Abstract
The longitudinal relationship between bone health and muscle health is scarcely explored. We aimed to explore the relationship between bone decline and muscle decline over 1 year in older individuals. We used data from the SarcoPhAge cohort, which aims to identify the consequences of sarcopenia. In this way, this study also highlights the yearly changes in muscle mass (by dual-energy absorptiometry), muscle weakness (by grip strength), and/or physical performance (by the short physical performance battery test). Measurements of areal bone mineral density (aBMD), enabling the diagnosis of osteoporosis, and bone microarchitecture (by means of the trabecular bone score) were also performed each year. A 1-year clinically relevant decline in bone and muscle health components was evidenced using the Edwards–Nunnally index. Among the 232 participants with complete data (75.5 ± 5.4 years, 57.8% women), we observed an association between a clinically relevant decline in the skeletal muscle mass index (SMI) and a decrease in aBMD (adjusted OR = 2.12 [1.14–2.51] for the spine, 2.42 [1.10–5.34] for the hip and 2.12 [1.04–5.81] for the neck), as well as a significant association between SMI and deterioration of the skeletal microarchitecture (aOR = 3.99 [2.07–7.70]). A clinically relevant decline in muscle strength was associated with a decrease in spine aBMD (aOR = 2.93 [1.21–7.12]) and hip aBMD (aOR = 3.42 [1.37–7.64]) only. The decline in muscle performance was related to the decline in bone microarchitecture only (aOR = 2.52 [1.23–5.17]). Individuals with incident sarcopenia had an approximately fivefold higher risk of concomitantly developing osteoporosis. A dynamic relationship between impaired muscle and bone health was observed, with an obvious association between the concomitant incidences of osteoporosis and sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction between bone and muscle is essential for individuals to move in their environment: muscle activity generates a biomechanical load stimulating adjustment to these strains on bone mass and microarchitecture. Bone and muscle also experience the simultaneous influence of other common factors, such as endocrine function, genetics, and environmental cues, as well as intrinsic and extrinsic health-related determinants [1, 2].
However, the aging process deeply affects the musculoskeletal system. Inevitably, there is a decline in muscle and bone tissue in terms of mass, structure, and function with aging. At a certain threshold of damage, the reductions in bone and muscle tissue are considered pathological, which are known as sarcopenia and osteoporosis, respectively. In our constantly aging population, this phenomenon, which will continue to significantly increase in prevalence [3, 4], requires specific attention from scientific research and health practitioners, given the complications that it engenders, which have an undeniable impact on the quality and quantity of life and, consequently, the burden of illness caused in terms of public health [3, 5].
More specifically, in its complete definition, osteoporosis is a disorder in which a decrease in bone mass and its microstructure is observed, predisposing the individual to an increased risk of fracture [6, 7]. Sarcopenia is a disorder in which muscle mass is diminished, accompanied by impaired muscle strength and physical function [8,9,10,11,12]. It has already been shown that these two conditions can co-exist [13,14,15], with the presence of one increasing the risk of the other by at least twofold [16]. This simultaneous bone and muscle impairment is currently referred to as osteosarcopenia [17], making the older individual even more susceptible to severe adverse health outcomes than each pathological entity considered separately [14]. Notwithstanding some studies exploring the cross-sectional association between osteoporosis and sarcopenia, with a reported risk increase from 2- to 12-fold [16], the prospective association between these two conditions, also taking into account most of the aspects defining them (i.e., mass, quality, and function), seems to be extremely underexplored. The evolution of the muscle and bone systems and the influences they have on each other during the aging process remain as challenging problems, with the question of whether the whole skeletal status indices decline in parallel to those of the muscle system or whether these two phenomena are initiated independently of one another.
Through the SarcoPhAge (for Sarcopenia and Physical Impairment with Advancing Age) study, we have already demonstrated the cross-sectional association between skeletal indices and muscle components, as well as between sarcopenia and osteoporosis [16]. Since then, within this framework, we have sought to examine the longitudinal interrelationships between the decline in skeletal status (i.e., decreased bone mass, altered microstructure, and increased fracture risk) and that of muscle components (i.e., decreased muscle mass, strength, and performance) in a cohort of older subjects, with the ultimate goal of emphasizing the pathways through which age-related musculoskeletal changes occur in order to optimize the prevention of osteosarcopenia and the management of individuals with this disease. Additionally, we also aim to highlight the individual relevance, intrinsic to each participant, of the variation in bone and muscle status over 1 year by using an original method that few have employed in the field, that of the Edwards–Nunnally index. This approach would allow us to determine, at the individual level, a threshold of clinically relevant change.
Methods
Study Population
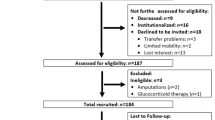
The SarcoPhAge (Sarcopenia and Physical impairment with advancing Age) study, implemented in 2013 in Liège, Belgium, involved the observational follow-up of 534 subjects aged 65 years and older recruited by convenience, both by advertisement and in clinical routine. Other methodological implications of the study were described previously [18]. Originally planned to determine the predictors and consequences of sarcopenia in the long term, this study also investigated, since October 2015, the bone health (i.e., bone mineral density, bone microstructure and fracture risk) of subjects. This baseline year of recruitment yielded 316 subjects for which all bone and muscle data were gathered [16]. We collected cross-sectional data covering a full year, and in October 2017, we obtained one complete year of follow-up data with regard to the evolution of bone parameters in our cohort of older subjects. Between baseline and 1-year follow-up, 84 subjects (26.6%) were not reviewed for various reasons: death (n = 7), physical or cognitive inability to attend the annual follow-up (n = 31), impossible to contact (n = 6), and refusal to participate again (= 40). As a result, this analysis covers a total sample of 232 participants with complete baseline and 1-year follow-up muscle and bone parameter data (Fig. 1).
Investigation of Muscle Health and Diagnosis of Sarcopenia
Dual-energy X-ray absorptiometry (DXA) (Hologic Discovery A, USA) was performed on each participant, ensuring that he/she was not wearing metal, wearing only light cotton clothing, and not wearing shoes. Quality control procedures were performed daily by scanning a spine phantom, according to the manufacturer’s instructions. The completion of this examination allowed us to determine the skeletal muscle mass index (SMI), calculated as the sum of the muscle mass of the four limbs divided by the squared height, indicated in kg/m2. To further analyze muscle health, we also determined handgrip strength using a hydraulic hand-dynamometer (Saehan Corporation, MSD Europe Bvba, Belgium), calibrated yearly. Subjects had to strongly squeeze the device three times with each hand, and the highest measurement was recorded [19]. Then, muscle function was established via an assessment of physical performance through the short physical performance battery (SPPB) test (maximum score of 12 points) [20] examining three skills: balance, 4-m walking speed and chair stand test. A diagnosis of sarcopenia was established on the basis of the criteria proposed by the European Working Group on Sarcopenia in Older People (EWGSOP) [8] when the following criteria were applied: a weak SMI (equal to or less than 5.5 kg/m2 for women, 7.23 kg/m2 for men) accompanied by a low muscle strength (less than 20 kg for women, 30 kg for men) and/or an impaired physical performance (score on the SPPB test equal to or less than 8 points for both men and women).
Investigation of Bone Health and Diagnosis of Osteoporosis
Using DXA technology and subsequent quality procedures, areal bone mineral density (aBMD) was evaluated at three sites, namely, the lumbar spine (L2–L4), total hip, and femoral neck, and is expressed in g/cm2 and as a T-score (sex-specific, derived from the National Health and Nutrition Examination Survey III normative data [21]). From these data, we define osteoporosis as an aBMD T-score equal to or less than − 2.5 for at least one of the three sites [6]. More specifically, the lumbar spine aBMD data allowed us to determine a trabecular bone score (TBS), an index of the bone texture obtained by analysis of the gray-level variation of the DXA image via the TBS iNsight software (Medimaps Group, Geneva, Switzerland, version 3.0.1). A weak TBS value reflects a poorer quality and fracture-prone bone microarchitecture, and inversely, a high TBS value is correlated with an improved and better fracture-resistant bone microstructure [22]. Another indicator of subject bone health is the measurement of his/her fracture risk. To perform this measurement, the FRAX tool, validated for Belgium [23], was applied. This tool consists of an algorithm that takes into account age, sex, clinical risk factor presence, and femoral neck aBMD to determine a 10-year probability of major osteoporotic fracture (MOF) and hip fracture (HF) for each individual.
Confounder Collection
At each annual interview, a set of parameters that could influence the musculoskeletal health of subjects was collected, including the following:
-
Body mass index (BMI), calculated as the weight (to the nearest 0.1 kg) divided by the height (to the nearest 0.1 mm) squared (kg/m2), measured by means of a standardized instrument that was calibrated regularly.
-
Number of conditions that the subjects were affected by and number of drugs consumed, self-reported by each individual.
-
Nutritional status was highlighted by the Mini-Nutritional Assessment [24]. The evaluation used a 30-point scale. Subjects were classified as well-nourished if their score was 24 points or more; those with scores between 23.5 and 17 points were classified as at risk of malnutrition; and those with scores below 17 points were classified as malnourished.
-
Cognitive function was assessed with the Mini-Mental State Examination (MMSE) [25], which consists of a 30-point questionnaire. A maximum score of 30 signifies no neuropsychological impairment.
-
Level of physical activity based on self-reported time spent on physical exercise in the past 7 days based on the Minnesota scale [26] proposing sex-specific cut-offs.
Statistical Methods
The data were processed using the SPSS Statistics 24 (IBM Corporation, Armonk, NY, USA) software package. First, descriptive statistics were used to describe the population of older subjects with regard to their main characteristics, with the results expressed as the mean ± standard deviation (SD) for continuous variables that followed a Gaussian distribution (as checked by examining the histogram, the quantile–quantile plot, the Shapiro–Wilk test, and the difference between the mean and the median). Absolute and relative (%) frequencies were described for qualitative variables. Annual change in the amount of each muscle and bone component was calculated between baseline and 1 year of follow-up and is expressed as the mean percentage of change. To determine the magnitude of the association between the percentage of losses of bone and muscle function, Pearson’s correlation coefficient was calculated for parametric data. Multivariable regression models were constructed to analyze the independent contribution of a factor on muscle status variables, yielding a β coefficient with standard error (SE). Next, we wanted to highlight changes in bone and muscle health, which are particularly meaningful in the clinic. Therefore, we used the notion of clinically significant change, measured by the Edwards–Nunnally (EN) index [27], as a method for computing clinically significant improvement and deterioration rates. We focus here on the degree of change in the health status of each individual patient rather than statistical comparisons of averages between conventional groups. This approach represents a considerable value in research because it bridges the gap between clinical research and clinical practice. Specifically, this is an index of regression to the mean by inserting an adjustment that minimizes the risk of exchange rates being factitiously bloated by regression to the sample mean. The formula of the applied EN is as follows:
where xpre is an individual’s score at pretest; µpre is the mean of the whole sample at pretest; σpre is the standard deviation of the whole sample at pretest; and rxx is the reliability of the measure.
More specifically, reliability measures were 0.990 for BMD and TBS assessment [28], 0.486 for MOF probability [29], 0.469 for HF probability [29], 0.997 for lean mass assessment [30], 0.976 for grip strength evaluation [31], 0.870 for the SPPB, and 0.750 for chair and stand test [32].
If the score obtained during the posttest is outside the two bounds of the confidence interval, then a clinically significant change will be noted. Because scientific literature has taught us that there were gender differences regarding age-associated bone and muscle mass changes [33], we have applied this EN index sex-specifically, and thus performed analyzes on men and women separately, before grouping them together. For categorical variables such as the SPPB test, this technique was not applicable. Therefore, scientific literature was used, and a clinically relevant decline in physical performance was defined as a loss of one unit over 1 year [34]. Chi-squared tests were applied for group comparisons of binary variables (i.e., clinically significant decline present or not). A stepwise binary logistic regression prediction model was developed to determine the influence of cofactors on a dependent binary variable (i.e., the presence or absence of a clinically relevant decline), and an odds ratio (OR) with a 95% confidence interval (CI) was calculated. These covariates were included in the multivariable model because they significantly differed between groups in the univariate analysis for at least one of the bone and muscle measurements (Model 1). Then, in view of the results obtained below, an additional adjustment (Model 2) was made taking into account the incidence or not of falls and fractures (Model 2).
Finally, we decided to determine the incidence (in percentages) of the pathological versant of muscle and bone decline and, subsequently, the association between the incidence of sarcopenia and osteoporosis by comparing the incidences in the two groups using Chi-squared tests.
Regarding all statistical stages of this work, adjustments were computed for covariates known to significantly impact muscle and bone health, including age, sex when applicable, BMI, comorbidity number, coprescription number, nutritional and cognitive status, and physical activity level. The overall results were statistically significant at the 0.05 critical threshold.
Results
Clinical Characteristics of Participants
After a 1-year follow-up, 232 older participants were interviewed a second time. The mean follow-up time between the two visits was 377 (± 44.9) days. They had a mean age of 75.5 (± 5.4) years, and the sample was 57.8% women. A summary of the baseline characteristics is available in Table 1. The most prevalent concomitant diseases were osteoarthritis (42.4%) and high blood pressure (20.6%). The medication was mainly used for indications concerning the nervous system (46.5%), the osteoarticular system (45.2%), as well as the cardiovascular system (40.9%). The general characteristics of participants interviewed after 1 year (n = 232) did not differ significantly from those of non-interviewees after 1 year (n = 84), all p values being greater than 0.05 (Supplementary Material).
Regarding the bone and muscle parameters, Table 2 provided the absolute values for each component at baseline and at follow-up as well as the percentage of annual change in these components. Furthermore, with the use of the EN index, we were able to determine that between 12.5% (lumbar spine aBMD) and 22.8% (TBS value) of the whole sample presented a clinically relevant decline over 1 year of at least one bone parameter and between 17.2% (grip strength) and 32.3% (SMI) presented a clinically relevant decline in muscle health.
Association Between the Mean Annual Percentage Change in Bone Health and That in Muscle Components
When we examined the association between the annual percentage change in skeletal status and the annual percentage change in muscle components, we observed that the annual percentage of 1-year decline in muscle mass was significantly correlated with the annual percentage of TBS value decline (r = 0.27, p value < 0.05), which remained statistically significant after adjustment for covariates (β(SE) = 0.20 (0.11), p value = 0.02). However, we did not observe any association between the annual percentage change in muscle mass and the annual change in aBMD values or the risk of fracture (all p values > 0.05). When performing the analysis in men and women separately, we could highlight an additional association: whereas the first findings remained in the same range when analyzed in men and women for association with the annual percentage change of TBS (i.e., coefficient correlation r around 0.27), and in women, the annual percentage change in muscle mass was also significantly correlated with the percentage change in hip aBMD (r = 0.26, p value = 0.02), remaining significant in multivariate analysis (β(SE) = 0.34 (0.40), p value = 0.04).
We also reported significant associations between the annual percentage of change in grip strength and that of hip aBMD (r = 0.24, p value < 0.05) and femoral neck aBMD (r = 0.37, p value < 0.05), which were still present after adjustments (β(SE) = 0.17 (0.04), p value = 0.04 and β(SE) = 0.21 (0.10), p value = 0.04, respectively). No association between muscle strength and risk of fracture or bone microstructure was detected (all p values > 0.05). Again, a sex-specific analysis has brought us a new element: in women, the annual percentage change in grip strength is also associated with the annual percentage change in TBS (r = 0.31, p value = 0.02), remaining significant after adjustment (β(SE) = 0.19 (0.13), p value = 0.03).
The annual percentage change in physical performance had a negative and significant correlation with the annual percentage change in the 10-year MOF probability (r = − 0.24, p value < 0.05). Multivariate analysis confirmed the significant influence of the change in physical performance on the 10-year MOF probability variation (β(SE) = − 0.21 (0.02), p value = 0.04). It should be noted that no significant association was established for the decline in physical performance with that of the aBMD values (all p values > 0.05). However, when analyzed sex-specifically, we found that the annual percentage change in physical performance in men was also associated with the annual percentage change in hip and femoral neck aBMD (respectively, r = 0.44, p value = 0.003 and r = 0.54, p value < 0.001, all results remaining significant in multivariate analysis).
Association Between the Relevant Decline of Bone Health and That of Each Muscle Component
We found that 75 subjects (32.3%) showed a clinically significant decline in their SMI, 40 (17.2%) presented a muscle strength decline, and 49 (21.1%) had a clinically relevant decline in physical performance. We therefore investigated whether this clinically significant decline in muscle function was associated with a parallel deterioration of skeletal status. The results are detailed in Table 3. We have described that when a subject has a decline in muscle mass (i.e., SMI), he/she has a 2.07 (1.05–4.09)-fold (MOF probability) to 3.99 (2.07–7.70)-fold (for TBS value) higher risk of presenting a decline in bone status in terms of bone mass, its microarchitecture, and the risk of fracture, even after full adjustment. In addition, individuals showing a significant decline in muscle strength have a significantly increased risk, after adjustment, of concomitantly presenting a decline in bone mass (Model 1 adjusted OR = 2.93 (1.21–7.12) for lumbar spine aBMD and Model 1 OR = 3.42 (1.37–7.64) for femoral neck aBMD). Finally, a clinically relevant decline in the physical function of individuals is associated, on the one hand, with a significant increase in fracture risk (Model 1 adjusted OR = 3.10 (1.29–7.44) for HF and Model 1 adjusted OR = 2.93 (1.37–6.25) for MOF) and, on the other hand, with a decline of bone microarchitecture (Model 1 adjusted OR = 2.52 (1.32–5.71)).
We thus noted that a large proportion of individuals showed a clinically relevant decline in muscle and bone health. We therefore investigated whether this could be related to a morbidity event. We found that a clinically relevant decline in physical performance was significantly associated with the occurrence of falls during the year of follow-up: 28 out of 49 subjects (57.1%) with a decline in performance experienced a fall, while 74 out of 183 subjects (40.4%) with no decline experienced a fall (p value = 0.04). In addition, a clinically relevant decline in lumbar spine aBMD was significantly associated with the occurrence of fractures: 6 out of 29 subjects (20.7%) with a decline in lumbar spine aBMD experienced a fracture in the year of follow-up compared to 16 out of 187 subjects (8.7%) with no decline which experienced a fracture (p value = 0.03). Based on these results, further adjustments (Model 2) were performed in order to see if the associations found between muscle and bone persist or not (Table 3). In most situations, we confirmed the results obtained using Model 1, with some associations even increasing in magnitude. However, we did encounter a case where the association was no longer significant: the relationship between the clinically relevant decline between muscle mass decline and lumbar spine aBMD decline (Model 2 adjusted OR = 2.16 [0.97–4.79]). We also found three new associations when we adjust for the incidence of falls and fractures (as binary variables): the association between the clinically relevant grip strength decline was then significantly associated with the clinically relevant femoral decline. aBMD (Model 2, OR = 2.74 [1.15–6.52]), as well as the decline in physical performance, which was then significantly associated with the clinically relevant decline in hip and femoral aBMD (respectively, Model 2 adjusted OR = 2.91 [1.31–6.46] and OR = 2.33 [1.07–5.31]).
Association Between Sarcopenia Incidence and Osteoporosis Incidence
A detailed description of baseline and 1-year follow-up prevalence of osteoporosis and sarcopenia, as well as their incidence, is available in Table 4. Within 1 year, 13 subjects (5.6%) became osteoporotic, and 16 became sarcopenic (6.9%). Individuals who became sarcopenic were significantly more likely to present concomitant osteoporosis compared to the sample who did not experience the occurrence of sarcopenia (incidence of osteoporosis amounting to 3 of 16 sarcopenic individuals (18.7%) versus the incidence of osteoporosis amounting to 10 of 216 non-sarcopenic patients (4.6%), p value < 0.001). Individuals with sarcopenia have a 4.75-fold higher risk of developing osteoporosis concomitantly: OR = 4.75 (1.16–19.41).
Discussion
Through this original study, we have provided further evidence that each muscle component that changed with advancing age was significantly associated with at least one change in bone parameters. This result shows a dynamic interaction between the two musculoskeletal slopes. This finding is true from a statistical point of view, but our research further demonstrates that, from a clinical view, with the use of the Edwards–Nunnally index, the interrelationships are even more numerous, marked, and relevant.
When we study our results in more detail, we first describe a positive longitudinal relationship between muscle and bone mass decline (adjusted ORs up to 2.1), as well as an increase in the risk of fracture (OR = 2.07). This was an anticipated result, which is in line with other longitudinal studies [33]. A decline in muscle mass seems to also be related to the TBS value, which is likely to reflect bone quality (OR = 3.99). TBS is not a direct measurement of bone architecture, but it is related to three-dimensional bone characteristics such as the trabecular number, the trabecular separation, and the connectivity density [35]. This relationship was previously demonstrated in a cross-sectional study in older individuals [16] and through a longitudinal design study in children [36] where bone microstructure was assessed using peripheral quantitative computed tomography. This observation is supported by the hypothesis of hormonal interaction between muscular quantity and quality of bone [37].
A second component of muscle health was prospectively assessed in our study: the decline in muscle strength was correlated with the decline in bone mass. This correlation has already been demonstrated in another study [33], where the rate of loss in leg muscle strength was significantly related (r = 0.25) to the rate of aBMD loss. Our results have an added value by also highlighting the change at the individual level: we showed a significant association between a clinically relevant decline in the two components in the same individuals (ORs > 2.90). These observations may be explained by multiple factors (mechanical, endocrine, genetic, etc.), and it could be hypothesized that improvement of muscle strength could possibly be protective against bone mass decline. However, care should be taken regarding findings related to handgrip strength, bearing in mind that this measure, taken alone, does not appear to totally reflect overall muscle strength [38].
Finally, the last muscle component assessed in our study was physical performance. Our results also highlight the well-known link between a decline in muscle physical function and an increased risk of fracture [39] (OR = 2.93 [1.37–6.25]). This close relationship could be explained by a mechanical link, that is, the structural strength of the bone adapting to the physical activity of subjects [40]. We also observed a parallel evolution between bone texture and physical performance (OR = 2.52 [1.23–5.17]). This association has already been proven crosswise [16, 41], reporting poor bone microstructure in subjects with impaired physical performance. Through our study, we extended these previous findings by incorporating a longitudinal and clinically relevant angle. By performing additional adjustments (i.e., incidence of falls and fractures as covariates), we also found that a clinically relevant decline in physical performance was significantly associated with a relevant decline in hip and neck aBMD. These results were not surprising starting from the fact that physical performance is related to the history of falls and fractures [42]. This phenomenon of association between physical performance decline and bone density decline seems to explain that physical activity generates muscle contraction on the adjacent bone surface, thus contributing to bone remodeling [42].
Overall, we also noted some differences in results when we analyzed the results separately between men and women. This does not seem surprising. Indeed, it appears that age-related changes in body composition are not progressing at the same rate in both sexes, according to a recent study [33].
Regarding the pathological aspect of poor muscle and bone health, we also established, despite the limited number of cases, that the occurrence of sarcopenia was significantly associated with the incidence of osteoporosis: participants with sarcopenia have a 4.75-fold higher risk of developing osteoporosis concomitantly. A recent meta-analysis had already shown that the association of their prevalence is evident [43], but to our knowledge, there is no study on the concomitant occurrence of these pathologies. This study brings a wealth of evidence to the existing evidence (e.g., genetic, hormonal, and mechanical factors) to support the hypothesis of a muscle–bone entity, whose progressive decline accompanying advancing age is known as “osteosarcopenia.” This kind of results is not, in the end—not so surprising. More and more evidence now proves the existence of a bone–muscle crosstalk. A recent review of the literature [44] has shown, more specifically during the aging process, the importance of the “mechanostat theory”: the bone adapts, from a morphological point of view, to mechanical load exercise by the muscle during its mobilization. However, this review showed that the relationship is also to look further: bone and muscle are also loosened by their adjacent surface [45], and, more specifically, there appear to exist muscle factors acting on bone formation and vice-versa. A molecular signaling between bone and muscle, going in both directions [44, 46] actually seem to allow communication between these two tissues, by the action of autocrine, paracrine, and endocrine mediators to coordinate their development [45]. Indeed, the endocrine function involves, for example, osteocalcin and osteoblast-derived protein, which could act specifically on the regulation of muscle mass [45]. In addition, together, molecules released by muscle secretome affect bone such as insulin-like growth factor-1, myostatin, and interleukin-6 [47]. Furthermore, the influence of paracrine on bone-muscle crosstalk is objectified by its localization on the muscle fiber insertion along periosteal interfaces [44].
Finding these numerous associations, at the group and individual levels, between each component of muscle and bone decline could pave the way for the development of a unique preventive and therapeutic treatment, targeting both structures simultaneously, aiming to drastically reduce impaired mobility, decline, and fractures. These results provide important insight into the parallel evolution and therefore have clinical implications. They indeed suggest an important role of good muscle health (with respect to its qualitative, quantitative, and functional aspects) in order to prevent the concomitant decline of skeletal status, thus hoping to decrease the risk of fracture. For example, a preclinical study already reported that dynamic muscle stimulation induced attenuation of bone loss and changes in trabecular bone microarchitecture [48]. A screening for sarcopenia during osteoporosis diagnosis could provide real added value and would therefore result in prediction of information that is instrumental to the clinical monitoring of bone mass.
We acknowledge some limitations of this study, and the interpretation of our results should therefore be done in this context. First, we adopted a convenience sample for this study, and thus, our sample may not be representative of the entire population over 65 years of age. Then, 26.6% of the baseline population was not interviewed again after 1 year of follow-up. A selection bias may therefore be present, as individuals presenting for maintenance are likely to be in better health because they are able to move, although the general characteristics of the participants interviewed after 1 year do not differ significantly from those who did not show up after 1 year. Furthermore, from this point on, the associations observed in our study were probably lessened. Moreover, the follow-up period was short. A longer follow-up duration would allow more statistical power. However, after 1 year, we already observe relevant associations, statistically and clinically, and are adding value in the field. Additionally, the accuracy of the technique used to measure body composition may impact the actual change detected. However, the DXA device shows good reproducibility even though a change in bone mineral density can often be detected after approximately 2 years [49]. This is also one reason why we recalculated the change in body composition using the EN index, which allows us to determine a clinically relevant decline at the individual level. Concerning this EN index, we chose this statistical technique by dichotomizing the information (i.e., decline: yes or no). There is then a loss of information with this approach. However, this technique is adapted to our data and our objectives and has proved its relevance from a clinical point of view. By binarizing the data through the EN index, the associations found were sometimes heightened with respect to the manipulation of quantitative data via correlations. This may seem counterintuitive, but, in the end, it is not so surprising: if, from a global point of view, at the level of the entire population, the indices of muscle and bone health did not seem to decrease considerably in 1 year, the decline felt at the individual level was more significant. Indeed, in clinical practice, many patients feel, at their individual level, a decline in their own health, but, relativized by the collective level, no longer seems significant for the clinician. It is therefore important to have a collective and individual vision of subjects’ health to allow optimal care. Finally, it is well known that the musculoskeletal system is influenced by a multitude of factors, such as hormonal, environmental, and behavioral factors. Therefore, the relationship between the decline in bone health and the decline in muscle components may be confounded by such potentially important factors (e.g., sex-hormone levels, calcium intake, 25OHD level, and inflammatory markers), which we did not have the opportunity to thoroughly investigate in this study. However, all our results take into account (and were adjusted for) a very large number of other known covariates.
In conclusion, a dynamic interaction was found between each component of bone and muscle health accompanying advancing age. We have shown that a decline in muscle function could be predicted by a deterioration of bone health indices, and vice-versa. Our results provide an additional substantial body of evidence for a pathological pathway in which bone and muscle entities are synchronized in the aging process. It is necessary to develop a better understanding of identifying and preventing these two diseases.
References
Cianferotti L, Brandi ML (2014) Muscle–bone interactions: basic and clinical aspects. Endocrine 45:165–177. https://doi.org/10.1007/s12020-013-0026-8
Gielen E, Bergmann P, Bruyère O et al (2017) Osteoporosis in frail patients: a consensus paper of the Belgian bone club. Calcif Tissue Int. https://doi.org/10.1007/s00223-017-0266-3
Reginster J-Y, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38:4–9. https://doi.org/10.1016/j.bone.2005.11.024
Ethgen O, Beaudart C, Buckinx F et al (2017) The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int 100:229–234. https://doi.org/10.1007/s00223-016-0220-9
Bruyère O, Beaudart C, Locquet M et al (2016) Sarcopenia as a public health problem. Eur Geriatr Med. https://doi.org/10.1016/j.eurger.2015.12.002
Kanis JA, McCloskey EV, Harvey NC et al (2015) Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res 30:1747–1753. https://doi.org/10.1002/jbmr.2531
Kanis JA, Cooper C, Rizzoli R et al (2017) Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28:2023–2034. https://doi.org/10.1007/s00198-017-4009-0
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Chen L-K, Liu L-K, Woo J et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A 69:547–558. https://doi.org/10.1093/gerona/glu010
Morley JE, Abbatecola AM, Argiles JM et al (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12:403–409. https://doi.org/10.1016/j.jamda.2011.04.014
Marzetti E, Calvani R, Tosato M et al (2017) Sarcopenia: an overview. Aging Clin Exp Res 29:11–17. https://doi.org/10.1007/s40520-016-0704-5
Locquet M, Beaudart C, Reginster J-Y et al (2017) Prevalence of concomitant bone and muscle wasting in elderly women from the SarcoPhAge cohort: preliminary results. J Frailty Aging 6:18–23. https://doi.org/10.14283/jfa.2016.111
Paintin J, Cooper C, Dennison E (2018) Osteosarcopenia. Br J Hosp Med 79:253–258. https://doi.org/10.12968/hmed.2018.79.5.253
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790. https://doi.org/10.1007/s00198-017-4151-8
Locquet M, Beaudart C, Bruyère O et al (2018) Bone health assessment in older people with or without muscle health impairment. Osteoporos Int 29:1057–1067. https://doi.org/10.1007/s00198-018-4384-1
Tarantino U, Baldi J, Celi M et al (2013) Osteoporosis and sarcopenia: the connections. Aging Clin Exp Res 25:93–95. https://doi.org/10.1007/s40520-013-0097-7
Beaudart C, Reginster JY, Petermans J et al (2015) Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol 69:103–110. https://doi.org/10.1016/j.exger.2015.05.003
Roberts HC, Denison HJ, Martin HJ et al (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429. https://doi.org/10.1093/ageing/afr051
Guralnik JM, Ferrucci L, Pieper CF et al (2000) Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A 55:M221–M231
Wright NC, Looker AC, Saag KG et al (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29:2520–2526. https://doi.org/10.1002/jbmr.2269
Harvey NC, Glüer CC, Binkley N et al (2015) Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78:216–224. https://doi.org/10.1016/j.bone.2015.05.016
Johansson H, Kanis JA, McCloskey EV et al (2011) A FRAX® model for the assessment of fracture probability in Belgium. Osteoporos Int 22:453–461. https://doi.org/10.1007/s00198-010-1218-1
Guigoz Y, Vellas B, Garry PJ (2009) Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev 54:S59–S65. https://doi.org/10.1111/j.1753-4887.1996.tb03793.x
Tombaugh TN, McIntyre NJ (1992) The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40:922–935
Elosua R, Marrugat J, Molina L et al (1994) Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. Am J Epidemiol 139:1197–1209. https://doi.org/10.1093/oxfordjournals.aje.a116966
Speer DC (1992) Clinically significant change: Jacobson and Truax (1991) revisited. J Consult Clin Psychol 60:402–408
Lodder MC, Lems WF, Ader HJ et al (2004) Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis 63:285–289. https://doi.org/10.1136/ARD.2002.005678
Imerci A, Yalın Kılınç C, Aydogan NH et al (2017) Fracture Risk Assessment tool (FRAX®) results calculated with and without bone mineral density values for the evaluation of fracture risk in postmenopausal women with osteopenia. J Clin Densitom. https://doi.org/10.1016/j.jocd.2017.06.027
Moreira OC, Oliveira CEP de, De Paz JA (2018) Dual energy X-ray absorptiometry (DXA) reliability and intraobserver reproducibility for segmental body composition measuring. Nutr Hosp 35:340–345. https://doi.org/10.20960/nh.1295
Savva C, Giakas G, Efstathiou M, Karagiannis C (2014) Test-retest reliability of handgrip strength measurement using a hydraulic hand dynamometer in patients with cervical radiculopathy. J Manipulative Physiol Ther 37:206–210. https://doi.org/10.1016/j.jmpt.2014.02.001
Gómez JF, Curcio C-L, Alvarado B et al (2013) Validity and reliability of the short physical performance battery (SPPB): a pilot study on mobility in the Colombian Andes. Colomb Med 44:165–171
Kim KM, Lim S, Oh TJ et al (2018) Longitudinal changes in muscle mass and strength, and bone mass in older adults: gender-specific associations between muscle and bone losses. J Gerontol A 73:1062–1069. https://doi.org/10.1093/gerona/glx188
Kwon S, Perera S, Pahor M et al (2009) What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 13:538–544
Hans D, Goertzen AL, Krieg M-A, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769. https://doi.org/10.1002/jbmr.499
Moon RJ, Cole ZA, Crozier SR et al (2015) Longitudinal changes in lean mass predict pQCT measures of tibial geometry and mineralisation at 6–7 years. Bone 75:105–110. https://doi.org/10.1016/j.bone.2015.02.015
Elkasrawy MN, Hamrick MW (2010) Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 10:56–63
Yeung SSY, Reijnierse EM, Trappenburg MC et al (2018) Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc 19:703–709. https://doi.org/10.1016/j.jamda.2018.04.019
Harvey NC, Odén A, Orwoll E et al (2018) Measures of physical performance and muscle strength as predictors of fracture risk independent of FRAX, falls and BMD: a meta-analysis of the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. https://doi.org/10.1002/jbmr.3556
Russo CR, MD (2009) The effects of exercise on bone. Basic concepts and implications for the prevention of fractures. Clin Cases Miner Bone Metab 6:223–228
Wagner P, Chapurlat R, Ecochard R, Szulc P (2018) Low muscle strength and mass is associated with the accelerated decline of bone microarchitecture at the distal radius in older men: the prospective STRAMBO study. J Bone Miner Res. https://doi.org/10.1002/jbmr.3456
Khazzani H, Allali F, Bennani L et al (2009) The relationship between physical performance measures, bone mineral density, falls, and the risk of peripheral fracture: a cross-sectional analysis. BMC Public Health 9:297. https://doi.org/10.1186/1471-2458-9-297
Nielsen BR, Abdulla J, Andersen HE et al (2018) Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med 9:419–434. https://doi.org/10.1007/s41999-018-0079-6
Picca A, Calvani R, Manes-Gravina E et al (2018) Bone-muscle crosstalk: unraveling new therapeutic targets for osteoporosis. Curr Pharm Des 23:6256–6263. https://doi.org/10.2174/1381612823666170526112300
Reginster J-Y, Beaudart C, Buckinx F, Bruyère O (2016) Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care 19:31–36. https://doi.org/10.1097/MCO.0000000000000230
Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481:314–320. https://doi.org/10.1038/nature10763
Tagliaferri C, Wittrant Y, Davicco M-J et al (2015) Muscle and bone, two interconnected tissues. Ageing Res Rev 21:55–70. https://doi.org/10.1016/j.arr.2015.03.002
Lam H, Qin Y-X (2008) The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. https://doi.org/10.1016/j.bone.2008.07.253
Bonnick SL (2008) Monitoring changes in bone density. Women’s Health 4:89–97. https://doi.org/10.2217/17455057.4.1.89
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Médéa Locquet, Charlotte Beaudart, Jean-Yves Reginster, and Olivier Bruyère declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The study protocol and related amendments received approval from the competent ethics authority of our institution (under reference 2012/277). All procedures were in accordance with the 1964 Helsinki declaration and its later amendments. Prior to the collection of baseline measurements and at each additional test added, the participants were rigorously informed about the study objectives and procedures, after which they provided informed written consent to the protocol itself and its amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Locquet, M., Beaudart, C., Reginster, JY. et al. Association Between the Decline in Muscle Health and the Decline in Bone Health in Older Individuals from the SarcoPhAge Cohort. Calcif Tissue Int 104, 273–284 (2019). https://doi.org/10.1007/s00223-018-0503-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0503-4