montevideo, Uruguay, pp.52. www.dinara.gub.uy/web_dinara/images/stories/file/Boletin_Estadistico_Pesquero_2009.pdf
Eder EB, Lewis MN, Campagna C, Koch PL (2010) Evidence of demersal foraging from stable isotope analysis of juvenile elephant seals from Patagonia. Mar Mamm Sci 26:430–442. doi:10.1111/j.1748-7692.2009.00347.x
Favero M, Silva Rodriguez MP (2005) Estado actual y conservación de aves pelágicas que utilizan la Plataforma Continental Argentina. El Hornero 20:95–110
Favero M, Khatchikian C, Arias A, Silva MP, Cañete G, Mariano-Jelicich R (2003) Estimates of seabird by-catch along the Patagonian Shelf by Argentine longline fishing vessels, 1999–2001. Bird Conserv Int 13:273–281
Favero M, Blanco G, García G, Copello S, Frere E, Quintana F, Yorio P, Rabuffetti F, Cañete G, Gandini P (2011) Seabird mortality associated with ice trawlers in the Patagonian shelf: effect of discards on the occurrence of interactions with fishing gear. Anim Conserv 14:131–139
Favero M, Blanco G, Copello S, Pablo J, Pon S, Patterlini C, Mariano-Jelicich R, García G, Berón MP (2013) Seabird bycatch in the Argentinean demersal longline fishery, 2001–2010. Endang Species Res 19:187–199
Forero MG, Bortolotti GR, Hobson KA, Donazar JA, Bertelloti M et al (2004) High trophic overlap within the seabird community of Argentinean Patagonia: a multiscale approach. J Anim Ecol 74:789–801
Forero MG, González-Solís J, Hobson KA, Donázar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Mar Ecol Prog Ser 296:107–113
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing non-ratite birds. J Avian Biol 30:116–121. doi:10.2307/3677252
Furness RW (2003) Impacts of fisheries on seabird communities. Sci Mar 67:33–45
Gales R (1998) Albatross populations: status and threats. In: Robertson G, Gales R (eds) Albatross biology and conservation. Surrey Beatty and Sons, Chipping Norton, pp 20–45
Galván DE, Sweeting CJ, Polunin NVC (2012) Methodological uncertainty in resource mixing models for generalist fishes. Oecologia 169:1083–1093
Gandini P, Frere E (2006) Spatial and temporal patterns in the bycatch of seabirds in the Argentinean longline fishery. Fish Bull 104:482–485
Gómez Laich A, Favero M (2007) Spatio-temporal variation in mortality rates of White-chinned Petrels Procellaria aequinoctialis interacting with longliners in the south-west Atlantic. Bird Conserv Int 17:359–366
Gómez Laich A, Favero M, Mariano-Jelicich R, Blanco G, Arias A, Silva Rodriguez MP, Brachetta H (2006) Environmental and operational variability affecting the mortality of Black-browed Albatrosses associated with long-liners in Argentina. Fish Res 106:21–28
Góngora ME, González-Zevallos D, Pettovello A, Mendía L (2012) Caracterización de las principales pesquerías del golfo San Jorge Patagonia, Argentina. Lat Am J Aquat Res 40:1–11
González-Zevallos D, Yorio P (2006) Seabird use of discards and incidental captures at the Argentine hake trawl fishery in the. Mar Ecol Prog Ser 316:175–183
González-Zevallos D, Yorio P (2011) Consumption of discards and interactions between Black-browed Albatrosses (Thalassarche melanophrys) and Kelp Gulls (Larus dominicanus) at trawl fisheries in Golfo San Jorge, Argentina. J Ornithol 152:827–838
Granadeiro JP, Phillips R, Brickle P, Catry P (2011) Albatrosses following fishing vessels: how badly hooked are they on an easy meal? PLoS One 6:1–7
Grémillet D, Wilson RP, Wanless S, Chater T (2000) Black-browed Albatrosses, international fisheries and the Patagonian Shelf. Mar Ecol Prog Ser 195:269–280
Hedd A, Montevecchi WA (2006) Diet and trophic position of Leach’s Storm-Petrel during breeding and molt, inferred from stable isotope analysis of feathers. Mar Ecol Prog Ser 322:291–301
Hobson KA, Gibbs HL, Gloutney ML (1997) Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can J Zool 75:1720–1723. doi:10.1139/z97-799
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
IUCN (2012) IUCN red list of threatened species, version 2012.2. www.iucnredlist.org. Accessed 20 Dec 2012
Jaeger A, Blanchard P, Richard P, Cherel Y (2009) Using carbon and nitrogen isotopic values of body feathers to infer inter- and intra-individual variations of seabird feeding ecology during moult. Mar Biol 156:1233–1240
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010a) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460
Jaeger A, Maëlle C, Pierre R, Cherel Y (2010b) Use of stable isotopes to quantify seasonal changes of trophic niche and levels of population and individual specialisation in seabirds. Mar Ecol Prog Ser 401:269–277
Jiménez S, Domingo A, Brazeiro A (2009) Seabird bycatch in the Southwest Atlantic: interaction with the Uruguayan pelagic longline fishery. Polar Biol 32:187–196
Jiménez S, Abreu M, Pons M, Ortiz M, Domingo A (2010) Assessing the impact of the pelagic longline fishery on albatrosses and petrels in the southwest Atlantic. Aquat Living Resour 23:49–64
Kelly B, Dempson JB, Power M (2006) The effects of preservation on fish tissue stable isotope signatures. J Fish Biol 69:1595–1611
Moreno R, Jover L, Velando A, Munilla I, Sanpera C (2011) Influence of trophic ecology and spatial variation on the isotopic fingerprints of seabirds. Mar Ecol Prog Ser 442:229–239
Navarro J, Oro D, Bertolero A, Genovart M, Delgado A, Forero MG (2010) Age and sexual differences in the exploitation of two anthropogenic food resources for an opportunistic seabird. Mar Biol 157:2453–2459
Neves T, Olmos F (1998) Albatross mortality in fisheries off the coast of Brazil. In: Robertson G, Gales R (eds) Albatross biology and conservation. Chipping Norton, Surrey Beatty & Sons, pp 214–219
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5:e9672
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc B 271:1283–1291
Phillips RA, Silk JRD, Croxall JP, Afanasyev V (2006) Year-round distribution of white-chinned petrels from South Georgia: relationships with oceanography and fisheries. Biol Consev 129:336–347
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Pierre JP, Abraham ER, Richard Y, Cleal J, Middleton DAJ (2012) Controlling trawler waste discharge to reduce seabird mortality. Fish Res 131–133:30–38
PNA-Aves (2010) Plan Nacional de Acción para reducir la interacción de aves con pesquerías en la República Argentina. http://www.minagri.gob.ar/site/pesca/pesca_maritima/01=plan%20de%20accion%20nacional/03-PAN-AVES/index.php. Accessed Aug 2 2013
Prince PA (1980) The food and feeding biology of Grey-headed albatross Diomedea chrysostoma and Black-browed albatross D. melanophrys. Ibis 122:476–488
Prince PA, Morgan RA (1987) Diet and feeding ecology of Procellariiformes. In: Croxall JP (ed) Seabirds: feeding, ecology and role in marine ecosystems. Cambridge University Press, UK, pp 135–171
Quillfeldt P, McGill R, Furness R (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304
Quillfeldt P, McGill R, Masello J, Weiss F, Strange I, Brickle P, Furness R (2008) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
Quillfeldt P, Masello JF, McGill RA, Adams M, Furness RW (2010a) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:15
Quillfeldt P, Michalik A, Veit-Köhler G, Strange IJ, Masello JF (2010b) Inter-annual changes in diet and foraging trip lengths in a small pelagic seabird, the thin-billed prion Pachyptila belcheri. Mar Biol 157:2043–2050
Quillfeldt P, Schroff S, van Noordwijk HJ, Michalik A, Ludynia K, Masello J (2011) Flexible foraging behaviour of a sexually dimorphic seabird: large males do not always dive deep. Mar Ecol Prog Ser 428:271–287
Ramos R, González-Solís J, Croxall JP, Oro D, Ruiz X (2009) Understanding oceanic migrations with intrinsic biogeochemical markers. PLoS One 4:e6236
Raya Rey A, Polito M, Archuby D, Coria N (2012) Stable isotopes identify age- and sex-specific dietary partitioning and foraging habitat segregation in southern giant petrels breeding in Antarctica and southern Patagonia. Mar Biol 159:1317–1326
Reid K, Croxall JP, Prince PA (1996) The fish diet of black-browed albatross Diomedea melanophris and grey-headed albatross D. chrysostoma at South Georgia. Polar Biol 16:469–477
Seco Pon JP, Gandini P, Favero M (2007) Effect of longline configuration on the seabird mortality in the Argentine semi-pelagic Kingclip Genypterus blacodes fishery. Fish Res 85:101–105
Stowasser G, Atkinson A, McGill RAR, Phillips RA, Collins MA, Pond DW (2012) Food web dynamics in the Scoti Sea in summer: a stable isotope study. Deep Sea Res Part II 59–60:208–221
Sullivan BJ, Reid TA, Bugoni L (2006) Seabird mortality on factory trawlers in the Falkland Islands and beyond. Biol Conserv 131:495–504
Thompson KR (1992) Quantitative analysis of the use of discards from squid trawlers by Black-browed Albatrosses Diomedea melanophris in the vicinity of the Falkland Islands. Ibis 134:11–21
Votier SC, Furness RW, Bearhop S, Crane JE, Caldow RWG, Catry P, Ensor K, Hamer KC, Hudson AV, Kalmbach E, Klomp NI, Pfeiffer S, Phillips RA, Prieto I, Thompson DR (2004) Changes in fisheries discard rates and seabird communities. Nature 427:727–730
Votier SC, Bearhop S, Witt MJ, Inger R, Thompson D, Newton J (2010) Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J Appl Ecol 47:487–497
Votier SC, Bicknell A, Cox SL, Scales KL, Patrick SC (2013) A bird’s eye view of discard reforms: bird-borne cameras reveal seabird/fishery interactions. PLoS One 8(3):e57376. doi:10.1371/journal.pone.0057376
Watkins BP, Petersen SL, Ryan PG (2008) Interactions between seabirds and deep-water hake trawl gear: an assessment of impacts in South African waters. Anim Conserv 11:247–254
Weiss F, Furness RW, McGill RAR, Strange IJ, Masello JF, Quillfeldt P (2009) Trophic segregation of Falkland Islands seabirds: insights from stable isotope analysis. Polar Biol 32:1753–1763
Zar J (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgments
We gratefully acknowledge the captain and the crew of the fishing vessel “Ur-ertza” and Lic. Gabriel Blanco from the National Observers Program (INIDEP) for facilitating the vessel access. P. Lértora and observers of the National Observers Program (INIDEP) for helping during sampling potential prey. This study was funded by the National Agency for the Promotion of Science and Technology (Agencia Nacional de Promoción Científica y Tecnológica, PICT 2008-0590), the National Research Council (PIP CONICET 00070), and the National University of Mar del Plata (Argentina). This study was conducted under approved animal use protocols and permits provided by the Argentinean Government. We would like to thank three anonymous reviewers for their valuable input that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Garthe.
Rights and permissions
About this article
Cite this article
Mariano-Jelicich, R., Copello, S., Seco Pon, J.P. et al. Contribution of fishery discards to the diet of the Black-browed albatross (Thalassarche melanophris) during the non-breeding season: an assessment through stable isotope analysis. Mar Biol 161, 119–129 (2014). https://doi.org/10.1007/s00227-013-2320-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2320-7