Abstract
Purpose
Declines in muscle mass and function are inevitable during the aging process. However, what is the “normal age appropriate” decline of muscle mass and function? Further, is this decline uniform for muscle mass versus functions or between different functional abilities? Using recognized Sarcopenia criteria [i.e. skeletal muscle mass index (SMI) defined as appendicular skeletal muscle mass/height (kg/m2), handgrip strength, gait velocity], the aim of the present project was to determine corresponding changes in community-dwelling men 70 years+ with low SMI over a 2-year period.
Methods
One hundred and seventy-seven (177) men within the lowest SMI quartile of a recent epidemiologic study (n = 965) were included in the 2-year follow-up analysis. Muscle mass was determined via direct-segmental, multi-frequency Bio-Impedance-Analysis, handgrip strength was tested with a Jamar hand-dynamometer and habitual gait speed was assessed with photo sensors applying the 10 m protocol.
Results
SMI, handgrip strength and gait velocity all declined significantly ( P< 0.001; effect size, d′ 0.39–1.17), however, with significantly higher reductions (P< 0.001) in functional compared with morphologic Sarcopenia criteria (P ≤ 0.006). Less expected, handgrip strength featured a fourfold higher decline compared with gait velocity (− 12.8 ± 10.9% versus − 3.5 ± 9.0%).
Conclusion
We provided evidence for significant non-uniform changes of Sarcopenia criteria in a cohort of community dwelling men 70 years+ with low SMI. We doubt that this result might be a particularity of the selected cohort; however, studies with other (older) cohorts should address this issue in more depth. Of practical relevance, our data further give implications for the prioritization of interventions that address Sarcopenia criteria in older community-dwelling men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia, characterized as the loss of muscle mass and function [13, 16, 64], was recently included in the international classification of diseases (ICD)-10 as a clinical condition (M62.84). However, declines in muscle mass and function are inevitable during the adulthood aging process. Further, what is the “normal age appropriate” decline of muscle mass and function? A large number of studies focus on this issue. Cross-sectional studies that determined average reduction of muscle mass or, more precisely, fat-free mass (FFM) across adulthood reported declines in the range of around 0.3–0.5% per annum (p.a.) in men [15, 49]. However, data vary “dramatically” [48] between studies depending on age ranges [37, 38] and regions of interest assessed.Footnote 1 Addressing Caucasian men 70 years+, the more reliable longitudinal studies that determined changes of FFM [14, 21, 35] reported reductions of 0.5–0.8% p.a. Although the relevance of morphometric muscle parameters for healthy aging might per se be consistently underestimated,Footnote 2 functional aspects are indisputably more important for older people’s well-being and independent living.
Defining muscle function “as a muscle’s ability to produce force and (joint) motion”, all recent Sarcopenia approaches [10, 13, 16, 31, 64] included “motion” (i.e. gait velocity”), “force” (i.e. grip strength) or both criteria in their Sarcopenia definition. Addressing strength, the few data that focus on this issue in men [2, 19, 24, 40, 44] reported an accelerated decline after the mid/late 1950s that is much more pronounced [14, 21, 35] than the decline in muscle mass. Further, and comparable to FFM, maximum strength reductions of the lower limbs were much higher compared with upper limbs [4, 14, 18, 39, 66]. There is considerable evidence that lower leg strength predicts gait speed in older people [53]. This aspect might contribute to the accelerated and very steep decline in gait speed in men 80–90 years [mean value (MV) 0.97 m/s; 0.83–1.10 m/s] compared with men 70–79 years old (MV 1.26 m/s; 1.21–1.32 m/s) reported by a descriptive meta-analysis [6]. Hence, although there is a consensus that functional and morphological criteria—included in the present Sarcopenia definitions—decline significantly with advanced age, their relative contribution in the development of Sarcopenia is unclear. Considering the highly relevant effect of muscle mass and function on healthy aging in older people [34, 41, 59], it is important to clearly determine age-dependent, potentially non-uniform changes of muscle mass and function in populations with or without increased risk for sarcopenia. Corresponding data might allow the physician to provide more specific preventive measures at an early stage of the decline of muscle mass and/or function. The aim of the present study was thus to quantify changes of muscle mass, handgrip strength and gait velocity over 24 months in community dwelling men 70 years+. In order to include a clinically relevant group, only men with a low skeletal muscle mass index were included.
Based on the present literature, our primary hypothesis was that the Sarcopenia-Z-Score will deteriorates significantly, with a significantly more prominent decline in functional compared with morphologic Sarcopenia criteria. Our secondary hypothesis was that the decline in gait velocity was significantly more pronounced compared with handgrip strength.
Methods
Study Design
This contribution is part of the ongoing Franconian Osteopenia and Sarcopenia Trial (FrOST) project. This 2 year observational study was initially based on epidemiologic data of the Franconian Sarcopenic Obesity study (FranSO) [29]. The present project focused on community dwelling men in the lowest quartile of skeletal muscle mass index as determined by the approach of the European Working Group on Sarcopenia in Older People (EWGSOP) [13] and aimed to determine changes of Sarcopenia in this cohort at risk. The Institute of Medical Physics and the Institute of Biomedicine of Aging, University of Erlangen-Nürnberg (FAU), Germany, initiated the project, which was approved by the University Ethics Committee of the FAU (Ethikantrag 67_15b and 4464b). After receiving detailed information, all study participants gave written informed consent. The Study was conducted in accordance with the HELSINKI declaration.
Setting and Participants
The FranSO-study recruited community-dwelling men 70 years and older living in the area of Erlangen/Nürnberg, Germany using citizen registers provided by the municipality. Altogether 965 men were eligible and were assessed between February and April 2016 for parameters that focus on Sarcopenia, obesity, physical functioning and disability. Details of the recruitment and eligibility assessments have been previously published [29].
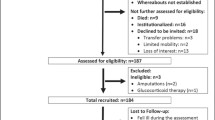
Two years later, in January/February 2018, men in the lowest SMIFootnote 3 quartile of FranSO (n = 242) were contacted by personal letters and subsequent phone calls. Of importance, we were unable to determine the status of 12 men who could not be contacted, although citizen registers and obituary columns were carefully checked (Fig. 1). Another 16 men were institutionalized, nine participants died during the 2-year observation period and 18 males refused to participate (Fig. 1). After application of the follow-up eligibility criteria: (1) community-dwelling men 72 years+; (2) no amputations of limbs or cardiac pacemaker implants during the last 2 years; (3) no new implementation of glucocorticoid therapy > 7.5 mg/day during the last 2 years; and (4) any cognitive impairments that confound assessment, 184 men were invited to the follow-up assessment. However, three men were unable to conduct the assessments due to acute diseases. A further four men initially willing to participate did not appear for their assessment dates although several appointments were made. Correspondingly, 177 out of 242 men (73%) in the lowest quartile of the FranSO study and with complete data sets were finally included in the analysis (Fig. 1). Of importance we did not observe differences for baseline values listed in Table 1 between participants included (n = 177) and men not included in the 2-year follow-up analysis. However, the 12 untraceable men were significantly older, had a significant less favorable Z-Score and suffered from more diseases compared with the cohort included (n = 177).
Table 1 gives baseline characteristics of this cohort within the lowest SMI quartile compared with the remaining part of the FranSO study.
Outcomes
Primary Outcome
-
Changes of Sarcopenia Z-Score applying the EWGSOP-I approach [13].
Secondary Outcome
-
Changes of Sarcopenia criteria constituting the Sarcopenia Z-Score i.e.
-
Changes of skeletal muscle mass index (SMI)
-
Changes of habitual gait velocity
-
Changes of handgrip strength.
-
Measurements
Baseline and follow-up assessments were performed using the identical calibrated devices, in exactly the same setting and at the same time of the day (± 90 min). However, research assistants who guided and supervised the tests were not consistently identical between baseline and follow-up.
The Sarcopenia Z-Score according to the EWGSOP-I approach included SMI, gait velocity and handgrip strength. Cut-off values applied were 0.8 m/s for gait velocity and 30 kg for handgrip strength. However, divergent from the cut off value for skeletal muscle mass index (SMI) suggested by the EWGSOP for BIA assessments [11, 26, 27]; we applied the “Weißenfels Score” (7.177 kg/m2),Footnote 4 specifically evaluated for this northern Bavarian cohort of community dwelling men 70 years+ [29]. Based on the corresponding cut offs and individual data, the Sarcopenia Z-Score was calculated:
Z = [(30 − individual handgrip strength)/SD handgrip strength] + [(0.8 − individual gait velocity)/SD gait velocity] + [(7.177 − individual SMI)/SD SMI].
Body mass and composition were determined via direct-segmental, multi-frequency Bio-Impedance-Analysis (InBody 770, Seoul, South Korea) applying standard protocols. Lean body mass was defined as fat-free body mass. Comparable to the calculation of the BMI (i.e. body mass/height2; kg/m2) and following the approach suggested by Baumgartner [5], SMI was calculated as fat free mass of the upper and lower extremities (= appendicular skeletal muscle mass) divided by square body height (kg/m2). Using the appendicular assessment approach, the vast majority of (soft) fat free mass can be considered as muscle mass.
In order to standardize the BIA assessment, we consistently used the same BIA test protocol that includes minor physical activity for 8 h and 15 min of rest in a supine position immediately before the BIA assessment. Further, all participants were provided with written specifications about dos and don’ts including basic nutritional guidance 24 before testing.
A standardized assessment of habitual gait speed [36] was performed using the 10 m protocol recommended for research [57]. Participants started in an upright position 3 m before the first photo sensor (HL 2-31, TagHeuer, La Chaux-de-Fonds, Switzerland), started walking and stopped 2 m after the second photo sensor. Tests were performed wearing regular shoes without any specific walking aids. The standardized instruction to the participants was consistently “walk at a speed just as if you were walking along the street to go to the shops”.
Handgrip strength was tested three times for the dominant and non-dominant hand using a Jamar handgrip dynamometer (Sammons Preston Inc., Bollington, USA). Handgrip width was adjusted individually to participant hand size. Tests were performed while standing upright, arms down by the side [45]. The standardized instruction to the participants was consistently “squeeze as strongly as possible”. The average result of the three trials for the dominant hand was included in the analysis.
General characteristics (e.g. family and educational status, occupational career), medication, diseases and lifestyle (including physical activity and exercise; [32]), falls, injurious falls, fractures, self-rated degree of independence were determined using a standardized questionnaire completed by the participants while visiting our lab. We also used the abridged version of the Late Life Function and Disability Instrument (LLFDI) [46]. Prior to the tests, participants were asked to list their medications and diseases in order to generate completeness and accuracy of the questionnaire. This summary was checked by research assistants in cooperation with the participants before the tests were conducted. During this interaction, degree of independence and autonomy, family status, social network and use of ambulatory nursing services was inquired more specifically. However, in this publication we focus on the effect of increasing age on dedicated Sarcopenia criteria, thus specific outcomes determined by the questionnaires are not addressed.
Risk of Bias
We undertook great efforts to contact people or their relatives who could not be reached by email or personal phone calls. The underlying reason for this approach was the fact that people who have lost their independence or died might have had greater declines of morphologic and functional Sarcopenia parameters compared with men still living in the community.
Statistical Analysis
Based on a statistically (Shapiro–Wilk test) and graphically (Q–Q plots) checked normal distribution, the outcomes presented in Table 1 were reported using mean values (MV) and standard deviation (SD). However, although most variables were normally distributed, we used the more conservative nonparametric Wilcoxon rank test and the Friedman test that is the non-parametric alternative to the one-way ANOVA with repeated measures. Of importance, for the first part of our hypothesis, we analyzed changes (baseline versus 24-month control) of Sarcopenia Z-Score and underlying criteria using the Wilcoxon rank test. In order to check differences between the Sarcopenia criteria we used the relative changes (%). After assessing whether there are changes in the three criteria by a Friedman test, we finally applied Wilcoxon tests that addressed the differences between SMI and gait velocity (primary hypothesis) as well as between gait velocity and handgrip strength (secondary hypothesis). We adjusted primary and secondary study endpoints for multiple testing using the Bonferroni procedure. We specified directed hypotheses, thus a single-tailed test was applied. Differences between baseline and follow-up for dichotomous variables were analyzed using the McNemar’s test. Significance was accepted at P < 0.05. Further, although percentage changes might be adequate to allow the reader to estimate the amount of the differences between the criteria we also calculated Cohens dz′ (i.e. MV/SD) in order to provide effect sizes (ES). Statistical procedures were performed using SPSS Statistics version 25.
Results
Table 1 shows baseline characteristics of the 177 men included in the analysis. At baseline, 10.2% of the cohort was classified as sarcopenic according to EWGSOP [13]. After 2 years, the number of participants with Sarcopenia increased significantly (P< 0.001) to 18.1%. Although the corresponding topic was not addressed by this study, Table 1 demonstrates that differences between the lowest (FrOST baseline cohort) versus the three highest quartiles of SMI of the FranSO-study were significant (all P < 0.001) for body height, body mass, lean body mass and of course SMI. With respect to functional parameters, handgrip strength was significantly lower in the FrOST baseline cohort; however, gait velocity was almost identical between both cohorts (Table 1). Further, significant differences in diseases, medication and lifestyle, including physical activity and exercise were not observed.
Figure 1 shows participant flow through the study. Of importance for the interpretation of the study 9 men were deceased, 16 men were no longer living independently and 12 men cannot be contacted even though strong emphasis was placed on the “whereabouts” of these subjects. Although citizen registers and obituary columns were carefully checked and relatives and neighbors were contacted, we were ultimately unable to identify the status of these men.
Average observational period was exactly 731 ± 5 days; individual variation ranged from 721 to 744 days.
The Sarcopenia Z-Score declined significantly (P < 0.001; ES dz′ 1.04) during the 2-year observation period (Table 2). In parallel SMI decreased by − 1.49 ± 2.75% ( P < 0.001, dz′ 0.54); handgrip strength declined by − 12.8% ± 10.9% (P < 0.001, ES dz′ 1.17) and gait velocity fell by − 3.5 ± 9.0% (P < 0.001, ES dz′ 0.39). When comparing the declines between the three criteria, the Friedman test resulted in significant differences (P < 0.001). Applying a pairwise comparison to test our primary hypothesis, the difference between SMI and gait velocity, as the functional criterion with the lower decline compared with handgrip strength, was significant (P = 0.006). Thus, our primary hypothesis that “Sarcopenia-Z-Score significantly deteriorates with a significantly more prominent decline in functional compared with morphologic Sarcopenia criteria” can be accepted.
Addressing our secondary hypothesis that “the decline in gait velocity was significantly more pronounced compared with handgrip strength”, we failed to determine a corresponding difference. In contrast, the decline in upper extremity function (i.e. “handgrip strength”) was significantly (P < 0.001) more pronounced (− 12.8 ± 10.9% versus − 3.5 ± 9.0%) than the decrease of gait velocity, which can be considered as a criterion for lower extremity function.
Confounding Variables
Changes in potentially confounding parameters were listed in Table 3. Additionally, no relevant changes were observed for the number of diseases. In parallel to SMI (Table 2), BMI decreased significantly during the 2-year observational period. Of importance, physical activity as reported by the study participants was maintained during the observation period. However, the number of men who exercised (P = 0.045) and more specifically, the number of participants who conducted a resistance type exercise training, decreased significantly during the 2-year period (P = 0.031).
Discussion
In this study we aimed to determine the age dependent decline in Sarcopenia criteria in community dwelling men 70 years+ with low skeletal muscle mass index (SMI ≤ − 1.2 SD T-Score [28]). Finally, we confirmed our hypothesis of a significant deterioration in Sarcopenia Z-Score and a significantly more pronounce decline in functional compared with morphologic criteria. However, when comparing the recently applied functional Sarcopenia criteria [10, 13, 16, 31, 64] “handgrip strength” and “gait velocity”, we observed about four times higher declines in handgrip strength compared with gait velocity (P < 0.001), a finding that strongly contrasts to our expectation.
Revisiting the first part of our hypothesis, we admit that our results of a significant overall worsening of “Sarcopenia” as determined by a dedicated Z-Score that averaged the changes of the individual Sarcopenia criteria during a 2-year period in men 70 years+ are far from spectacular. However, more pronounced than the corresponding Z-Score, Sarcopenia prevalence is almost double (10.2 to 18.1%; P < 0.001). This finding can be largely attributed to the 12.8% decline in handgrip strengthFootnote 5 that can be considered as the functional “killer criterion”Footnote 6 in the Sarcopenia approaches of the EWGSOP [13] and the FNIH [64]. Applying the IWGS approach [16] that focuses on gait speed as the only functional criterion, changes of Sarcopenia prevalence were non-significant (4.0–6.2%; P = 0.219).Footnote 7 Indeed, differences in sarcopenia prevalence due to the application of varying sarcopenia criteria, cut-off and assessment tools are a common and comprehensible finding [28, 30]. Correspondingly, considering the different developments of Sarcopenia criteria observed in the present study, changes in Sarcopenia prevalence were highly dependent on the Sarcopenia definition applied. Taking these features and our focus on differences in the development of Sarcopenia criteria into account, we prefer not to embark on a further discussion of changes of Sarcopenia prevalence in community dwelling men 70 years+ with low SMI. Addressing the latter criterion, however, there is no evidence for a pronounced further decline of SMI in this cohort. In actual fact, the annual SMI loss of 0.75% corresponds to longitudinal changes reported by other studies that determined changes of 0.4–0.9% p.a. of lean body mass/fat free mass, appendicular skeletal muscle mass or skeletal muscle mass index as assessed by direct-segmental, multi-frequency Bio-Impedance-Analysis or Dual Energy X-Ray Absorptiometry in men 70 years and older [14, 21, 35, 52, 61].
Notably, changes of strength in older age (i.e. 70 years+) were reported to be much higher. Longitudinal studies showed that at age 75 years, men lost 3–4% p.a. strength (review in [49]). However, since age-dependent strength loss is not uniform across the body [49], it is important to focus on the region of interest. Most longitudinal studies in older people reported lower annual changes of handgrip strength (2–4% p.a.; e.g. [14, 41, 42, 63]) than the 6% p.a. decline in the present study. Dey et al. [14], contrasts its 0.8% p.a. decline in fat free mass to a 4.0% decrease in handgrip strength in his cohort of men 75 years, observed for 5 years and thus confirmed our result of significantly higher functional compared with anthropologic Sarcopenia criteria changes in people 70 years+. Nevertheless, the outstanding decline in handgrip strength observed in the present study is difficult to explain. Methodical reasons related to the proper standardization and realization of the test were unlikely, since considerable emphasis was placed on a high repetition accuracy of the test procedure.
Comparable to handgrip strength (e.g. [3, 8, 41, 51]), low habitual gait speed is closely related with frailty, hospitalization, morbidity and mortality [1, 8, 22, 41, 47, 54, 55]. Of relevance, the first important finding of our study was that men in the lowest SMI quartile of the study featured similar gait velocity performance compared with their “SMI-normal” peers (Table 1). Additionally, gait speed of this cohort (1.24 m/s) is in the range of reference values (1.26 m/s) reported for men 70–79 years old [6] and, independent of age, gender and anthropometrics, in the normal range of gait speed (1.2–1.4 m/s) [17]. Further, during the 2-year period, we observed an annual loss of 1.7% (0.023 m/s). Compared with the gait speed cut-offs of 0.8 m/s and 1.0 m/s provided by the EWGSOP [13], the IWGS [16], and the AWGS [10], the 24 month follow-up value of 1.20 ± 0.17 m/s of the present study is still within the normal range. Considering further that 0.12 m/sFootnote 8 was calculated as a substantial meaningful change (ES 0.5) of gait speed in community dwelling people 74 ± 6 years [56], the fivefold lower annual decline in our community dwelling men 70 years+ is far from alarming. Nevertheless, revisiting our secondary hypothesis, we had hypothesized higher declines in gait velocity compared with handgrip strength due to the higher strength and power reductions of the lower compared with the upper limbs [4, 14, 18, 39, 66]. However, although most studies (e.g. [9, 25, 58, 60]) emphasize the close correlation (up to r = 0.939) between knee extensor strength/power and habitual or maximum gait speed in 60–85 years old healthy adults, there are a large variety of determinants which impact gait speed in older people. While lower extremity muscle sizeFootnote 9 appears not to be a predictor for walking speed [12], ROM of the lower limb joints [9, 60] and motor control [12, 23] are closely related to gait velocity. Moreover, several non-musculoskeletal factors e.g. several domains of cognitive status [20, 62, 65], depression [7, 43] and of course motivation/test compliance relevantly contribute to explaining the variance of gait speed in older adults. Thus, although a determinant of muscle function i.e. muscle’s ability to produce force and motion, it might be inadequate to subsume gait speed under the topic “lower extremity force”.
However, our study features some particularities and limitations that may prevent an adequate comparison with other studies in this field. (1) In this observational study, we focus on the subgroup of community dwelling men within the lowest SMI quartile of the FranSO study. Comparing this cohort with their peers with normal-high SMI might have provided further insight in the age-dependent development of Sarcopenia criteria. (2) In parallel, additional tests e.g. leg/hip extensor/flexor strength, cognitive status, etc. might have explained the minor changes in gait velocity in more depth. However, economic reasons prevent both approaches. (3) There is a considerable loss to follow-up, predominately due to men who died, were institutionalized, or were unable to reach our lab. Considering that these reasons were, at least to a high proportion, related with functional impairments, our results may underestimate the true decline of morphologic and functional decline of Sarcopenia criteria in older people. As discussed below, this might be a limitation of the generalizability, however, due to the aim of this ongoing project, we opt to focus on the target group of community dwelling men. (4) Due to the big sample size, significance (P ≤ 0.05) should not be confounded with clinical relevance. This refers particularly to the “significant” but limited clinically relevant decline in gait speed in this cohort (see above). (5) High emphasis was placed on exact reproducibility of baseline and 2-year follow-up tests. We consistently applied the identical calibrated devices, in exactly the same order and setting at the same time of the day. Further, tests were introduced consistently with the identical standardized instruction i.e. “walk at a speed just as if you were walking along the street to go to the shops” (gait velocity) and “squeeze as strongly as possible” (handgrip strength). However, we have to admit that baseline and follow-up-assessments tests were not consistently guided by the same research assistants. Although research assistants were carefully briefed and supervised by the principal investigator, even small differences in instructions might affect the results of functional testing. This may be the case particularly for habitual gait speed,Footnote 10 although we did not observe differences when two research assistants subsequently instruct the same participant. (6) It is difficult to estimate the external validity of our study with respect to its generalizability on other older cohorts. We however aimed to focus on a cohort at higher risk for—or already with established Sarcopenia—to monitor changes in morphologic and functional Sarcopenia criteria over 2 years.
In conclusion, we provided evidence for non-uniform changes of Sarcopenia criteria in a cohort of community dwelling men 70 years+ with low skeletal muscle mass index (≤ − 1.2 T-Score). Our finding that appendicular muscle mass reductions were much less pronounced compared with functional changes was in line with the present literature. However, what we did not expect was the very steep decline in handgrip strength we observed, while the corresponding fall in gait velocity was clinically less relevant. We doubt that this result might be a particularity of the selected cohort; however, studies with other (older) cohorts should address this issue in more depth. However, what is the practical application of our finding? Principally, one has to consider the aspect that type and composition of interventions that address muscle mass, handgrip-strength, gait speed or related abilities vary widely. Thus, the knowledge in kinetics of muscle mass and function is of high clinical relevance to schedule optimum therapies and consider their prioritization in older people.
Notes
SMI was calculated as fat free mass of the upper and lower extremities (= appendicular skeletal muscle mass) divided by square body height (kg/m2) [5].
T-Score based approach of SMI (ASMM/height2) based on 2 SD below the mean value of a young reference cohort of 1189 healthy Caucasian men 18–35 years old [28].
…along with baseline values close to the cut-off value of 30 kg [13].
…correspondingly non-significant changes would result when applying the AWGS approach [10], that is however not suitable for this Caucasian cohort.
Smallest meaningful change (ES 0.2) was calculated to be 0.05 m/s.
i.e. Cross sectional area of the triceps surae, quadriceps and hamstrings as assessed by MRI.
Nasciemiento et al. [50] reported significant differences in maximum gait speed after different instructions. Considering that instructions for habitual, i.e. “normal” gait speed have to be even more sophisticated, the importance of standardized and consistently identical test instructions is obvious.
References
Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–9.
Akima H, Kano Y, Enomoto Y, Ishizu M, Okada M, Oishi Y, Katsuta S, Kuno S. Muscle function in 164 men and women aged 20–84 yr. Med Sci Sports Exerc. 2001;33(2):220–6.
Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50(7):1250–6.
Amaral JF, Alvim FC, Castro EA, Doimo LA, Silva MV, Novo Junior JM. Influence of aging on isometric muscle strength, fat-free mass and electromyographic signal power of the upper and lower limbs in women. Braz J Phys Ther. 2014;18(2):183–90.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63.
Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–9.
Brown PJ, Roose SP, Zhang J, Wall M, Rutherford BR, Ayonayon HN, Butters MA, Harris T, Newman AB, Satterfield S, Simonsick EM, Yaffe K. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci. 2016;71(2):221–7.
Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, Sewall A, Goodpaster B, Satterfield S, Cummings SR, Harris TB, Health, Aging and Body Composition Study. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–9.
Chang RW, Dunlop D, Gibbs J, Hughes S. The determinants of walking velocity in the elderly. An evaluation using regression trees. Arthritis Rheum. 1995;38(3):343–50.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101.
Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56(9):1710–5.
Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48(3):358–63.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg, Sweden. Nutrition. 2009;25(6):613–9.
Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. 2018;8(3):a029785.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56.
Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32(2):46–9.
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000;88(4):1321–6.
Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985). 1991;71(2):644–50.
Gonzales JU, James CR, Yang HS, Jensen D, Atkins L, Thompson BJ, Al-Khalil K, O'Boyle M. Different cognitive functions discriminate gait performance in younger and older women: a pilot study. Gait Posture. 2016;50:89–95.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64.
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31.
Hall CD, Echt KV, Wolf SL, Rogers WA. Cognitive and motor mechanisms underlying older adults’ ability to divide attention while walking. Phys Ther. 2011;91(7):1039–50.
Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112(1):267–75.
Hollman JH, Beed KC, Buus RJ, Schleicher KL, Lanzino DJ. Does upper limb coordination predict walking speed in older adults? A cross-sectional study. J Geriatr Phys Ther. 2014;37(3):106–15.
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–21.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96.
Kemmler W, Teschler M, Weissenfels A, Sieber C, Freiberger E, von Stengel S. Prevalence of sarcopenia and sarcopenic obesity in older German men using recognized definitions: high accordance but low overlap! Osteoporos Int. 2017;28(6):1881–91.
Kemmler W, Von Stengel S, Bebenek M, Sieber C, Freiberger E. Prevalence of sarcopenic obesity in Germany using established definitions. Baseline data of the FORMOsA Study. Osteoporos Int. 2016;27(1):275–81.
Kemmler W, Von Stengel S, Engelke K, Sieber C, Freiberger E. Prevalence of sarcopenic obesity in Germany using established definitions. Baseline data of the FORMOsA Study. Osteoporos Int. 2015;27(1):275–81.
Kemmler W, von Stengel S, Kohl M. Developing sarcopenia criteria and cutoffs for an older Caucasian cohort—a strictly biometrical approach. Clin Interv Aging. 2018;13:1365–73.
Kemmler W, Weineck J, Kalender WA, Engelke K. The effect of habitual physical activity, non-athletic exercise, muscle strength, and VO2max on bone mineral density is rather low in early postmenopausal osteopenic women. J Musculoskelet Neuronal Interact. 2004;4(3):325–34.
Kenney WI, Buskirk ER. Functional consequences of sarcopenia: effects on thermoregulation. J Gerontol A Biol Sci Med Sci. 1995;50:78–85.
Kim S, Welsh DA, Ravussin E, Welsch MA, Cherry KE, Myers L, Jazwinski SM. An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively. J Gerontol A Biol Sci Med Sci. 2014;69(6):650–6.
Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Health ABC study. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66(8):888–95.
Kressig RW, Beauchet O, European GNG. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res. 2006;18(2):174–6.
Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55(8):663–72.
Kyle UG, Genton L, Hans D, Karsegard VL, Michel JP, Slosman DO, Pichard C. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc. 2001;49(12):1633–40.
Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56(10):B443–8.
Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(3):451–6.
Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24(6):468–73.
Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63(4):376–83.
Lemke MR, Wendorff T, Mieth B, Buhl K, Linnemann M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J Psychiatr Res. 2000;34(4–5):277–83.
Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol (1985). 1997;83(5):1581–7.
Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am]. 1984;9(2):222–6.
McAuley E, Konopack JF, Motl RW, Rosengren K, Morris KS. Measuring disability and function in older women: psychometric properties of the late-life function and disability instrument. J Gerontol A Biol Sci Med Sci. 2005;60(7):901–9.
Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314–22.
Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS ONE. 2013;8(10):e78636.
Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260.
Nascimento MAD, Gerage AM, Silva D, Ribeiro AS, Machado D, Pina FLC, Tomeleri CM, Venturini D, Barbosa DS, Mayhew JL, Cyrino ES. Effect of resistance training with different frequencies and subsequent detraining on muscle mass and appendicular lean soft tissue, IGF-1, and testosterone in older women. Eur J Sport Sci. 2019;19(2):1–9.
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7.
Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–8 (quiz 915–876).
Ohta J, Seki M, Ao M, Okajima R, Kuwabara A, Takaoka H, Aoyama K, Tanaka K. Comparison of lower leg muscle strength and grip strength for diagnosing slower gait speed in the elderly. Osteoporos Sarcopenia. 2017;3:128–31.
Pamoukdjian F, Paillaud E, Zelek L, Laurent M, Levy V, Landre T, Sebbane G. Measurement of gait speed in older adults to identify complications associated with frailty: a systematic review. J Geriatr Oncol. 2015;6(6):484–96.
Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39–46.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–9.
Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24–30.
Rantanen T, Avela J. Leg extension power and walking speed in very old people living independently. J Gerontol A Biol Sci Med Sci. 1997;52(4):M225–31.
Roshanravan B, Patel KV, Fried LF, Robinson-Cohen C, de Boer IH, Harris T, Murphy RA, Satterfield S, Goodpaster BH, Shlipak M, Newman AB, Kestenbaum B. Association of muscle endurance, fatigability, and strength with functional limitation and mortality in the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2017;72(2):284–91.
Sakari R, Era P, Rantanen T, Leskinen E, Laukkanen P, Heikkinen E. Mobility performance and its sensory, psychomotor and musculoskeletal determinants from age 75 to age 80. Aging Clin Exp Res. 2010;22(1):47–53.
Shimokata H, Ando F, Yuki A, Otsuka R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: a 12-year longitudinal study. Geriatr Gerontol Int. 2014;14(Suppl 1):85–92.
Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. J Gerontol B Psychol Sci Soc Sci. 2015;70(3):357–66.
Stenholm S, Harkanen T, Sainio P, Heliovaara M, Koskinen S. Long-term changes in handgrip strength in men and women—accounting the effect of right censoring due to death. J Gerontol A Biol Sci Med Sci. 2012;67(10):1068–74.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58.
van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Olde Rikkert MG. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63(12):1344–9.
Viitasalo JT, Era P, Leskinen AL, Heikkinen E. Muscular strength profiles and anthropometry in random samples of men aged 31–35, 51–55, and 71–75 years. Ergonomics. 1985;28(11):1563–74.
Acknowledgements
This study was exclusively funded by resources of the Institute of Medical Physics, University of Erlangen-Nürnberg, Germany. All the authors state that they have no conflicts of interest.
Author information
Authors and Affiliations
Contributions
Wolfgang Kemmler (WK), Simon von Stengel, Stephanie Kast, Cornel Sieber and Ellen Freiberger designed the study, completed data analysis and/or interpretation and drafted the manuscript. WK accepts full responsibility for the integrity of the data sampling, analysis and interpretation.
Corresponding author
Rights and permissions
About this article
Cite this article
Kemmler, W., von Stengel, S., Kast, S. et al. Longitudinal Changes in Sarcopenia Criteria in Older Men with Low Skeletal Muscle Mass Index: A 2-Year Observational Study. J. of SCI. IN SPORT AND EXERCISE 1, 59–68 (2019). https://doi.org/10.1007/s42978-019-0006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-019-0006-7