Abstract
Patients suffering a low-energy fracture are at high risk of subsequent fractures. Investigation of all fragility fracture patients above the age of 50 years is recommended in order to prevent further fractures. The aim of this study was to investigate alternative strategies including known risk factors (age, sex, and body weight) for selecting fracture patients for osteoporosis assessment and investigate how these strategies would affect the proportion of patients with osteoporosis identified and the number of patients referred for Dual X-ray Absorptiometry (DXA) compared with the original FLS strategy. From OFELIA; a cohort study comprising 794 fragility fracture patients we included 622 patients aged 18 + years (mean age 56 ± 17) with fragility fractures. We investigated the predictive value of clinical risk factors using ROC curves and AUC analyses. The revised strategies were compared by analyzing sensitivity and specificity of different strategies based on sex, age, BMI, and bodyweight. For patients 50 + years, a strategy investigating men and women with body weight ≤ 85 kg resulted in sensitivity and specificity of 94% and 25%, respectively, reducing the number of DXAs by 21%. For patients < 50 years, the prevalence of osteoporosis was low and it was, therefore, difficult to develop an acceptable strategy. We found that the original FLS strategy can be modified to include substantially fewer patients and still only miss the osteoporosis diagnosis in a very few patients. A modified strategy would potentially save costs and concerns in many patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical consequence of osteoporosis is fractures happening after no or minimal trauma [1], but patients suffering a fragility fracture are often not offered appropriate assessment of the risk of subsequent fractures, investigation of underlying osteoporosis or treatment to prevent such fractures. This well-known phenomenon, “the osteoporosis care gap” in post-fracture management, exists worldwide [2, 3]. Despite global campaigns recommending coordinator-based fracture prevention programs known as Fracture Liaison Service (FLS) targeting fragility fracture patients aged 50 + years [4,5,6], fracture prevention programs are only sporadically implemented in Denmark. Approximately, 50,000 individuals aged 50 years or older are annually treated for a fracture in Denmark [7]. In light of the fact that a fracture begets subsequent fractures [8, 9] and that more than 45% of disabling hip fractures occurs in persons with at least one prior fracture [10], it can be argued that fracture prevention programs are needed. According to the World Health Organization, approximately 500,000 Danish women and men (of a total population of 5.7 million) above the age of 50 years suffer from osteoporosis, but only one-fifth receive treatment according to the Danish register of prescribed medications [11]. A few hospitals in Denmark have implemented FLS; however, at Aarhus University Hospital (AUH), the second biggest hospital in Denmark serving local and regional patients, no systematic fracture prevention program exists, with the exception of hip fracture patients older than 65 years who are managed by the department of geriatric medicine. It has been argued that implementing the original FLS strategy, recommending osteoporosis screening in all fragility fracture patients aged 50 years or older [12], is too cumbersome and inefficient. We recently investigated the prevalence of osteoporosis in a Danish adult cohort of fragility fracture patients treated at AUH; the OFELIA study [13], and found that one in five patients above 50 years of age had osteoporosis. The aim of this current study was to investigate how modifications to the original FLS-model would affect the probability of identifying osteoporosis in low-energy fracture patients.
Methods
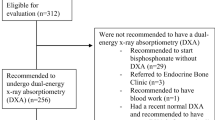
This study was based on data from the OFELIA cohort [13], which included 794 adult patients (18–93 years) suffering fragility fractures at any sites except skull and face treated at AUH between 1st of May 2014 and 30th of April 2015. Fractures of hands (including fingers) and feet (including toes) are not generally categorized as osteoporotic fractures as they are not consistently found to be associated with a higher risk of subsequent fractures [14, 15]. From the original OFELIA cohort, we, therefore, excluded patients with only hands/finger fractures (n = 101) or fractures of the feet/toes (n = 71). A total of 622 fracture patients could, therefore, be included in the present study.
The outcome of interest was osteoporosis, based on Bone Mineral Density (BMD) at the total hip or lumbar spine measured by Dual energy X-ray Absorptiometry (DXA) by trained and experienced technicians using one of 3 Hologic discovery systems (Hologic Inc., Waltham, MA, USA). Data were categorized following WHO’s definitions based on DXA measured BMD of the hip or spine (the lowest value was considered). Normal bone status was defined as T-score > − 1.0; osteopenia as − 2.5 < T-score ≤ − 1.0; and osteoporosis as T-score ≤ − 2.5 [16].
Variables found to be associated with osteoporosis in OFELIA (age, sex, body weight, and body mass index) were used to suggest possible models for identifying osteoporosis in fractures patients. In order to identify the variables and the combination of variables that best predicted osteoporosis, logistic regression models were used in order to draw estimates of ROC curves based on the predicted probabilities and the observed real osteoporosis status for each individual. The Area Under the Curve (AUC) was calculated, and models with the largest AUC were chosen for further analysis. The performance of the models was compared with the original FLS model, in which the only variable used is age dichotomized as < 50/≥ 50 years.
Sensitivity (percentage of osteoporotic patients being diagnosed with osteoporosis by DXA) and specificity (percentage of non-osteoporotic patients not being investigated) of the derived strategies for selecting patients for osteoporosis investigation were analyzed for the original FLS strategy as well as the strategies based on the models with higher AUC. In order to investigate robustness of the suggested strategies a sensitivity analysis in the OFELIA cohort of 794 fracture patients including patients with fractures of hands and feet was performed.
All analyses were performed using STATA 13 (Stata Corporation, College Station, TX, USA).
Results
Characteristics of the study population are presented in Table 1. A total of 174 men and 448 women were included, with mean (SD) age of 56 (16) years, 48 (17) years in men, and 59 (14) years in women. A total of 108 patients (including 13 men) were diagnosed with osteoporosis based on a T-score at either lumbar spine or total hip ≤ − 2.5.
Applying the original FLS strategy (investigating all patients aged ≥ 50 years), 446 patients would have been referred for DXA, from which 100 would have been diagnosed with osteoporosis (22.4%). However, the OFELIA cohort also included 176 fragility fracture patients younger than 50 years of age. Applying the original FLS strategy, these patients would not have been referred to DXA and 8 of these patients were diagnosed with osteoporosis (4.5%). The sensitivity and specificity of the original FLS model (model 1) in our cohort were, therefore, 92.6% and 36.7%, respectively, leading to an estimated AUC of 62.6% (95% CI 59.4–65.8%).
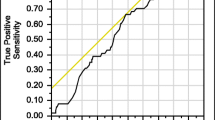
We investigated different models including age, sex, and body weight and the interactions between variables for selecting patients for osteoporosis investigation (Table 2). As a selection tool age was included in different ways: dichotomized with different cut points between 40 and 60 years, grouped into 3 age groups as well as a continuous variable or using cubic splines. The AUCs for all models were similar and we, therefore, chose the most commonly used age cut point in FLS research and clinical practice; age < 50/50 + for further analyses. We then added sex and body weight or BMI to the model. The model with highest AUC was model 13, including sex, age, body weight, and interactions between these variables (AUC 79.4 (74.8; 83.9)). But since model 9, with AUC 79.0 (74.5;83.6), was very similar (Fig. 1), we chose this more parsimonious model without interactions between variables as the best and most simple model for identifying patients for osteoporosis investigation in a clinical setting.
To further examine the effect of the parameters included in model 9, we estimated the predicted probability of osteoporosis depending on body weight for 4 different groups depending on sex and age (above or below 50 years) (Fig. 2). We saw that the probability of being diagnosed with osteoporosis was generally higher in older individuals and decreased with increasing body weight. In addition, the predicted probabilities for osteoporosis were similar in men and women above the age of 50 years when taking body weight into account; however, the difference between men and women < 50 years remained.
Strategies for selecting patients for osteoporosis investigation using combinations of sex and different cut points for body weight guided by the probability curves were investigated. Given that the prevalence of osteoporosis differed markedly between younger and older patients (4.6% and 22.4%, respectively), we investigated the different strategies in two separate groups, namely patients aged 50 years or older (n = 446, 83% women) and patients younger than 50 years (n = 176, 50% women).
Fracture Patients Above the Age of 50 Years
Analyses were performed in the 446 fracture patients aged ≥ 50 years. This group consisted of 86 men of whom 10 (12%) were diagnosed with osteoporosis and 360 women of whom 90 (25%) diagnosed with osteoporosis. AUCs were calculated for the same combinations of variables as done in the total cohort (Supplemental Table A) and the findings were similar; adding body weight or BMI to age improves the AUC markedly.
Table 3 presents the outcome of different strategies of selecting patients for osteoporosis evaluation in the 50 + years old patients. Strategies C and E have the same outcome, 94 patients diagnosed correctly and 6 patients with osteoporosis not identified; however, there was a reduction of 40 DXAs compared with the original strategy (A) (bold) for strategy E, but a reduction of 93 DXAs for strategy C, reflected in the different specificity of the two strategies (9.8% and 25.1%, respectively). Furthermore, 87 non-osteoporotic patients would not be targeted applying strategy C, whereas the same number for strategy E was only 34. Strategies B and C have almost the same outcome, respectively, 96/94 patients diagnosed correctly and 4/6 patients with osteoporosis not identified; however, the number of DXAs saved compared with the original strategy (A) was 63 for strategy B and 93 for strategy C, reflected in the different specificity of the two strategies (17.1% and 25.1%, respectively). We then tried different strategies with different cut-points in women and men for age and body weight, strategy F saved a similar number of DXAs as strategy C, however, missed the osteoporosis diagnosis in a substantially higher number of patients, reflected in the lower sensitivity. Therefore, strategy C (bold): bodyweight less than 85 kg as a cut-off point for DXA independent of sex seem to be the best strategy for identifying patient for DXA in patients above 50 years of age. This strategy reduced the number of DXA’s from 446 to 353 (20.9%) at the cost of 6 osteoporotic patients not being identified.
Patients Below the Age of 50 Years
Models for identifying osteoporosis in patients younger than 50 years of age (Supplemental Table B) were investigated and based on the AUCs, models including bodyweight and sex were investigated. Based on the probability graph (Fig. 2), we estimated that body weight below 50–60 kg in women and below 60–70 kg in men would identify patients at high risk of osteoporosis. We, therefore, tested strategies based on combinations of different cut points (Table 4). It proved difficult to develop a strategy in this group of patients with satisfactory sensitivity and specificity. We found that best strategy for identification of osteoporosis in patients below 50 years of age was strategy J (bold) investigating women with body weight below 55 kg and men with body weight below 70 kg, which identified 4 of 8 patients with osteoporosis despite referring only a total of 13 patients for DXA.
Sensitivity analyses performed in the entire OFELIA cohort of 794 patients including patients with fractures of hands or feet showed similar results (data not shown).
Discussion
In this cohort of 622 adult patients with fragility fractures at all sites except skull, face, fingers, and toes investigated for osteoporosis, we applied the generally accepted FLS strategy (investigation of all patients older than 50 years with a low-energy fracture) and found a sensitivity of 92.6% and specificity of 32.7%. By combining risk factors associated with osteoporosis [13]—especially bodyweight and age—we identified other strategies that could reduce the number of fracture patients being referred for investigation and at the same time still identify almost all of the patients with osteoporosis. The choice of strategy is not only depending on the sensitivity and the specificity of the strategy but also on the financial implications, the complexity of the strategy to be implemented in a busy clinical setting, as well as the psychological effects of being confronted with the possibility of having a chronic disease have to be considered. The scenarios can be maximizing the number of low-energy fracture patients with underlying osteoporosis being diagnosed at the expense of investigating many non-osteoporotic patients or accepting a strategy where fewer patients are referred for DXA, resulting in fewer non-osteoporotic patients being referred but at the expense of more patients with underlying osteoporosis not being diagnosed. A qualitative study on the experiences of fracture patients being screened for osteoporosis suggested, that being confronted with a potential osteoporosis diagnosis caused worries in healthy people to but all considered, the patients preferred “to know what was hidden in the bones” [17]. In the group of fracture patients aged 50 years or more, an example of an optimized strategy could be strategy C (including women and men, 50 + years, and body weight ≤ 85 kg) in which 79% of 446 patients with low-energy fractures were referred for DXA. Overall, 94 patients would have been diagnosed with osteoporosis, but the diagnosis would have been missed in 6 patients. In addition, DXA was not performed in 93 patients of whom 87 were non-osteoporotic. As almost all patients with osteoporosis were identified and more than 20% of DXAs indicated according to the original FLS strategy could be saved, this modified strategy, which should be easy to implement in the clinic, would be even more cost effective than the original FLS [4]when only considering the cost per diagnosed patient. However, there may also be costs associated with missing the diagnosis. Strategy D where only men and women with a bodyweight ≤ 80 kg were referred for DXA missed the diagnosis in an additional 3 patients but saved an additional 43 DXAs and increased the specificity of the strategy to 26.7%. In both strategy C and D, the sensitivity is high and the decision of using one or the other strategy should be based on the capacity of the DXA and resources in the FLS.
Should FLS also include younger patients (below the age of 50 years)? The difficulty is that osteoporosis is rare in young fracture patients and although low body weight seems to be a strong predictor of osteoporosis among younger fracture patients, it proved difficult to identify other common risk factors among the younger patients with osteoporosis. However, when selecting young patients with low body weight, we were able to identify 50% of patients with osteoporosis by referring only 13 patients for DXA. Only rarely diagnosing osteoporosis in younger fracture patients leads to pharmacological treatment [18], but as optimising diet and increasing physical activity have been demonstrated to increase bone mineral density in younger adults [13, 19] being diagnosed with osteoporosis may still be beneficial for the individual. Furthermore, as most of the fracture patients younger than 50 years in the investigated cohort were women above the age of 40 years and therefore approaching menopause, a diagnosis of osteoporosis would enable considerations of anti-osteoporosis treatment at menopause. It is important to consider that the numbers of patients below 50 years diagnosed with osteoporosis was very low, and results need to be interpreted with caution.
We observed that the significant difference in the risk of osteoporosis between men and women aged ≥ 50 years diminished when the estimates were adjusted for body weight, which is somewhat surprising [20]. However, our data suggest that the well-known difference in osteoporosis and fracture risk between men and women may be explained, at least partly by the differences in weight between the two sexes.
In this study, we used ROC curves and AUC for investigating how well clinical risk factors or combinations thereof predicted the outcome. Traditionally, an AUC greater than 0.9 has been suggested to indicate that the factors or the combination of factors included in the model predict the outcome very well (high accuracy) [21]. Moderate accuracy has been suggested to be indicated by AUC between 0.7–0.9 and low accuracy by AUC 0.5–0.7. An AUC = 0.5 means that the model predicts the outcome randomly. When using the original FLS strategy in our cohort, the AUC was 62.5% which could be interpreted as low accuracy. By modifying the model by including body weight and sex, the AUC was improved to 79.1%, suggesting moderate accuracy. However, it is important to take into consideration that the ROC curve of the original FLS-model includes only one cut-off point (at age 50 years), so the sensitivity and specificity might be better measures to estimate the validity of a diagnostic tool. In terms of sensitivity, the model we suggest is similar to the original FLS-model; however, the specificity was improved resulting in a marked reduction in unnecessary DXAs.
The original FLS strategy comprises men and women above the age of 50 years; however, it could be argued that postmenopausal women below the age of 50 years also should be included. In addition, premenopausal women, despite being above 50 years are at low risk of osteoporosis. From a clinical point of view, we suggest that it is much easier to implement a cut point of age 50 regardless menopausal status. Questioning women about their menopausal status may be inconvenient in the orthopedic setting.
This study has strengths and limitations. The strengths are that the fracture patients are recruited in a real world setting at a single university emergency and trauma center and comprise a homogenous population. The sample size is relatively large for the population above the age of 50 years with comprehensive information about patient characteristics, clinical risk factors, and fracture types. However, at the same time, the sample size is also a limitation as it did not allow us to divide the population into a population used for the development of the strategies and another for testing the strategies.
There are also limitations to the FLS in it self. It is well-known that for example older and fragile patients with comorbidities are often not able to show up for DXA and other procedures [13, 22]. Therefore, when implementing fracture prevention programs special offers of investigation and intervention should be in place for fragile patients at high risk of having osteoporosis.
The original FLS, including low-energy fracture patients above the age of 50 years, has in numerous papers been shown to be cost-effective. We systematically investigated alternative strategies based on risk factors suggested to predict osteoporosis in a cohort of fracture patients and found that the original FLS strategy can be modified to include substantially fewer patients and still only miss the osteoporosis diagnosis in a very small number of patients. This modified strategy would save costs and concerns in many patients unnecessarily confronted with osteoporosis being the underlying cause of their fracture. The suggested strategy (Fig. 3) needs to be verified in an independent population of fracture patients before it can be recommended for implementation.
References
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporosis Int 16:S3–S7
Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD (2006) Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum 35:293–305
Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E (2012) Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner R 27:2039–2046
McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, Adekunle F, Roberts D (2011) Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 22:2083–2098
Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, March L, Seibel MJ (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24:393–406
Åkesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporosis Int 24:2135–2152
Hansen L, Petersen KD, Eriksen SA, Langdahl BL, Eiken PA, Brixen K, Abrahamsen B, Jensen JB, Harsløf T, Vestergaard P (2014) Subsequent fracture rates in a nationwide population-based cohort study with a 10-year perspective. Osteoporos Int 26:513–519
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Cooper C, Mitchell P, Kanis JA (2011) Breaking the fragility fracture cycle. Osteoporos Int 22:2049–2050
Health Government in Denmark (2018) http://www.medstat.dk. Accessed Nov 2018
McLellan AR, Gallacher SJ, Fraser M, McQuillian C (2003) The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 14:1028–1034
Tei RMH, Ramlau-Hansen CH, Plana-Ripoll O, Brink O, Langdahl BL (2018) OFELIA: prevalence of Osteoporosis in Fragility Fracture Patients. Calcif Tissue Int. https://doi.org/10.1007/s00223-018-0476-3
Kanis JA, McCloskey EV, Harvey NC, Johansson H, Leslie WD (2015) Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res 30:1747–1753
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ III, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Tei R, Langdahl B, Brink O, Dreyer P (2019) Screening for underlying osteoporosis in fragility fracture patients: the patients’ perspectives. Open Nurs J 13:3–11
Langdahl BL (2017) Osteoporosis in premenopausal women. Curr Opin Rheumatol 29:410–415
Ellen GHM, van den Heuvel EGHM, Steijns JMjM (2018) Dairy products and bone health: how strong is the scientific evidence? Nutr Res Rev 31:167–178
Favus MJ (1993) Primer on the metabolic bone diseases and disorders of mineral metabolism, In: Harvey N, Dennison E, Cooper C (eds) Raven, New York, pp 348–356
Fischer JE, Bachmann LM, Jaeschke R (2003) A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med 29:1043–1051
Eekman DA, van Helden SH, Huisman AM, Verhaar HJJ, Bultink IEM, Geusens PP, Lips P, Lems WF (2014) Optimizing fracture prevention: the fracture liaison service, an observational study. Osteoporos Int 25:701–709
Acknowledgements
We wish to express our gratitude to all participants in OFELIA for their willingness to make this study possible and to our colleagues at the Department of Orthopaedic Surgery and the Department emergency and acute medicine (FAA) at Aarhus University Hospital (AUH) for identifying fracture patients for this study. Also, many thanks to the staff at The Osteoporosis Clinic, AUH for their great work performing the DXAs.
Funding
For financial support, we wish to thank the Health Research Fund of Central Denmark Region, Aarhus University Hospital (“Spydspidspuljen”), Department of Endocrinology and Internal Medicine, AUH and the Danish Osteoporosis Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RMHT, OP-R, and OB have no conflict of interests. BLL has received honoraria for consulting and lecturing for Amgen, Merck, UCB, and TEVA and received research grants from Amgen and Novo Nordisk. The main author; Tei RMT has full control of all primary data and she agrees to allow the journal to review their data if requested.
Ethical Approval
The study complies with the principles outlined in the Declaration of Helsinki. The study was approved by the Danish Data Protection Agency (J. No. 2007-58-0016). AIn accordance to the OFELIA study all patients gave written informed consent to participate in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tei, R.M.H., Plana-Ripoll, O., Brink, O. et al. An Optimised Fracture Liaison Service Model: Maintained Diagnostic Sensitivity Despite Reduced Number of Diagnostic Tests Performed. Calcif Tissue Int 104, 641–649 (2019). https://doi.org/10.1007/s00223-019-00535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00535-y