Abstract
The fine link between a particular dive pattern and a specific prey item represents a challenging task in the analysis of marine predator–prey relationships. There is growing evidence that prey type affects diving seabirds’ foraging strategies, dive shapes and underwater activity costs. This study investigates whether a generalist diver, the Great Cormorant Phalacrocorax carbo, modifies the time budget allocated to prey-capture behaviour and breathing strategies (reactive vs. anticipatory) with respect to the prey type (pelagic vs. benthic). Video recordings of 91 Great Cormorants show how the ecology and behaviour of their main prey, Mullets (Mugilidae) and Flounders Platichthys flesus, affect dive/surface durations and the diving pattern. The demersal habit and the low mobility of Flounders leads to an easy access to prey with an anticipatory strategy. Moreover, the patchy distribution of this fish species increases prey-capture rates. Conversely, Mullets exploit the whole water column and are highly mobile, and this is reflected in the need of performing two sequential dives to capture a prey, both longer and likely more expensive, with a consequent switch of strategy from reactive in the searching phase to anticipatory breathing during prey-capture events. This study provides evidence that a generalist diver may switch between different foraging strategies, and it shows how each of them may be optimal under particular ecological conditions. These constraints influence the dynamics that operate within the marine food chains and have relevant implications in managing lagoon areas, including fish ponds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of predator–prey relationships in seabirds is a challenging task, since most seabird species are highly mobile predators, and their foraging sites are widespread (e.g. Kato et al. 1998; Bost et al. 2008; but see Rayner et al. 2008), so it is difficult to link a behavioural foraging pattern with a given prey item. This is particularly true for species that rely on a wide prey spectrum (generalists). While many specialist species seem to carry out defined behavioural strategies to search and capture prey (Watanuki et al. 1993; Davoren et al. 2003; Weimerskirch et al. 2005; Wilson et al. 2005), it is little known whether generalists modify their foraging behaviour with respect to the prey type (Ropert-Coudert et al. 2002). Understanding the foraging behaviour of generalist diving species is more difficult, since foraging processes occur underwater and are therefore difficult to observe.
During foraging, divers have to face a trade-off between time spent foraging at depth (bottom time) and time spent breathing at the surface (surface time). The oxygen stores are also depleted while travelling through the water column to and from the foraging patches (travel time). The way in which divers partition their time between these components of the dive cycle is subjected to both physiological and ecological constraints (Green et al. 2005). Divers are predicted to deploy behavioural strategies that best use their oxygen reserves in order to optimise dive time versus surface time (Kramer 1988; Houston and Carbone 1992). Moreover, prey distribution in the water column, affecting predator–prey relationships (Baldwin 1988), plays a fundamental role in divers’ foraging strategies and time allocation within dives (Wilson et al. 2002; Ropert-Coudert et al. 2006a; Elliott et al. 2008a, b).
Lea et al. (1996) and Jodice and Collopy (1999) have identified two diving strategies by the best-fit-regression between dive times and the relative preceding or succeeding surface durations. On the one hand, if dive times correlated better with the preceding surface pauses, the authors identified an anticipatory breathing strategy, as if divers could anticipate the dive duration and so the oxygen load relied on the preceding surface pause. This is a typical strategy of benthic foragers, assumed to foresee the foraging depth. On the other hand, if dive times correlated better with the subsequent surface durations, they identified the reactive breathing strategy, as divers could immerse with a constant air amount and so the oxygen reloading depended on dive duration. This latter strategy characterises pelagic divers that forage within the water column. Nevertheless, surface pauses in Brünnich Guillemots Uria lomvia have both an anticipatory and a reactive component (Elliott et al. 2008a), suggesting that each surface duration would likely represent a trade-off between recovering from the preceding dive and preparing for the next. So during shallow dive bouts the anticipatory component could prevail, whereas deep dive bouts are more likely to be reactive (Elliott et al. 2008a). In order to minimise the behavioural variability, Mori et al. (2002) analysed the regression between each dive time and the shortest preceding and succeeding surface time. This approach allowed removal of all surface durations not strictly linked to physiological constraints. The authors identified a logarithmic function as the best fitting between dive and surface times.
The layer of the water column where divers forage can also be inferred by the analysis of dive shapes at different depths, obtained by bird borne time-depth recorders (reviewed in Ropert-Coudert and Wilson 2005). Two main dive profiles are identified: benthic foraging is often characterised by series of U-shaped flat dives to the same depth (Ropert-Coudert et al. 2002; Takahashi et al. 2003; Lescroël and Bost 2005; Elliott et al. 2008b), whereas pelagic foraging is often categorised by V-shaped bounce dives to different depths (Tremblay and Cherel 2000; Elliott et al. 2008b). While time-depth recorders provide extensive data about feeding locations, dive depth and duration, the fine link between a particular dive pattern and a specific prey item is still poorly understood (Enstipp et al. 2007). Nonetheless, there is growing evidence that prey type affects (1) diving strategies (Elliott et al. 2008a), (2) dive shapes (Bost et al. 2007; Elliott et al. 2008b) and (3) underwater activity costs (Elliott et al. 2009).
The Great Cormorant Phalacrocorax carbo forages mostly in shallow coastal areas (Cooper 1986; Johnsgard 1993; Nelson Bryan 2005), feeding on a wide range of fish species (Baccetti et al. 1993). This generalist diver relies mainly on demersal fish species (Ashmole 1971; Cramp and Simmons 1977; Grémillet et al. 2004, 2006), captured with U-shaped benthic dives (Grémillet et al. 1999; Ropert-Coudert et al. 2006b). However, the Great Cormorant can easily switch between benthic and pelagic feeding (Grémillet et al. 1998, 1999). We investigated whether Great Cormorants modify diving behaviour and foraging strategies with respect to the prey type (pelagic vs. benthic). The following biological questions were tested: (a) is there an increase in dive and surface times according to foraging depth? (b) Is there a difference in dive and surface time budget with respect to prey characteristics and behaviour? (c) Does prey type affect the diving strategy (anticipatory vs. reactive)? To answer these questions, we studied Great Cormorants directly at feeding areas, so behavioural foraging patterns were associated with visible prey captured (including records of prey species).
Study area and methods
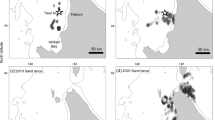
Field observations were carried out along the coastal area of Friuli Venezia Giulia, upper Adriatic Sea (NE Italy). The study area includes the Grado and Marano lagoon (between 45°41′–45°46′N, 13°05′–13°25′E), a shallow tidal wetland of about 16,000 ha (1,700 ha managed as fish ponds) and a stretch of sea among Isonzo and Timavo river mouths (between 45°43′–45°47′N, 13°32′–13°36′E) (Fig. 1). The area is characterised by large tidal mudflats (about 1 m deep), intertwined with natural and artificial channels (up to 5 m deep). The lagoon is of national importance for wintering Great Cormorants (Baccetti et al. 2002). In January 2008, a total of 1,455 birds were observed (Cosolo 2008).
Prey type and diving behaviour
We analysed diving sequences of 91 single-foraging Great Cormorants (N = 3,470 dives) on the basis of two prey types, Mullets (Mugilidae) and Flounders Platichthys flesus, handled by the birds and recognisable at the surface by a distant observer. Mullets exploit the whole water column (pelagic) and are highly mobile (Kailola et al. 1993). This fish species usually occurs in small schools (Eschmeyer et al. 1983), mainly in shallow coastal waters (Rheman et al. 2002). In contrast, Flounders are typically demersal (benthic) and low-mobile species (Muus and Dahlström 1974). Mullets and Flounders make up the predominant part of the prey biomass ingested by Great Cormorants in the coastal area of Friuli Venezia Giulia (Privileggi 2003; Cosolo 2008). Moreover, in this area Mullets and Flounders are common fish species and are characterised by a high socio-economic value: the landings of commercial capture fisheries in winters 2005/2006 and 2006/2007 (period September–April) ranged between 19,590 and 25,799 kg for Mullets and between 12,087 and 13,027 kg for Flounders (Marchesan 2008). Focusing on the Grado and Marano lagoon area, the range of Mullets’ commercial captures remained higher (11,022–12,692 kg vs. 7,769–7,920 kg of Flounders). Since within tidal mudflats (about 1 m deep) both fish species are caught by traditional and unselective bottom gillnets, the commercial captures data approximate the relative abundance of these fish species.
Video recordings were carried out from October 2004 to April 2007 and were performed from the coastline and by boat with a digital camera with telephoto lens (max. 50×). Foraging birds were categorised on the basis of the two foraging depths: the higher in channels 4–5 m deep (N = 43) and the lower in tidal mudflats about 1 m deep (N = 48). Depths of all foraging sites were measured, taking into account the tidal level. The probability of recording the same individual was negligible, given that the video recordings were carried out in different years, coastal areas and winter periods. At both depths, Great Cormorants captured 207 prey items. We never observed the capture of different prey species within a single diving sequence, so we discriminated Mullet (N = 29; N prey = 37) from Flounder sequences (N = 62; N prey = 170) (Table 1). In this regard, it is often assumed that cormorants bring most prey to the surface to swallow. In some cases, this behaviour may include small fish, often taken at high rate (Carss and The Diet Assessment and Food Intake Working Group 1997). However, we considered the possibility that birds swallowed prey items underwater. Strod et al. (2003) addressed this issue during captive trials of 5 Great Cormorants. They considered this behaviour as infrequent and occurring when fish were small in size. Enstipp et al. (2007), during captive trials of 9 Double-crested Cormorants Phalacrocorax auritus, observed that underwater swallowing occurred just in 9 cases out of 257 prey captures and mostly in the two largest birds. Moreover, Carss and The Diet Assessment and Food Intake Working Group (1997) highlighted that birds surfacing without a visible fish but which shook their heads, gaped, exhibited throat fluttering or took a drink, implied that they had just swallowed fish underwater. In our study, we observed such behaviour only in 12 out of 3,470 dives. On the basis of the results from Strod et al. (2003) and Enstipp et al. (2007) and according to our field observations, we maintained that the effects of this behaviour in our analysis were negligible, acting as a ‘background noise’.
For each diving cormorant, we collected the following parameters: total foraging time (dive sequence duration), number of dives, dive times and the relative preceding and succeeding surface durations. To assess the differences between diving parameters at the two depths, we used the Mann–Whitney U Test. The significance threshold was set at α < 0.05.
Prey-capture behaviour analysis
We analysed the following parameters characterising Flounder and Mullet sequences: unsuccessful dive times (‘ud’), unsuccessful surface durations (‘sud’), dive times immediately preceding the predatory dive (‘dpP’), surface durations preceding the predatory dive (‘spP’), predatory dive times (‘Pd’), surface durations succeeding the predatory dive (‘ssP’) (Fig. 2). To assess the differences between diving parameters within a predatory sequence, we used the Wilcoxon Test. Otherwise, to assess the differences between diving parameters with respect to the prey type and the relative foraging depth, we used the Mann–Whitney U Test. In sequences where we observed more than one prey item captured, we averaged the values of diving parameters. The significance threshold was set at α < 0.05.
Diving strategies with respect to prey type
In order to identify Great Cormorant’s diving strategies with respect to the prey type (Flounders or Mullets), we carried out a multiple regression analysis (stepwise method) (Sokal and Rohlf 1969) between each dive time and the shortest preceding and succeeding surface durations, considering all data (Mori et al. 2002; Sponza et al. 2010). The shortest surface pause needed to recover from a given dive time (in 1 s steps) allows the elimination of all surface durations not strictly dependent on physiological limits. We processed separately predatory dive sequences at the two study depths. The best fitting relationship and, consequently, the prevalent diving component (anticipatory or reactive) were identified by the higher value of the parameter Beta. In the regression analysis, we discriminated the different phases of the predatory sequence: (a) all dive times (not linked to a predatory event) (‘ud’), (b) dive times preceding the predatory dive (‘dpP’) and (c) predatory dives (‘Pd’) with their own shortest preceding and succeeding surface durations. In the latter case, the shortest succeeding surface durations allow to minimise prey handling time.
The regression curves were set passing through the origin, and models were tested with ANOVA. All surface data were ln-transformed, because the function between dive and surface times tended in all cases to a logarithmic. For each regression, we verified the conditions of validity such as the normal distribution of residuals, the homogeneity of the variance and the homoscedasticity. The significance threshold was set at α < 0.05.
Results
Dive and surface times
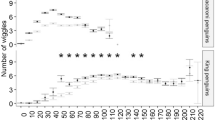
Mean dive and surface times (±SD) at 4–5 m depth were significantly higher than at 1 m depth (Table 2). The effect of depth was also revealed by the distribution of dive times, set in 5 s classes (Fig. 3). At shallower depth, Great Cormorants performed shorter dives. The modes of the two distributions settled at well-separated dive classes (11–15 s for 1 m depth and 26–30 s for 4–5 m depth).
Prey-capture behaviour
Flounders’ capture rate (0.19 prey min−1, N = 62) was significantly higher than Mullets’ capture rate (0.07 prey min−1, N = 29) (Mann–Whitney U Test: N Mullets = 29, N Flounders = 62, z = −5.37, α < 0.0001) (Table 1). This trend remained at 4–5 m depth (0.12 Flounders min−1, N = 18 vs. 0.06 Mullets min−1, N = 25) (Mann–Whitney U Test: N Mullets = 25, N Flounders = 18, z = −2.3, α < 0.05) (Table 1). At 1 m depth, Mullet sequences (N = 4) were too small a sample to be compared with Flounder sequences (N = 44). Flounders’ capture rate at 1 m depth (0.22 prey min−1) was significantly higher than at 4–5 m depth (Mann–Whitney U Test: N 4−5m = 18, N 1m = 44, z = −3.3, α < 0.001).
In order to discriminate Flounder from Mullet sequences, we compared the relative diving parameters (Figs. 2, 4; Tables 3, 4). At 4–5 m depth, unsuccessful dives (‘ud’) did not differ between Mullet and Flounder sequences (Table 4). Unlike Flounder sequences, dives preceding the predatory dive (‘dpP’) of a Mullet were significantly longer than unsuccessful dives (Table 3). Both in Flounder and Mullet sequences, predatory dives (‘Pd’) were significantly shorter than unsuccessful dives and dives preceding the predatory dive (Table 3). Overall, Flounders were captured during shorter dives than Mullets (Table 4). In Mullet sequences, surface times preceding the predatory dive (‘spP’) did not differ from surface times following an unsuccessful dive (‘sud’). In both prey sequences, surface times succeeding the predatory dive (‘ssP’) were significantly longer than any other surface duration (Table 3).
Average (±SD) diving parameters characterising at 4–5 m depth the prey-capture behaviour with respect to Mullets (N = 25; unsuccessful dives ‘ud’ = 27.71 ± 4.31 s; unsuccessful surface times ‘sud’ = 10.14 ± 2.59 s; dives preceding the predatory dive ‘dpP’ = 32.12 ± 4.98 s; surface times preceding the predatory dive ‘spP’ = 9.80 ± 2.83 s; predatory dives ‘Pd’ = 22.26 ± 5.02 s; surface times succeeding the predatory dive ‘ssP’ = 17.82 ± 7.81 s) and Flounders (N = 18; unsuccessful dives ‘ud’ = 27.40 ± 4.66 s; unsuccessful surface times ‘sud’ = 8.58 ± 2.08 s; dives preceding the predatory dive ‘dpP’ = 27.49 ± 4.88 s; surface times preceding the predatory dive ‘spP’ = 8.93 ± 6.48 s; predatory dives ‘Pd’ = 16.45 ± 9.14 s; surface times succeeding the predatory dive ‘ssP’ = 23.93 ± 10.45 s)
At 1 m depth, the small sample of Mullet sequences (N = 4) did not allow us to highlight the effect of depth. In Flounder sequences (N = 44), both dive and surface times were significantly shorter with respect to 4–5 m depth (Table 4). As seen at 4–5 depth, predatory dives were shorter than any other dive time, and unsuccessful dives did not differ from dives preceding the predatory dive (Table 3).
Foraging strategies to capture Mullets and Flounders
Given that dives preceding Mullet captures had the largest durations (Fig. 4; Table 3), we investigated how this variation could be linked with the diving strategies. So we analysed the relationship between dive times and the shortest preceding and succeeding surface durations (Mori et al. 2002) in the different phases of Mullet and Flounder predatory sequences (Tables 5, 6). In Mullet sequences, we found a strong relationship between all dive times with both shortest preceding and succeeding surface durations (Table 5). The higher values of Beta for the latter (0.597 vs. 0.398) predicted a slight prevalence of the reactive breathing component. Focusing on dives preceding the predatory dive, regression analysis provided only a relationship with the succeeding surface duration (reactive breathing) (Table 5), whereas for predatory dives only the anticipatory breathing component (Table 5).
As regards Flounder sequences at 4–5 m depth, all dive times had a strong relationship with both shortest preceding and succeeding surface durations (Table 6). The higher value of Beta (0.547 vs. 0.452) however predicted a slight prevalence of the anticipatory breathing component. Preceding surface pauses become predictive of dive durations (anticipatory) analysing both dives preceding the predatory dive and the predatory dives (Table 6).
The surface duration succeeding the predatory dive was the parameter with the greatest variability both in Mullet and in Flounder sequences (Fig. 4). Given this variability, the use of the shortest succeeding surface time allowed us to minimise the effect of prey ingestion in the regressions with the predatory dives. This is particularly true in Flounder sequences, given the roughly oval shape of this flatfish. In fact, Enstipp et al. (2007) highlight that handling times at the surface (from surfacing to prey ingestion) for pelagic prey (as Mullets) are on average particularly short.
At 1 m depth, we could only investigate Flounder sequences (Table 6). Regression analysis with all dive times provided a relationship with the succeeding surface durations only. The reactive component prevailed also in the regression analysis with dives preceding the predatory dive (Table 6), whereas for predatory dives we obtained a prevalence of the anticipatory component (Table 6).
The capture of Flounders
Given Flounders’ benthic habits and higher prey-capture rate at 1 m depth, we investigated whether this value was affected by a particular spatial distribution of this species along the bottom. In fact, Modin and Pihl (1996) and Zwanette (2001) showed that juvenile Flounders tend to aggregate in groups. We analysed the prey-capture latency, which is the period between the arrival of a Great Cormorant at the feeding site and the first capture, and the mean time (±SE) between a capture and the successive one. Both parameters decreased significantly at 1 m depth (respectively ANOVA: R 2 = 0.62, F 1,6 = 9.63, α < 0.05 and R 2 = 0.52, F 1,6 = 6.58, α < 0.05) with the increase in the number of Flounders captured. It was then checked whether time spent at the foraging site correlated with the number of prey captured. For this analysis, we considered only the sequences where we had both the arrival and the departure of a Great Cormorant from the foraging site (N = 5). Although the data available were few, the number of Flounders captured increased with time spent at the foraging patch. The best fitting plot was an exponential function (ANOVA: R 2 = 0.97, F 1,3 = 115.81, α < 0.01) (Fig. 5). If we removed the single data point where a Great Cormorant captured 12 Flounders, the best fitting remained an exponential function (R 2 = 0.95).
Discussion
Foraging times
Most studies on Great Cormorant’s foraging behaviour highlight the species’ preference for shallow waters (Cooper 1986; Wilson and Wilson 1988; Lea et al. 1996; Grémillet et al. 1998, 1999, 2006; Kato et al. 2006; Ropert-Coudert et al. 2006b). Given the positive correlation between water depth and dive duration (Kramer 1988; Houston and Carbone 1992), foraging in shallow waters is reflected in short dives and consequent surface times. Mean (±SD) dive duration (19.5 ± 9.2 s) of the 91 single-foraging Great Cormorants observed is similar to 21.1 ± 8.9 s durations calculated by Lea et al. (1996) at comparable depths (ranging between 1 and 5 m). At 4–5 m depth, dive duration (28.2 ± 4.5 s) is equivalent to 28 s recorded by Grémillet et al. (1998), while it is higher than 18.9 ± 3.7 s and 22.8 ± 5.8 s, respectively, calculated by Kato et al. (2006) and Ropert-Coudert et al. (2006b) at comparable depths. Mean (±SD) surface time (6.9 ± 6.4 s) at both depths was similar to 7.1 s recorded by Lea et al. (1996).
The increase in dive durations according with water depth is typical in benthic divers (Wilson and Wilson 1988; Croxall et al. 1991; Jodice and Collopy 1999). In our data, we provide support for such an increase for cormorants foraging on Flounders (Table 4).
Pelagic versus benthic feeding
The Great Cormorant is mainly a benthic feeder (Ashmole 1971; Cramp and Simmons 1977; Grémillet et al. 2004, 2006). The species forages mostly on benthic fish (60–75%), even in situations of higher pelagic prey abundance (Grémillet et al. 2004). Despite fisheries data (see “Study area and methods”), we record a higher capture rate for Flounders with respect to Mullets at 4–5 m depth (Table 1). Consistent with Grémillet et al. (2004), this difference is linked with the different ecological characteristics of these prey species beyond their abundance. Flounders burrow in the substrate and rely on mimicry as anti-predator behaviour (Muus and Dahlström 1974). Given their very limited mobility, they are likely easy to capture (Sponza et al. 2010). In contrast, Mullets exploit the whole water column and are highly mobile (Kailola et al. 1993). Halsey et al. (2007) emphasise a clear difference in the energetic costs of diving for benthic (sedentary) and pelagic (motile) prey. Across dives of all durations in Double-crested Cormorants Phalacrocorax auritus, the foraging phase was more expensive (higher oxygen consumption) when birds hunted motile prey (Halsey et al. 2007), due to the increased costs of a higher swimming speed and underwater activity (Schmid et al. 1995). Moreover, pelagic fish species usually form aggregations that are ephemeral and unpredictable, while benthic prey patches follow predictable bathymetric gradients or bottom sediment characteristics (Coyle et al. 1992; Montevecchi 1993; Davoren 2000; Litzow et al. 2000; Carscadden and Wilhjalmsson 2002; Watanuki et al. 2004).
Prey-capture behaviour and diving strategies
At 4–5 m depth, unsuccessful dive times for Flounders and Mullets do not differ (Fig. 4; Table 4), indicating that Great Cormorants prefer a definite dive duration in order to maximise time spent underwater searching for prey. In Mullet sequences, this occurs until the dive preceding the predatory dive. These latter dives are indeed the longest (Fig. 4; Table 3). In contrast, in Flounder sequences there are no differences between dive preceding the predatory dive and unsuccessful dive durations. Thereafter, dives to capture both Mullets and Flounders are significantly shorter than any other dive (Fig. 4; Table 3). Flounders, which sit still and hide in the substrate, are caught during random searching behaviour of steady search duration. On the contrary, why dives to capture Mullets are shorter is more difficult to explain, as during such dives Great Cormorants have to search, pursue and capture a Mullet. Pelagic prey like Mullets usually form schools that are unpredictable and undergo an encounter-dilution effect (Pitcher and Parrish 1993). Enstipp et al. (2007) found, during trials on captive Double-crested Cormorants, that underwater search time increased significantly at low prey densities. According to Walton et al. (1998), during searching dives, relative time underwater is maximised, and when a pelagic prey is located dive duration can be extended, due to the time required to pursue and capture the prey. Enstipp et al. (2007) recorded an average pursuit duration of 10.2 ± 2.9 s when a pelagic fish was part of a school and 5.2 ± 1.4 s when cormorants attacked a solitary pelagic fish. On average, just 58.3% of pursuits were successful. A similar value (63.2%) was recorded for Great Cormorants (Grémillet et al. 2006). In both situations, the foraging success increased only when cormorants initiated more than one prey pursuit per dive. Therefore, we maintain that the decrease in predatory dive times to capture Mullets depends on the longer duration of dives preceding the predatory dive. During these dives, Great Cormorants likely search, detect and, eventually, pursuit the prey. Enstipp et al. (2007) stressed how, in response to a predator attack, schools moved towards the bottom of the experimental tank, forcing cormorants to dive deeper and typically remained there until trials were terminated. This behaviour might represent a strategy that relies on camouflage rather than actively swimming away. While pursuits are initiated throughout the water column, 60% of captures occurred near the bottom of the tank, where light levels were lowest (Enstipp et al. 2007). We suggest that such prey behaviour that facilitates Great Cormorants as the bottom, compared to the water column, is a predictable landmark. In this regard, all dive times in Mullet sequences correlated with both preceding and succeeding surface pauses. The prevalence of the latter component (reactive breathing) (Table 5) assumes that divers are not aware beforehand of the position of the prey within the water column (Lea et al. 1996; Jodice and Collopy 1999). Such strategy is kept in dives preceding the predatory dive; hence cormorants are still working within the water column (Table 5). During predatory dives, we emphasise a switch in the diving strategy. Indeed, the regression analysis fits the preceding surface component only and hence an anticipatory breathing strategy, which characterises divers that can predict the foraging depth and, consequently, travel time duration. Therefore, divers adjust air volume for that given dive depth. Such variation of strategy corroborates the hypothesis that Great Cormorants increase the predictability of predatory dives just in dives preceding the capture. Consistent with Enstipp et al. (2007), the interaction with a Great Cormorant during these dives forces Mullets to the bottom, which is quickly reached in the subsequent dive. For this reason, predatory dives are the shortest (Fig. 4; Table 3).
The difficulty in coping with Mullets is reflected in performing two sequential dives to capture the prey, much longer and likely more expensive (i.e. higher oxygen consumption) (Schmid et al. 1995; Wilson et al. 2006; Halsey et al. 2007) with respect to the dives to capture Flounders. Surface durations preceding the predatory dive are indeed significantly longer in Mullet than in Flounder sequences (Table 4). Given the short distance between the surface and the bottom, the switch of strategy avoids Mullets’ escape response. These fish species can easily reach 4.3 m s−1 swim speed (Rulifson 1977; Sambilay 1990), much higher than underwater mean speed (1.5 m s−1) by Great Cormorants (Schmid et al. 1995; Ribak et al. 2004; Kato et al. 2006; Ropert-Coudert et al. 2006b). This difference in swim speed explains why the angle of attack of Double-crested Cormorants during prey capture is about perpendicular to fish orientation (Enstipp et al. 2007).
At 1 m depth, Great Cormorants cannot ‘anticipate’ Mullets’ movements. In fact, the lack of a proper water column forces Great Cormorants to pursue the prey within an horizontal shallow layer. Therefore the shallowest depths should not be suitable for catching Mullets. The limited number of Mullets captured (5), compared with the number of Flounders caught (141, Table 1), would support this hypothesis.
Great Cormorants foraging on Flounders at 4–5 m show a different foraging pattern. The regression analysis substantiates the anticipatory breathing strategy both considering all dives (see Sponza et al. 2010), dives preceding the predatory dive and predatory dives (Table 6). This strategy characterises benthic divers able to predict the foraging depth, and the occurrence of such strategy is supported by Flounders’ demersal habits. We thus validate the methodology proposed by Mori et al. (2002) also in waters 4–5 m deep. Great Cormorants foraging on Flounders perform highly predictable dives: they have just to reach the bottom and start to search. For a such stationary, cryptic prey cormorants randomly detect prey items only at short distance or even through direct contact with the beak (Voslamber et al. 1995; Grémillet et al. 2005; Ropert-Coudert et al. 2006b) and may use a prey-flushing strategy that forces prey to make an escape response (White et al. 2007). The ability of Great Cormorants to perform brief accelerations and strike rapidly with their neck might be a way to capture prey without extended pursuits (Ropert-Coudert et al. 2006b).
In Flounder sequences at 1 m depth, we record the occurrence of the reactive component within all dives, and the prevalence of such component during dives preceding the predatory dive (Table 6). Furthermore, these results support Mori et al. (2002), as the model records just the bottom time duration and its intrinsic unpredictability, hence the prevalence of the reactive component. In fact, the definition of anticipatory breathing strategy implies that the travel time (not present at 1 m depth) is a significant and constant component of the dive cycle, which conceals the variability of searching and pursuing durations on the bottom (bottom time).
Finally, the increase in capture rate (0.22 prey min−1) at 1 m depth with respect to 4–5 m (0.12 prey min−1) corroborates a patchy distribution of Flounders at the lowest depth (Modin and Pihl 1996; Zwanette 2001). Although the data were limited, both prey-capture latency and time between a capture and the successive one decreased with the number of Flounders captured. Moreover, the longer duration of a dive bout was reflected in a higher number of prey items captured, according to an exponential function (Fig. 5). This curve is in agreement with the Marginal Value Theorem (Charnov 1976), as a positive correlation between patch quality and patch residence times (Mori 1998; Mori et al. 2002).
In conclusion, we suggest that Great Cormorants adjust foraging pattern with respect to prey type (pelagic vs. benthic). Flounders, as low-mobile demersal species, are easy to capture with an anticipatory breathing strategy. Moreover, they tend to a patchy distribution, which allows Great Cormorants to increase prey-capture rate. Conversely Mullets, as high-mobile species, are more difficult to capture because escape movements can be performed. As a result, the searching phase is described by a reactive breathing strategy, whereas during prey-capture events Great Cormorant must switch to an anticipatory strategy.
Implications
We suggest that this study has important implications in Great Cormorant management, which represents a challenging issue in European wetlands managed as fish ponds (Carss 2003). In the North Adriatic Sea, the commercial fish ponds (extensive aquaculture) are characterised by large shallow depth basins (about 1 m), delimited by a deeper artificial canal. All reared fish species (Sparus aurata, Dicentrarchus labrax, Liza ramada, Chelon labrosus, Mugil sp.) are pelagic and highly mobile. Our results explain the low occurrence of foraging cormorants in these environments for most of the winter (Cosolo et al. 2009). High numbers of Great Cormorants, hence an impact on the fish production, were observed only within the deeper artificial canal and typically in November and December, coinciding with the times when fishery managers concentrated fish stocks in the canal for harvesting.
References
Ashmole NP (1971) Seabird ecology and marine environment. In: Fraser DS, King JR (eds) Avian biology, vol I. Academic, New York, pp 221–271
Baccetti N, Boldreghini P, Santolini R (1993) Le Grand Cormoran en Italie: effectif, régime alimentaire et conflits avec la pisciculture. Bull Mens Off Nat Chasse 178:22–25
Baccetti N, Dall’Antonia P, Magagnoli P, Melega L, Serra L, Soldatini C, Zenatello M (2002) Risultati dei censimenti degli uccelli acquatici svernanti in Italia: distribuzione, stima e trend delle popolazioni nel 1991–2000. Biol Cons Fauna 111:1–240
Baldwin J (1988) Predicting the swimming and diving behaviour of penguins from muscle biochemistry. Hydrobiologia 34:255–261
Bost CA, Handrich Y, Butler PJ, Fahlman A, Halsey LG, Woakes AJ, Ropert-Coudert Y (2007) Changes in dive profiles as an indicator of feeding success in king and Adélie penguins. Deep-Sea Res Pt II 54:248–255
Bost CA, Jaeger A, Huin W, Koubbi P, Halsey LG, Hanuise N, Handrich Y (2008) Monitoring prey availability via data loggers deployed on seabirds: advances and present limitations. In: Tsukamoto K, Kawamura T, Takeuchi T, Beard TD, Kaiser MJ (eds) Fisheries for global welfare and environment, 5th World Fisheries Congress 2008: pp 121–137
Carscadden JE, Wilhjalmsson H (2002) Capelin: what are they good for? ICES J Mar Sci 59:863–869
Carss DN (ed) (2003) Reducing the conflict between Cormorants and fisheries on a pan-European scale. REDCAFE Final Report. Report of a Concerted Action funded by the European Union. Study contract no. Q5CA-2000-31387
Carss DN, The Diet Assessment and Food Intake Working Group (1997) Techniques for assessing Cormorant diet and food intake: towards a consensus view. Suppl Ric Biol Selv 26:197–230
Charnov EL (1976) Optimal foraging and the marginal value theorem. Theor Pop Biol 9:129–136
Cooper J (1986) Diving patterns of Cormorants (Phalacrocoracidae). Ibis 128:562–570
Cosolo M (2008) Interazioni tra avifauna ittiofaga ed attività produttive nella laguna di Grado e Marano. PhD thesis. University of Trieste, 114 pp
Cosolo M, Utmar P, Roppa F, Sponza S (2009) Interactions between fish-eating birds and fish ponds in the Grado and Marano lagoon: the case of the Great cormorant (Phalacrocorax carbo). Acrocephalus 29(140):17–23
Coyle KO, Hunt GL Jr, Decker MB, Weingartner TJ (1992) Murre foraging, epibenthic sound scattering and tidal advection over a shoal near St. George Island, Bering Sea. Mar Ecol Prog Ser 83:1–14
Cramp S, Simmons KLS (1977) The birds of the western paleartic: ostrich to ducks. Oxford University Press, Oxford
Croxall JP, Naito Y, Kato A, Rothery P, Briggs DR (1991) Diving patterns and performance in the Antarctic blue-eyed shag Phalacrocorax atriceps. J Zool Lond 225:177–199
Davoren GK (2000) Variability in foraging in response to changing prey distribution in rhinoceros auklets. Mar Ecol Prog Ser 198:283–292
Davoren GK, Montevecchi WA, Anderson JT (2003) Search strategies of a pursuit-diving marine bird and the persistence of prey patches. Ecol Monogr 73:463–481
Elliott KH, Davoren GK, Gaston AJ (2008a) Time allocation by a deep-diving bird reflects prey type and energy gain. Anim Behav 75:1301–1310
Elliott KH, Woo K, Gaston AJ, Benvenuti S, Dall’Antonia L, Davoren GK (2008b) Seabird foraging behaviour indicates prey type. Mar Ecol Prog Ser 354:289–303
Elliott KH, Woo K, Benvenuti S (2009) Do activity costs determine foraging tactics for an arctic seabird? Mar Biol 156:1809–1816
Enstipp MR, Grémillet D, Jones DR (2007) Investigating the functional link between prey abundance and seabird predatory performance. Mar Ecol Prog Ser 331:267–279
Eschmeyer WN, Herald ES, Hammann H (1983) A field guide to Pacific coast fishes of North America. Houghton Mifflin Company, Boston, p 336
Green JA, Halsey LG, Butler PJ (2005) To what extent is the foraging behaviour of aquatic birds constrained by their physiology? Physiol Biochem Zool 78:766–781
Grémillet D, Argentin G, Schulte B, Culik BM (1998) Flexible foraging techniques in breeding Cormorants Phalacrocorax carbo and Shags Phalacrocorax aristotelis: benthic or pelagic feeding? Ibis 140:113–119
Grémillet D, Wilson RP, Storch S, Gary Y (1999) Three dimensional space utilization by a marine predator. Mar Ecol Prog Ser 183:263–273
Grémillet D, Kuntz G, Delbart F, Mellet M, Kato A, Robin JP, Chaillon PE, Gendner JP, Lorentsen SH, Le Maho Y (2004) Linking the foraging performance of a marine top predator to local prey abundance. Funct Ecol 18:793–801
Grémillet D, Kuntz G, Gilbert C, Woakes AJ, Gilbert C, Butler PJ, Le Maho Y (2005) Cormorants dive through the Polar night. Biol Lett 1:469–471
Grémillet D, Enstipp MR, Boudiffa M, Liu H (2006) Do Cormorants injure fish without eating them? An underwater video study. Mar Biol 148:1081–1087
Halsey LG, White CR, Enstipp MR, Jones DR, Martin GR, Butler PJ (2007) When cormorants go fishing: the differing costs of hunting for sessile and motile prey. Biol Lett 3:574–576
Houston AI, Carbone C (1992) The optimal allocation of time during the dive cycle. Behav Ecol 3:233–262
Jodice PGR, Collopy MW (1999) Diving and foraging patterns of Marbled murrelets (Brachyramphus marmoratus): testing predictions from optimal breathing models. Can J Zool 77:1409–1418
Johnsgard PA (1993) Cormorants, darters and pelicans of the world. Smithsonian Institution Press, Washington
Kailola PJ, Williams MJ, Stewart PC, Reichelt RE, McNee A, Grieve C (1993) Australian fisheries resources. Bureau of Resource Sciences, Canberra 422 pp
Kato A, Watanuki Y, Naito Y (1998) Benthic and pelagic foraging of two Japanese cormorants, determined by simultaneous recording of location and diving activity. J Yamashina Inst Ornithol 30:101–108
Kato A, Ropert-Coudert Y, Grémillet D, Cannell B (2006) Locomotion and foraging strategy in foot-propelled and wing-propelled shallow-diving seabirds. Mar Ecol Prog Ser 308:293–301
Kramer DL (1988) Behavioural ecology of air breathing by aquatic mammals. Can J Zool 66:89–94
Lea SEG, Daley C, Boddington PJC, Morison V (1996) Diving patterns in Shags and Cormorants (Phalacrocoracidae): test of an optimal breathing model. Ibis 138:391–398
Lescroël A, Bost CA (2005) Foraging under contrasting oceanographic conditions: the gentoo penguin at Kerguelen Archipelago. Mar Ecol Prog Ser 302:245–261
Litzow MA, Piatt JF, Abookire AA, Prichard AK, Robards MD (2000) Monitoring temporal and spatial variability in sand eel (Ammodytes hexapterus) abundance with pigeon guillemot (Cepphus columba) diets. ICES J Mar Sci 57:976–986
Marchesan M (2008) Analisi quali-quantitativa e distribuzione del pescato in Laguna di Grado e Marano. In: Benassi MC, Facchin G, Fabro C, Florit F, Ferrero E, Iacumin C, Serra L, Sponza S, Susmel P, Zanetti M (eds) Progetto ANSER. Ruolo ecologico delle zone umide per la sosta e lo svernamento degli uccelli acquatici nell’Adriatico settentrionale: linee guida per la conservazione e la gestione del patrimonio marino costiero. Relazione progettuale finale. Regione Autonoma Friuli Venezia Giulia—Direzione centrale risorse agricole, naturali e forestali, Udine, pp 184–190
Modin J, Pihl L (1996) Small-scale distribution of juvenile plaice and flounder in relation to predatory shrimp in a shallow Swedish bay. J Fish Biol 49(6):1070–1085
Montevecchi WA (1993) Birds as indicators of change in marine prey stocks. In: Furness RW, Greenwood JJD (eds) Birds as monitors of environmental change. Chapman and Hall, London, pp 217–265
Mori Y (1998) The optimal patch use in divers: optimal time budget and the number of dive cycles during bout. J Theor Biol 190:187–199
Mori Y, Takahashi A, Mehlum F, Watanuki Y (2002) An application of optimal diving models to diving behaviour of Brünnich’s guillemots. Anim Behav 64:739–745
Muus BJ, Dahlström P (1974) Collins guide to the sea fishes of Britain and North-Western Europe. Collins, London, p 244
Nelson Bryan J (2005) Pelicans, cormorants, and their relatives. The Pelecaniformes. Oxford University Press, Oxford, p 661
Pitcher TJ, Parrish JK (1993) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) Behaviour of teleost fishes, 2nd edn. Chapman & Hall, London, pp 363–439
Privileggi N (2003) Great cormorants (Phalacrocorax carbo sinensis) wintering in Friuli Venezia Giulia, Northern Adriatic: specific and quantitative diet composition. Vogelwelt 124:237–243
Rayner MJ, Hauber ME, Clout MN, Seldon DS, Van Dijken S, Bury S, Phillips RA (2008) Foraging ecology of the Cook’s petrel Pterodroma cookii during the austral breeding season: a comparison of its two populations. Mar Ecol Prog Ser 370:271–284
Rheman S, Islam ML, Shah MMR, Mondal S, Alan MJ (2002) Observation on the fecundity and Gonadosomatic Index (GSI) of Grey mullet Liza parsia (Ham.). J Biol Sci 2(10):690–693. (Online)
Ribak G, Weihs D, Arad Z (2004) How do Cormorants counter buoyancy during submerged swimming? J Exp Biol 207:2101–2114
Ropert-Coudert Y, Wilson RP (2005) Trends and perspectives in animal-attached remote sensing. Front Ecol Environ 3:437–444
Ropert-Coudert Y, Kato A, Bost CA, Rodary D, Sato K, Le Maho Y, Naito Y (2002) Do Adélie penguins modify their foraging behaviour in pursuit of different prey? Mar Biol 140:647–652
Ropert-Coudert Y, Kato A, Wilson RP, Cannell B (2006a) Foraging strategies and prey encounter rate of free-ranging Little Penguins. Mar Biol 149:139–148
Ropert-Coudert Y, Grémillet D, Kato A (2006b) Swim speeds of free-ranging Great cormorants Phalacrocorax carbo. Mar Biol 149:415–422
Rulifson RA (1977) Temperature and water velocity effects on the swimming performance of young of-the-year Striped mullet (Mugil cephalus), Spot (Leiostomus xanthurus) and Pinfish (Lagodon rhomboids). J Fish Res Board Canada 34:2316–2322
Sambilay VC (1990) Interrelationships between swimming speed, caudal fin aspect ratio and body length of fishes. Fishbyte 8(3):16–20
Schmid D, Grémillet D, Culik BM (1995) Energetics of underwater swimming in the Great cormorant (Phalacrocorax carbo sinensis). Mar Biol 123:875–881
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman and Co, San Francisco
Sponza S, Cimador B, Cosolo M, Ferrero EA (2010) Diving costs and benefits during post-breeding movements of the Mediterranean shag in the North Adriatic Sea. Mar Biol 157:1203–1213
Strod T, Izhaki I, Arad Z, Weihs D, Katzir G (2003) Cormorants Phalacrocorax carbo swallow fish under water. Vogelwelt 124:270–276
Takahashi A, Dunn MJ, Trathan PN, Sato K, Naito Y, Croxall JP (2003) Foraging strategies of chinstrap penguins at Signy Island, Antarctica: importance of benthic feeding on Antarctic krill. Mar Ecol Prog Ser 250:279–289
Tremblay Y, Cherel Y (2000) Benthic and pelagic dives: a new foraging behaviour in rockhopper penguins. Mar Ecol Prog Ser 204:257–267
Voslamber B, Platteew M, Van Eerden MR (1995) Solitary foraging in sand pits by breeding Cormorants Phalacrocorax carbo sinensis: does specialized knowledge about fishing sites and fish behaviour pay off? Ardea 83(1):199–212
Walton P, Ruxton GD, Monaghan P (1998) Avian diving, respiratory physiology and the marginal value theorem. Anim Behav 56:165–174
Watanuki Y, Kato A, Mori Y, Naito Y (1993) Diving performance of Adélie penguins in relation to food availability in fast sea ice areas: comparison between years. J Anim Ecol 62:634–646
Watanuki Y, Ishikawa K, Takahashi A, Kato A (2004) Foraging behaviour of a generalist marine top predator, Japanese cormorants (Phalacrocorax filamentosus), in years of demersal versus epipelagic prey. Mar Biol 145:427–434
Weimerskirch H, Gault A, Cherel Y (2005) Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86:2611–2622
White CR, Day N, Butler PJ, Martin GR (2007) Vision and foraging in Cormorants: more like herons than hawks? PloSOne doi:10.1371/journal.pone.0000639
Wilson RP, Wilson MPT (1988) Foraging behaviour in four sympatric Cormorants. J Anim Ecol 57:943–955
Wilson RP, Ropert-Coudert Y, Kato A (2002) Rush and grab strategies in foraging marine endotherms: the case for haste in penguins? Anim Behav 63:85–95
Wilson RP, Scolaro JA, Grémillet D, Kierspel MAM, Laurenti S, Upton J, Gallelli H, Quintana F, Frere E, Müller G, Straten MT, Zimmer I (2005) How do Magellanic Penguins cope with variability in their access to prey? Ecol Monogr 75:379–401
Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ (2006) Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol 75:1081–1090
Zwanette J (2001) Transport and retention of Flounder larvae (Platichthys flesus) in the Dollard nursery (Ems estuary). J Sea Res 45:153–171
Acknowledgments
We thank P. Utmar for extensive help in the field. F. Roppa, N. Ventolini and N. Privileggi helped with data collection. M. Tofful and C. Trani contributed to Great Cormorant population monthly surveys. We thank also the staff of the ‘Office for Studies on Wild Fauna’ of Friuli Venezia Giulia Region and the personnel of Valle Noghera fishing ‘valli’ and Valle Cavanata Natural Reserve for data and support. PhD scholarship to M. Cosolo was partially granted by the European Community Initiative INTERREG IIIB ‘CADSES’ Project “Management and sustainable development of protected transitional waters”. Finally, we would like to thank the anonymous referees and the Associate Editor for valuable criticism.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. E. Hauber.
Rights and permissions
About this article
Cite this article
Cosolo, M., Ferrero, E.A. & Sponza, S. Prey ecology and behaviour affect foraging strategies in the Great Cormorant. Mar Biol 157, 2533–2544 (2010). https://doi.org/10.1007/s00227-010-1517-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1517-2