Abstract
There is little information on the effort put into foraging by seabirds, even though it is fundamental to many issues in behavioural ecology. Recent researchers have used changes in the underwater cruising speed of penguins to allude to prey ingestion since accelerations are thought to reflect the encounter and pursuit of prey. In this study, we attached minute accelerometers, to determine flipper beat frequency as a proxy for prey pursuit, to Little Penguins Eudyptula minor foraging in shallow waters in Western Australia. During diving, Little Penguins flapped continuously and at a regular pace of 3.16 Hz while descending the water column and throughout the bottom phase of most dives. However, the frequency and amplitude of wingbeats increased transitorily, reaching 3.5–5.5 Hz, during some dives indicating prey pursuit. Pursuit phases lasted a mean of 2.9±3.3 s and occurred principally during the bottom phases of dives (75.4%). Most dives in all birds (86%) had a clear square-shaped depth profile indicating feeding activity near the seabed in the shallow waters of the bays. Hourly maximum depth, time spent underwater, percentage of dives with pursuit events and catch per unit effort showed an overall increase from zero at ca. 0500 h to a maximum during the hours around mid-day before decreasing to zero by 1900 h. During pursuit phases, Little Penguins headed predominantly downward, probably using the seabed to assist them in trapping their prey. In the light of our results, we discuss depth use by Little Penguins and their allocation of foraging effort and prey capture success as a function of environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feeding and activity patterns are central themes to studies of life-history evolution (Stearns 1992). The rate of energy acquisition via feeding determines how energy can be allocated to the exclusive processes of growth or reproduction (Levins 1968). Seabirds are central place foragers, which gather food at sea but reproduce on land. Their feeding is thus limited by the time they spend at sea, and their foraging efficiency constrained by prey encounter rate and their hunting and prey processing abilities (Ropert-Coudert et al. 2004a). The constraints imposed on seabirds make them interesting models for behavioural ecology work, recently enhanced by recent advances and miniaturization of electronics that can continuously monitor their activity at sea (cf. Naito 2004 for details).
Little Penguins Eudyptula minor are largely visual hunters (Cannell and Cullen 1998), a capacity that limits foraging to daylight hours. However, little is known about their actual foraging effort. The few studies that have investigated the at-sea activity of Little Penguins in Western Australia have revealed that during breeding, these birds forage almost exclusively within 15–20 km of the colony, where waters are shallow (seabed at a maximum depth of ca. 17 m, Klomp and Wooller 1988). Time-depth recorders mounted on Little Penguins have shown that they recurrently exploit specific depth zones from trip to trip (Ropert-Coudert et al. 2003). Dietary studies indicate that these birds eat primarily small, schooling fish such as sandy sprat (Hyperlophus vittatus), pilchard (Sardinops sagax), garfish (Hyporhamphus melanochir), blue sprat (Spratelloides robustus), anchovy (Engraulis australis) and occasionally squid (Idiosepius notoides) (Klomp and Wooller 1988; Wienecke et al. 1995). Aside from this, nothing is known about prey encounter rates and the portion of underwater time devoted to feeding and travelling.
Measurement of speed in free-ranging penguins has shown that individuals swim at a roughly constant speed during the majority of their dives and during all travelling phases of dives (Wilson et al. 2002; Ropert-Coudert et al. 2001a; Bethge et al. 1997). Departures from this cruising speed have therefore been thought to reflect the encounter and pursuit of prey (Ropert-Coudert et al. 2000; Wilson et al. 2002) and, in keeping with this, Little Penguins have been observed to accelerate when capturing fish prey in both shallow and deep waters and when capturing either a solitary fish or one from a shoal (Schulz 1987; Cannell 1994). Therefore, measurement of changes of swimming speed of Little Penguins should help elucidate their foraging strategies and prey encounter rate. Since reliable swim speed recorders are generally too large for such a small animal (0.6–1.4 kg, but see Bethge et al. 1997), we used minute accelerometers that record simultaneously the body posture and the dynamic motion of animals (Yoda et al. 2001; Ropert-Coudert et al. 2004b, c; Watanuki et al. 2003) to determine flipper beat frequency as a proxy for acceleration.
This study aimed to reconstruct the time/activity budget of free-ranging Little Penguins from Penguin Island, Western Australia as they foraged in the shallow waters of Comet Bay. Using acceleration as an index of prey pursuit, we provide a first attempt to describe the foraging strategies of this bird and its rate of prey encounter. Based on the rate of prey encounter and the birds’ diving activity, we discuss prey availability and Little Penguins foraging efficiency and how both might vary according to environmental conditions.
Materials and methods
Data-loggers were fitted to four Little Penguins in August 2002. All birds were nesting on the central part of Penguin Island, Rockingham (32°16′S, 115°21′E), Western Australia, in rectangular, wooden nest-boxes (see Klomp et al. 1991 for details), allowing easy capture of the adults and checking of the chicks. Nautical dusk and dawn for the study period were taken to be 0550 h and 1848 h (local time), respectively, (http://lychnis.imcce.fr/cgi-bin/levcou.cgi). Birds were captured at their nest site, either at night or before departure for sea early in the morning. The sex (determined from the bill depth; Gales 1989), breeding status and the mass of both the adult and its chicks were noted. Loggers were then attached using waterproof (Tesa) tape (Wilson et al. 1997a) on the median line of the birds’ back, near the tail so as to minimize drag (Bannasch et al. 1994). The attachment of the logger was completed in <5 min and birds were released at the entrance of their nest-box.
Time-budgets and activity patterns of birds were recorded using miniaturized, cylindrical, four-channel data loggers (M190-D2GT, 12 bit resolution, 52×15 mm, 16 g, Little Leonardo, Tokyo, Japan). The absolute accuracy for the depth sensor was 0.1 m. The devices simultaneously monitored depth (1 Hz) and acceleration (16 Hz) along the longitudinal (surging) and dorso-ventral (heaving) axes of the birds. The units contained sensors capable of measuring both dynamic acceleration (e.g. vibration) and static acceleration (gravity). In the absence of movement, values of static acceleration ranged from +1 to −1 G. For instance, a vertically upright logger would correspond to values of 0 G on the heaving axis and −1 or +1 G on the surging axis, depending on whether the logger was head-up or head down, respectively, (see Yoda et al. 1999 for technical details).
Equipped birds were recaptured after one foraging trip at sea. The instruments and tape were removed, and the penguins were reweighed and released. The status of the birds was then checked weekly until the next moulting period.
Calibration sessions were conducted at the Perth Zoo, Western Australia. One Little Penguin was equipped with an accelerometer, attached using tape in same position as used on free-ranging individuals. This bird was then released in the pool where it swam with conspecifics. The behaviour of the bird was filmed using a digital video-camera (Handycam, Sony Ltd., 30 frames/s) while it fed on dead pilchards S. sagax. An exhibit window comprising one complete side of the pool allowed us to track the bird for the whole period it was submerged. These video sessions were subsequently used to confirm the relationship between the signals recorded by the logger and the posture and activity of the birds.
Data were downloaded into a computer and analysed using IGOR Pro (Wavemetrics Inc., USA, 2000, Version 4.01). A dive started when birds departed from the water surface and ended when they returned to it. Only dives >1 m were analysed. The bottom phase of dives was considered to have started and ended the first and last times that the rate of change of depth did not exceed 0.25 m/s. All depth changes occurring within this bottom phase are subsequently termed undulations (see Wilson 1995; Simeone and Wilson 2003).
Since surging acceleration was recorded along the main body axis of the birds, the static component of this acceleration channel was the most sensitive to changes in body orientation and was consequently used to calculate dive angle. Body angle was defined using the method described by Watanuki et al. (2003). Briefly, we used a low-pass filter (Tanaka et al. 2001) to separate the component of the gravity acceleration along the surging axis from the high frequency component resulting from wing beat activity. Body angle (θ) was then calculated using the following equation:
where A is gravity acceleration along the surging axis, g is gravity and α is logger attachment angle. Attachment angle was calculated assuming that body angle was 0 when birds were at the sea surface between two dives.
The accurate start time of the foraging trip was inferred from body angle values since the birds’ body angles differed between standing on land and swimming at sea, with distinctive acceleration values of ca. 0 G and ca. +0.4 G, respectively, on the surging axis.
Flipper beats were apparent in the acceleration signals as an oscillating pattern present simultaneously on both axes, with each propulsive stroke recorded on the heaving axis resulting in a forward acceleration recorded on the surging axis. This oscillating pattern has been identified as limb beating in several studies using similar loggers on a variety of birds’ species (Yoda et al. 2001; Sato et al. 2002; Watanuki et al. 2003; Ropert-Coudert et al. 2004b, c; Weimerskirch et al. 2005). All parts of the Little Penguin dives lacking these distinctive oscillating patterns were considered to be passive swimming phases where birds use buoyancy to ascend (cf. Sato et al. 2002). The amplitude and frequency of each wingbeat were analysed using the heaving acceleration signal (the most sensitive to undulation in the birds body resulting from flipper beats).
Hourly irradiation data measured by a solar panel (with 0° of inclination and a reflectivity of 0.1) were downloaded from the web page of the Australian National University (http://solar.anu.edu.au/Sun/Irrad/Irradiation.html) for Perth, Western Australia during August.
Flipper beat frequencies were calculated using a Power Spectrum Density analysis (Igor Procedure Version 1.1, LH 971028, Wavemetrics Inc., USA, 2000, Version 4.01). Simple regression was used to highlight trends between variables. Comparisons of dive parameters were conducted using the Student’s t-test. All statistical tests were performed using Statview (Version 4.57, Abacus Concepts Inc., Berkeley, CA, USA, 1996) following Sokal and Rohlf (1969). When data did not follow a normal distribution, non-parametric tests were used. The statistical threshold was 0.05. Values are presented as means ± SD.
Results
Three of four Little Penguins foraged over a single day, restricting their diving activity to daylight hours (Fig. 1a). Birds entered the water between 0330 h and 0400 h and exited the water between 1745 h and 1930 h. The last bird (B18) entered the water relatively late, at 0517 h, and foraged throughout the day but stayed at sea overnight, resuming its activities on the next day. For this bird, we only analysed the diving activity on the first day because the accelerometer’s memory was full before the end of the second day. Three penguins started to dive about 2 h after entering the water but B18 began diving 4 h after entering the water (Table 1). The time spent by the penguins underwater varied according to the time of day, with birds spending a higher percentage of their time underwater during the mid-day period than they did closer to dawn or dusk (Fig. 4a). Most dives (86%) had a clear square-shaped depth profile (Fig. 1b).
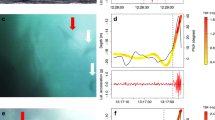
Little Penguins flapped continuously and at a regular frequency while descending the water column. They maintained this pace throughout the bottom phase of most dives, before progressively reducing their wingbeat frequency towards the beginning of the ascent (Fig. 2a). Penguins stopped beating their flippers completely during the ascent phase, a mean of 5.69±4.55 m (vertical distance from the surface) before surfacing, i.e. at 56±29% of the maximum depth of their dives (n=2,114 dives for all birds, Table 2). We applied a power spectrum density analysis on the heaving acceleration signals during diving phases only. We found that about a main wing-beat frequency peak of 3.16 Hz there were two clear points of inflection located at 2.25 and 3.94 Hz (Fig. 2b). Inspection of the dives showed that values below the lower threshold of 2.25 Hz corresponded to decelerations preceding the final gliding phase when the penguins were returning to the surface (cf. Sato et al. 2002–see later). Values above the upper threshold of 3.95 Hz generally occurred in discrete groups. We assumed that all frequencies exceeding 3.94 Hz for more than three consecutive wing beats were due to prey pursuit. Note that we assume that any ‘encounter’ with a prey during the course of a dive will result in a pursuit of this prey, and that this is illustrated by an increase in the flipper beat frequency as defined. Frequencies <2.25 Hz were subsequently termed ‘decelerations’. Overall, birds beat their flippers between 87.3 and 97.6% of their underwater time swimming, 86% of this flapping time at the cruising frequency between 2.25 and 3.94 Hz.
a Dive depth, smoothed heaving acceleration data and dive angle illustrated during the course of one dive; and b distribution curve resulting from a Power Spectrum Density analysis (PSD) applied to the heaving acceleration signals recorded during the dive phases of all Little Penguins. The main flipper beat frequency of Little Penguins was 3.16 Hz. All frequencies below 2.25 and above 3.94 Hz were termed deceleration and pursuits, respectively. These three frequencies are also visible on the heaving acceleration data in a
Based on the presence or absence of pursuits, dives were separated into pursuit and cruising dives, respectively. Overall, the ratio of cruise to pursuit dives was 1.16±0.57 but this ratio was highly variable among birds (Table 3). However, this ratio changed systematically during the day as the proportion of pursuit dives increased in the morning to peak around mid-day before decreasing again in the late afternoon (Fig. 4c). Long, uninterrupted successions of cruising dives were mainly observed at the beginning and end of foraging trips. Prey encounter rates were similar for the four birds although rates were notably lower for B19 than in conspecifics (Fig. 3). Catch per unit effort, a measure of the ease with which birds encountered prey (Wilson et al. 1994; Grémillet 1997), was derived by dividing the number of pursuits per time interval by the total time spent underwater during that time interval. This calculation showed an overall increase from zero at ca. 0500 h to a maximum during the hours around mid-day before decreasing to zero by 1900 h (Fig. 4c). A sixth order polynomial fit of the data (y=−18.24x 6+56.17x 5−61.31x 4+27.17x 3−3.91x 2+0.12x+0.002; R 2=0.71) showed a peak at mid-day and 90% limits between 0540 h and 1840 h (Fig. 4d).
Cumulative index of prey encounter (see Results for details) as a function of the time of day for birds B35, B65, B19 and B18. Arrows indicate substantial sequences of cruising dives. White circles show the departure from and arrival at the colony. Losanges on the curves indicate the first and last dive >1 m, respectively
a Hourly time spent underwater, b mean maximum dive depth per 10 min, c proportions of pursuit dives, d Catch per Unit Effort (CPUE, calculated as the proportion of the underwater time spent engaged in prey pursuit over a 10 min interval) and e average irradiation recorded in the Perth region in August, all against time of day. Vertical bars show SD
Little Penguins performed 2.17±1.53 pursuit phases per pursuit dive (maximum=11 distinct accelerations in one dive, Table 2). These pursuit phases lasted a mean of 2.9±3.3 s (range 0.19–31.6 s, n=4 birds), with 86% of the pursuits lasting <5 s. They occurred principally during the bottom phases of dives (75.4% of all dives on all birds, range 67.4–82.9%) and only occasionally during the descent (13.7%) and ascent (10.9%) phases. During pursuit phases, Little Penguins headed predominantly downward (Fig. 3b) but, overall, the amplitudes of depth changes during pursuit phases were extremely small, being on average 0.74±1.71 m (max. 11.8 m, n=457 pursuits) and 0.73±0.94 m (max. 8.0 m, n=935 pursuits) during upward and downward pursuit phases, respectively. The distribution of the depth at which acceleration occurred revealed intra-specific variability in the way Little Penguins use the water column with individuals apparently exploiting rather narrow depth bands (Fig. 5).
The descent angle of a dive was significantly more acute when a pursuit had occurred during the course of the previous dive in all birds (Fig. 6) although there was substantial inter-individual variability in the way descent angles changed. This variability was probably related to the differences in the maximum depth reached during the foraging trip by birds.
Discussion
Our data represent, to our knowledge, the first account of the fine-scale time budget of Little Penguins, with a detailed description of the species’ underwater activities (but see also Bethge et al. 1997; Gales et al. 1990). This study also provides the first report of the diving angle for this species. Although our sample size was too small to allow us to be too specific about the quantitative aspects of the foraging efficiency of Little Penguins, we have highlighted general trends in the foraging activity and strategies of the smallest of all the Spheniscids.
Assumptions about flipper beat frequency being a proxy for prey pursuit
One critical assumption in this study is that Little Penguins that occasionally depart from their cruising flipper-beat frequency during the course of a dive do so in order to increase their speed to pursue prey. Such observations have been made by one of the authors during feeding experiments of Little Penguins in captivity (B.L. Cannell, unpublished data). We expect free-living Little Penguins to behave similarly when they encounter prey in the wild, although we cannot define the extent to which such pursuits were successful. However, data from Magellanic penguins (Spheniscus magellanicus) fitted with speed and flipper beat frequency/amplitude sensors in tandem with beak angle sensors show that most (>90%) sudden increases in acceleration result in prey capture (R.P. Wilson, unpublished data). We cannot exclude the possibility that birds accelerated to avoid predators or as a result of agonistic behaviour from conspecifics. We believe, none-the-less, that should this occur, it would only reflect a very small proportion of the acceleration events recorded in our study. The very short accelerations observed (0.75–1 s) may correspond to either very short pursuits or chases abandoned quickly. In both cases, accelerations should still generally reflect a prey encounter but do not necessarily represent prey capture and ingestion.
Depth use by Little Penguins
The increase in mean maximum depth from dawn to mid-day and decrease from mid-day to dusk shown by our equipped penguins (Fig. 4b) typifies general patterns of depth use by other penguin species (Wilson et al. 1993; Ropert-Coudert et al. 2004a, etc.). Diel changes in the vertical distribution of prey may play some role in this. However, a primary determinant of maximum dive depth in penguins seems to be the availability of light since these birds rely primarily on vision to hunt (Wilson et al. 1993; Pütz and Bost 1994; Cannell and Cullen 1998) and this would appear to be the case in our study (Fig. 4e). However, a notable difference between our study birds and other penguin species is the difference in the absolute values of the depth maxima as a function of time of day (and therefore light intensity). Little Penguins do not dive as deep as other species (maximum depth so far recorded: 69 m, Montague 1985) at any time of the day even though the general diel pattern is apparent. Aside from the general allometric relationship between maximum dive depth and body mass (e.g. Kooyman and Davis 1987; Wilson et al. 1997b), this implies that something is different for Little Penguins. They may have, for instance, poorer vision at low light intensity than other species (e.g. the special anatomical adaptation of the eye of the deeper diving king penguins Aptenodytes patagonicus, Martin 1999). In addition, the water turbidity is markedly higher in our study area than in areas where studies have been conducted on other species. This would lead to more rapid reduction in light intensity with increasing depth. Although there is indeed evidence that waters in the Perth area result in more rapid light attenuation with depth than many sub-Antarctic and Antarctic sites where other penguin species forage (Jerlov 1968), bottom topography may compound the observed patterns. The square ‘U-shaped’ dive profiles (see Le Boeuf et al. 1988 for general definition) and the consistency between maximum depths in adjacent dives makes it highly likely that the dives are made close to the sea bed (cf. also Tremblay and Cherel 2000). This is given further credence by the fact that water depths in the foraging areas of these birds generally concur closely with maximum dive depths (which are around 15–20 m in Cockburn Sound, Warnbro Sound and Shoalwater Bay). Thus, Little Penguins leaving their breeding site and moving out into deeper waters during the day before reversing the process towards evening, will have a depth use pattern that should approximate that shown in Fig. 4b. The depth limits at any time will thus be defined by the movements of the birds and the bottom topography. The apparent correlation of depth with light may be a consequence of this and the general penguin condition of needing light to forage (Cannell and Cullen 1998).
Allocation of foraging effort and prey capture success
In tandem with diel changes in maximum dive depth (Fig. 4b), the apparent foraging success of the birds also increased, as evidenced by their CPUE (Fig. 4c) and this, in turn, was paralleled by an increase in the proportion of time that the birds spent underwater (Fig. 4a). The clear, apparently light-dependent, patterns need to be considered in a hierarchical manner so that proper dependencies can be ascertained. As discussed earlier, the absolute maximum dive depth, ultimately, is likely to be dependent on light levels because Little Penguins are visual hunters (Cannell and Cullen 1998). The fact that Little Penguins encounter more prey over mid-day (Fig. 4c) may be due to greater light at depth, which would enhance visual capacities and increase the probability of prey being perceived, or simply because it is at this time, roughly half way through the foraging trip, that the birds have moved to the areas of greatest prey abundance. The final, ostensibly light-related, pattern of percentage time spent underwater (Fig. 4a) may simply be a correlate of prey abundance, with penguins working harder in areas where prey are most encountered. This behaviour is a well-known feature of animal foraging (Krebs 1978; Schoener 1971; Pyke 1984) and has obvious advantages.
Specifics of prey capture
Little penguins apparently pursued most prey during the bottom phase of the dives. Pursuit of prey during bottom phases of dives in penguins that forage in the water column has been noted for Magellanic Penguins (cf. Simeone and Wilson 2003). Benthic or demersal feeding has already been noted in emperor Aptenodytes forsteri (Rodary et al. 2000), chinstrap Pygoscelis antarctica (Takahashi et al. 2003), gentoo P. papua (Croxall et al. 1988; Wilson et al. 1991; Robinson and Hindell 1996), rockhopper Eudyptes chrysocome (Tremblay and Cherel 2000) and Adélie penguins P. adeliae, although in this last species benthic dives are extremely rare (Ropert-Coudert et al. 2001b). It is, therefore, little surprising that benthic diving activity was also observed in the Little Penguin. Interestingly, penguins foraging in the mid-water, i.e. king penguins A. patagonicus (Ropert-Coudert et al. 2000) and Adélie penguins for most of their diving activity (Ropert-Coudert et al. 2001a) tend to capture prey from the underneath. In contrast, Little Penguins headed downward for most of their pursuits. This, together with the small amplitude of the depth changes during pursuit, suggests that birds use the sea bed to trap prey. The overall picture, therefore, is one where birds encountered most of their prey in the demersal zone, a few meters above the seabed, and force them down closer to the seabed to reduce the prey’s escape field. A similar, but reverse effect, has been noted for auks manoeuvering prey against the sea surface (Duffy et al. 1987).
Interestingly, descent angles were more acute when a prey had been encountered in the previous dive than when no prey was encountered. In Adélie penguins, this has been regarded as a strategy to reduce transit times between the surface and the prey patch without having to depart from the COTmin speed (Wilson et al. 1996; Ropert-Coudert et al. 2001b). Such a strategy is not expected to occur in the case of benthic diving activity since the profitable depth here is the seabed. Indeed, benthic divers are expected to use the same angle to reach the seabed whether the presence of prey at the bottom is certain or not. In our study, although Little Penguins dived substantially close to the seabed, an appreciable proportion of their dives terminated in mid-water. This, together with the purported 25% of prey catches occurring during transit warrant the change in angle observed. The predominant dependence on near-bottom-dwelling fish, however, may account for the fact that the difference in dive angles is small.
The use of miniature accelerometers has allowed us access to the fine-scale underwater activity and to allude to prey encounter rate in free-ranging Little Penguins. The possibility of inferring prey–predator interactions from the analysis of the behaviour of the predators means that we are now potentially able to assess the foraging efficiency of individuals. Future studies should focus on verifying the extent to which our use of accelerometry is justified as a measure of prey encounter so that large numbers of similar datasets could subsequently be collected.
References
Bannasch R, Wilson RP, Culik BM (1994) Hydrodynamics aspects of design and attachment of a back-mounted device in penguins. J Exp Biol 194:83–96
Bethge P, Nicol S, Culik BM, Wilson RP (1997) Diving behaviour and energetics in breeding little penguins (Eudyptula minor). J Zool Lond 242:483–502
Cannell BL (1994) The feeding behaviour of Little Penguins, Eudyptula minor. PhD Thesis, Monash University, Melbourne
Cannell BL, Cullen J (1998) The foraging behaviour of Little Penguins Eudyptula minor at different light levels. Ibis 140:467–471
Croxall JP, Davis RW, O’Connell MJ (1988) Diving patterns in relation to diet of gentoo and macaroni penguins at South Georgia. Condor 90:157–167
Duffy DC, Todd FS, Siegfried WR (1987) Submarine foraging behavior of alcids in an artificial environment. Zoo Biol 6:373–378
Gales RP (1989) Feeding ecology and free-living energetics of the Little Penguin, Eudyptula minor, in Tasmania. PhD Dissertation, University of Tasmania, Hobart, Australia
Gales RC, Williams C, Ritz D (1990) Foraging behaviour of the little penguin, Eudyptula minor: initial results and assessment of instrument effect. J Zool Lond 220:61–85
Grémillet D (1997) Catch per unit effort, foraging efficiency, and parental investment in breeding great cormorant (Phalacrocorax carbo carbo). ICES J Mar Sci 54:635–644
Jerlov NG (1968) Optical oceanography. Elsevier, Amsterdam, pp 194
Klomp NI, Wooller RD (1988) Diet of Little Penguins, Eudyptula minor, from Penguin Island, Western Australia. Aust J Mar Freshw Res 39:633–639
Kooyman GL, Davis RW (1987) Diving behaviour and performance, with special reference to penguins. In: Croxall JP (ed) Seabirds: feeding biology and role in marine ecosystem. Cambridge University Press, London, pp 63–75
Krebs JR (1978) Optimal foraging: decision rules for predators. In: Krebs JR, Davies NB (eds) Behavioral ecology: an evolutionary approach. Blackwell Press, Oxford
Le Boeuf BJ, Costa DP, Huntley AC, Feldkamp SD (1988) Continuous, deep diving in female northern elephant seals Mirounga angustirostris. Can J Zool 66:446–458
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton, NJ
Martin GR (1999) Eye structure and foraging in king penguins Aptenodytes patagonicus. Ibis 141:444–450
Montague T (1985) A maximum dive recorder for little penguins. Emu 85:264–267
Naito Y (2004) New steps in bio-logging science. Mem Natl Inst Polar Res Spec Issue 58:50–57
Pütz K, Bost CA (1994) Feeding behaviour of free-ranging king penguins (Aptenodytes patagonicus). Ecology 75:489–497
Pyke GH (1984) Optimal foraging theory: a critical review. Ann Rev Ecol Syst 15:523–575
Robinson SA, Hindell MA (1996) Foraging ecology of gentoo penguins Pygoscelis papua at Macquarie Island during the period of chick care. Ibis 138:722–731
Rodary D, Bonneau W, Maho YL, Bost CA (2000) Benthic diving in male emperor penguins Aptenodytes forsteri foraging in winter. Mar Ecol Prog Ser 207:171–181
Ropert-Coudert Y, Sato K, Kato A, Charrassin J-B, Bost C-A, Le Maho Y, Naito Y (2000) Preliminary investigations of prey pursuit and capture by king penguins at sea. Polar Biosci 13:102–113
Ropert-Coudert Y, Kato A, Baudat J, Bost C-A, Le Maho Y, Naito Y (2001a) Feeding strategies of free-ranging Adélie penguins, Pygoscelis adeliae, analysed by multiple data recording. Polar Biol 24:460–466
Ropert-Coudert Y, Kato A, Baudat J, Bost C-A, Le Maho Y, Naito Y (2001b) Time/depth usage of Adélie penguins; an approach based on dive angles. Polar Biol 24:467–470
Ropert-Coudert Y, Kato A, Naito Y, Cannell B (2003) Individual diving strategies in the Little Penguins. Waterbirds 26:403–408
Ropert-Coudert Y, Wilson RP, Daunt F, Kato A (2004a) Patterns of energy acquisition by a central place forager: benefits of alternating short and long foraging trips. Behav Ecol 15:824–830
Ropert-Coudert Y, Grémillet D, Ryan PG, Kato A, Naito Y, Le Maho Y (2004b) Between air and water: the gannet’s plunge dive. Ibis 146:281–290
Ropert-Coudert Y, Grémillet D, Kato A, Ryan PG, Naito Y, Le Maho Y (2004c) A fine-scale time budget of cape gannets provides insights into their foraging strategies. Anim Behav 67:985–992
Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin J-B, Bost C-A, Handrich Y, Le Maho Y (2002) Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J Exp Biol 205:1189–1197
Schoener TW (1971) Theory of feeding strategies. Ann Rev Ecol Syst 2:369–404
Schulz M (1987) Observations of feeding of a Little Penguin Eudyptula minor. Emu 87:186–187
Simeone A, Wilson RP (2003) In depth studies of Magellanic foraging behaviour: can we estimate prey consumption by perturbations in the profile? Mar Biol 143:825–831
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman Press, San Francisco, USA
Stearns SC (1992) The evolution of life history. Oxford University Press, New York, USA
Tanaka H, Takagi Y, Naito Y (2001) Swimming speeds and buoyancy compensation of migrating adult chum salmon Onchorhynchus keta revealed by speed/depth/acceleration data logger. J Exp Biol 204:3895–3904
Takahashi A, Dunn M, Trathan PN, Sato K, Naito Y, Croxall JP (2003) Foraging strategies of Chinstrap penguins at Signy Island, Antarctica: importance of benthic feeding on Antarctic krill. Mar Ecol Prog Ser 250:279–289
Tremblay Y, Cherel Y (2000) Benthic and pelagic dives: a new foraging behaviour in rockhopper penguins. Mar Ecol Prog Ser 204:257–267
Watanuki Y, Niizuma Y, Gabrielsen GW, Sato K, Naito Y (2003) Stroke and glide of wing-propelled divers: deep diving seabirds adjust surge frequency to buoyancy change with depth. Proc R Soc Lond B 270:483–488
Weimerskirch H, Le Corre M, Ropert-Coudert Y, Kato A, Marsac F (2005) The three dimensional flight of red-footed boobies: adaptations to foraging in a tropical environment. Proc R Soc Lond 272:53–61
Wienecke BC, Wooller RD, Klomp NI (1995) The ecology and management of Little Penguins on Penguin Island, Western Australia. In: Dann P, Norman I, Reilly R (eds) The penguins: ecology and management. Surrey Beatty, Sydney, pp 440–467
Wilson RP (1995) Foraging ecology. In: Perrins C, Bock W, Kikkawa J (eds) Bird families of the world: the penguins spheniscidae. Oxford University Press, Oxford, pp 81–106
Wilson RP, Pütz K, Bost C-A, Culik BM, Bannasch R, Reins T, Adelung D (1993) Diel dive depth in penguins in relation to diel vertical migration of prey: whose dinner by candlelight? Mar Ecol Prog Ser 94:101–104
Wilson RP, Culik BM, Bannasch R, Lage J (1994) Monitoring Antarctic environmental variables using penguins. Mar Ecol Prog Ser 106:199–202
Wilson RP, Pütz K, Peters G, Culik B, Scolaro JA, Charrassin J-B, Ropert-Coudert Y (1997a) Long-term attachment of transmitting and recording devices to penguins and other seabirds. Wildl Soc Bull 25:101–106
Wilson RP, Bost C-A, Pütz K, Charrassin J-B, Culik BM, Adelung D (1997b) Southern Rockhopper penguin Eudyptes chrysocome chrysocome foraging at Possession Island. Polar Biol 17:323–329
Wilson RP, Ropert-Coudert Y, Kato A (2002) Rush and grab strategies in foraging marine endotherms: the case for haste in penguins? Anim Behav 63:85–95
Wilson RP, Culik B, Spairani HJ, Coria NR, Adelung D (1991) Depth utilization by penguins and gentoo penguin dive patterns. J Ornithol 132:47–60
Wilson RP, Culik BM, Peters G, Bannasch R (1996) Diving behaviour of Gentoo penguins, Pygoscelis papua; factors keeping dive profiles in shape. Mar Biol 126:153–162
Yoda K, Sato K, Niizuma Y, Kurita M, Bost C-A, Le Maho Y, Naito Y (1999) Precise monitoring of porpoising behaviour of Adélie penguins determined using acceleration data loggers. J Exp Biol 202:3121–3126
Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y (2001) A new technique for monitoring the behaviour of free-ranging Adélie penguins. J Exp Biol 204:685–694
Acknowledgements
This study was financially supported by the Japanese Society for Promotion of Science and Murdoch University. Field experiments were conducted while Y.R-.C. was visiting Murdoch University, Western Australia. The authors wish to acknowledge M. Mitchell, C. Lamont, M. Banks, T. Goodlich, all the wardens of Penguin Island, the staff from the Penguin experience on Penguin Island for their help in the field and the staff from the Perth Zoo for their help during the calibration experiments. J. Holder at the Department for Planning and Infrastructure and three anonymous referees for their helpful comments. Special thanks to A-M. Kato-Ropert and the Cannell-Lunn family. All research was covered by the appropriate Department of Conservation and Land Management permits.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Ropert-Coudert, Y., Kato, A., Wilson, R.P. et al. Foraging strategies and prey encounter rate of free-ranging Little Penguins. Marine Biology 149, 139–148 (2006). https://doi.org/10.1007/s00227-005-0188-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0188-x