Abstract
Repeated measures design. This study examined recovery of postural equilibrium (centre of pressure (COP) excursion, time to recover balance, and the number of postural adjustments) following unexpected support surface perturbation in healthy participants with and without a rigid lumbar corset to reduce lumbar motion. Lumbar spine movement is thought to aid postural stability, especially when a “hip” (lumbopelvic) strategy is required, such as in response to large and fast perturbations. Delayed onset of lumbar spine movement in association with prolonged postural recovery in chronic low back pain implies reduced spinal motion could underpin balance deficits in this group. However, other explanations such as poor proprioception cannot be excluded, and the relationship between lumbar movement and postural stability remains unclear. We hypothesized restricted lumbar spine movement would impair control of postural recovery following support surface perturbation. Participants regained postural stability following unexpected support surface perturbations in different directions (forward and backward), with different amplitudes (small, medium, and large), with and without restriction of spine motion by a hard lumbar corset. Although the latency of the postural adjustment was unaffected by the corset, the quality of postural recovery was compromised (increased COP range, time taken for postural recovery, and number of postural adjustments) during recovery, especially in response to large perturbation. Restriction of lumbar spine movement adversely affects postural recovery. The results suggest movement of the lumbar spine, although small in amplitude, is critical for efficient recovery of standing balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maintenance of postural stability involves movement and moments at multiple body segments. Classical stereotypical movement synergies to maintain postural equilibrium are defined as hip and ankle strategies and refer to dominant movement or moments at either the hip or the ankle joint (Horak and Nashner 1986), respectively, but failed to address the contribution of the lumbar spine. During quiet stance on a fixed base of firm support, postural stability in the antero-posterior (AP) direction is typically maintained by movement/moments at the ankle. To overcome quick and large postural perturbation, the hip strategy is used, involving hip movement/moments to assist recovery of postural stability (Horak and Nashner 1986). Although motion/moments at the lumbar spine are likely to be an essential component of the hip strategy, the contribution of this region is seldom examined in detail. We hypothesized that reduced lumbar motion would adversely affect standing balance control and that this may underpin commonly reported (Mok et al. 2011a, b) balance deficits in people with chronic low back pain (LBP).
Spinal movement as a component of postural adjustments, although small in amplitude, has been measured in quiet standing and in association with voluntary arm movements in healthy individuals (Hodges et al. 1999, 2002; Mok et al. 2007). When people with LBP perform voluntary arm movements or are unexpectedly perturbed by an external load, the onset of lumbar spine movement is delayed and the time to postural recovery is prolonged, with more postural adjustments (Hodges et al. 1999, 2002; Mok et al. 2007, 2011a, b). This is particularly evident when standing on a short base, which limits the potential to use an ankle strategy forcing the participant to use a “hip strategy”. Preliminary data suggest that delayed movement of the lumbar spine could explain the longer time to regain postural stability and greater number of postural adjustments during the recovery period in that context (Mok et al. 2011a). These data imply an association between movement of the lumbar spine and the quality of postural control, especially when a hip strategy is required. Although it is plausible that reduced spinal motion may underpin the balance deficit in this group, it is not possible to conclude whether this relationship is explained by increased stiffness of the lumbar spine [potentially as a result of a protective response of the trunk muscles (Hodges et al. 2013)] or changes in control secondary to compromised proprioception, which is a common finding in LBP (Sheeran et al. 2012; Willigenburg et al. 2012).
Two studies that restricted spine movement with external braces provide further evidence that compromised lumbar motion may underpin the balance deficit in LBP, but cannot resolve the question. First, Gruneberg et al. (2004) examined postural reaction to support surface perturbation with a corset that either blocked movement of the hip–pelvis–lumbar spine complex (half corset) or hip–pelvis–thoracolumbar spine complex (full corset). Although the data showed increased likelihood of a loss of balance when wearing a corset (consistent with the multi-link pendulum model of balance control involving movement/moment of hip and trunk) (Gruneberg et al. 2004), the corset restricted hip motion, and the potential-specific contribution of lumbar spine motion to postural stability remains unknown. This is important as other work shows that hip motion can increase to compensate for changes in lumbar motion (Smith et al. 2005). Second, application of a lumbar brace (to maintain lumbar lordosis) in individuals with LBP and degenerative lumbar disc pathology increased the frequency of postural correction, but did not affect COP excursion during quiet standing. As the brace may have restricted lumbar motion in a group with already reduced motion, the data cannot resolve the effect of increased spine stiffness on postural control.
This study aimed to investigate the effect of specific restriction of lumbar spine mobility on postural steadiness and recovery in response to unexpected support surface perturbation. The quality of postural recovery was quantified by the latency to the onset of the postural response, the time taken to regain balance, the number of postural adjustments during postural recovery, and the excursion of centre of pressure (COP) (postural steadiness) during postural recovery (Mackey and Robinovitch 2005).
Methods
Participants
Twenty (11 male and 9 female) participants with a mean (SD) age of 20 (1) years were included if they had no recent history of significant LBP or neck pain (defined as episodes that required treatment or sick leave) in the past 6 months and body mass index (BMI) between 18.5 and 25 kg/m2 (WHO Expert Consultation 2004). Participants were excluded if they had history of spinal surgery, any significant structural abnormality, deformity or mal-alignment of the spine, unresolved lower limb musculoskeletal pathology, any known sensory or neurologic disorders, or any condition or medication that could affect balance. Participants completed a Habitual Physical Activity Questionnaire (HPA) (Baecke et al. 1982). Participant characteristics are presented in Table 1. All procedures were approved by Institutional Medical Research Ethics Committee and were conducted in accordance with the Declaration of Helsinki.
Ground reaction forces
A dynamic 18″ × 18″ strain gauge dual force platform system embedded in the SMART EquiTest system (NeuroCom, USA) was used to detect ground reaction forces. Data were acquired at 500 Hz.
Procedure
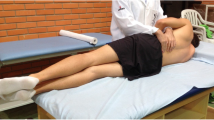
A rigid (thermoplastic—Eco Nat 3.2 mm, Orfit Industries, Belgium) thoracolumbar corset was tailor-made by an orthotist to fit each participant prior to testing with the intention to restrict lumbar motion without affecting hip motion or breathing. The corset was custom moulded as a two-piece (anterior and posterior) bivalve thoracolumbar orthosis and secured with three Velcro straps on each side. The posterior piece spanned between S2 inferiorly to T10 superiorly, and the anterior piece spanned from the superior edge of the pelvis to within 4 fingers width below the xyphoid process (Fig. 1). Participants were tested with and without the corset in random order with group allocation in a sealed envelope.
The rigid lumbar corset—two-piece (anterior and posterior) bivalve thoracolumbar orthosis, and secured with three Velcro straps on each side. The posterior piece spanned between S2 inferiorly to T10 superiorly, and the anterior piece spanned from the superior edge of the pelvis to within 4 fingers width below the xyphoid process
Support surface translation was provided using a SMART EquiTest system (NeuroCom, USA). Participants were instructed to regain upright posture as soon as possible after the perturbation. The “Motor Control Test” protocol (a standard measurement method of the EquiTest system, version 8.4, NeuroCom. USA) was used to measure the postural response to antero-posterior support surface translations in two directions (backward or forward) at three amplitudes (large, medium, and small). The amplitude of the translations was scaled according to the individual’s height. Small, medium, and large translations were designed by the apparatus manufacturer to produce an angular displacement of the centre of gravity (based on a single-pendulum model) of 0.7° in 250 ms, 1.8° in 300 ms, and 3.2° in 400 ms, respectively. Each translation occurred at a constant velocity and transferred constant forward or backward angular momentum to the participant’s body. Three trials for each translation condition (2 directions × 3 amplitude) were tested for 18 trials, with and without the corset (i.e. 36 trials in total) with a randomized translation condition sequence.
The test was conducted in a quiet environment, and earplugs reduced audio cues from the system. Participants stood relaxed with bare feet on the force plate with the distance between the lateral malleoli determined by the participant’s height. Participants were attached to a safety harness, and a trial was considered as “fail” if the participant took a step or grabbed an object to maintain balance. Participants rested for >10 s between trials.
Data processing
COP displacement and the velocity of COP (COPV, calculated by differentiation of the COP position data) were analysed using Matlab 6.0 software (The Mathworks, USA). Data were only analysed in the antero-posterior plane (i.e. COPap and COPapV) as postural perturbation induced by the system occurred primarily in this direction. COPapV data were rectified, and the mean amplitude from 100 to 400 ms before the onset of perturbation was calculated as a measure of baseline postural stability (Fig. 2).
a Quantification of the temporal parameters for postural recovery. The onset of platform movement is derived from the shear force data. Postural reaction latency: the time period between the onset of platform movement and the onset of postural reaction (dash line). Length of time for postural stabilization: the time period between onset of platform movement and point of stabilization. b Quantification of the number of postural adjustments from onset of postural reaction to the time of postural stabilization. Times at which the COPapV crossed zero are marked with circles. Five crossings before postural stabilization are identified in this trial
The time between the onset of the platform translation and onset of postural response (“Postural reaction latency”) was calculated. The onset of platform translation was determined as the instant the shear force (recorded from the EquiTest system) deviated from baseline. The onset of the postural response was identified as the point at which COPapV increased by >2SD above amplitude of baseline (mean-rectified COPapV from 100 to 400 ms before onset of perturbation (Brauer et al. 2001) (Fig. 2a).
The “time to postural stabilization” was defined as the time between the onset of platform translation and the time at which COPapV returned to a pre-perturbation level (Brauer et al. 2001), i.e. when COPapV remains below a threshold velocity (mean baseline velocity plus 2 SD) for 30 ms following postural perturbation (Fig. 2a).
The “number of postural adjustments” was recorded as the number of times the unrectified COPapV crossed zero (which represents major direction change of the COPap trajectory) in the period from platform translation until the time to stabilization as described above (Fig. 2b).
The COP range in antero-posterior direction (COPEap) was defined as the difference between the maximum and minimum antero-posterior position of COP during the period of postural recovery.
Statistical analysis
Data from “fail” trials were not analysed. Distribution of the data was tested with Shapiro–Wilk test. All measures were compared between lumbar corset conditions (with vs. without corset), directions (forward vs. backward), and amplitudes (small, medium and large) using a linear mixed model. Significance was tested using F statistics of the analysis of variance table (ANOVA). Differences in the number of successful trials and postural adjustments were analysed using chi-square and Wilcoxon’s ranked sum test, respectively. Post hoc testing involved paired tests with Bonferroni correction for multiple comparisons. SPSS v16.0 (SPSS Inc, Chicago, IL) was used for all analysis, and the significance was set at P ≤ 0.05. Data are presented as mean [SD] values throughout the text and figures.
Results
Number of “fail” trials
Participants were more likely to lose balance and grab for an object (“fail”) in trials with lumbar corset than without (χ 2 = 4.52, P = 0.03; Table 2). Individual platform translation conditions were not analysed separately because of the small number of fail trials in this healthy participant group.
Baseline COPapV
There was no difference in the baseline-rectified COPapV between trials with (3.2 [0.3] mm/s) and without (3.4 [0.2] mm/s, P = 0.93; Table 3) a corset. This suggests the corset had no effect on balance in quiet stance.
Postural reaction latency
The mean postural reaction latency did not differ between conditions with (132 [16] ms) and without (136 [12] ms) lumbar corset (P = 0.34; Table 4). Postural latency was significantly increased (P = 0.03) during forward (143 [11] ms) translation than backward (125 [74] ms). There was no difference in postural latency between amplitudes (P = 0.07).
Time to postural stabilization
In response to sudden support surface translation, the time taken to regain postural stability was longer when motion of the lumbar spine was restricted with the corset (952 [182] ms) than trials without a corset (832 [125] ms; P < 0.001). Time to postural stabilization was longer with large (main effect: amplitude—P < 0.001; post hoc—P < 0.01) and medium (post hoc—P = 0.01) translation than small-amplitude translation, but no difference between the large and medium translation (P = 0.76). There was no difference in time to postural stabilization between directions (P = 0.26). The absence of a significant interaction between corset condition × direction × amplitude (P = 0.47) indicates that restriction of movement of the lumbar spine consistently prolongs the time to regain postural equilibrium, irrespective of the direction and amplitude of the support surface translation (Fig. 3a).
Group COP data during postural recovery. Data for trials in forward and backward direction are displayed in the left and right panel, respectively. a Time to postural stabilization. The time taken to restore postural equilibrium and the number of postural adjustments were significantly greater with lumbar corset application in the large translation condition for both forward and backward perturbations. b The number of postural adjustments was significantly greater with lumbar corset application in the large translation condition for both forward and backward. c COPap excursion during postural recovery was significantly greater with lumbar corset application in the medium and large translation conditions with both forward and backward perturbations. Mean and SD are shown
Number of postural adjustments
The number of postural adjustments was greater during the time to stabilization when the corset was worn (3.4 [1.2]) than without the corset (2.6 [0.8]; P < 0.001). More postural adjustments were also required during large (main effect P < 0.001, post hoc P < 0.001) and medium translation (P = 0.02) than with small translation, with no difference between large and medium translation (P = 0.14). There was no interaction between corset condition, amplitude, and direction (P = 0.14) (Fig. 3b).
Range of COP
The range of the COP excursion in antero-posterior direction during postural stabilization was greater with the corset (6.7 [3.10] cm) than without the corset (5.5 [2.5] cm; P < 0.001). The COP excursion was greater with the large (main effect P < 0.001, post hoc P < 0.001) and medium (P < 0.001) translations than the small-amplitude translation. There was no interaction between corset condition and amplitude or direction (all: P = 0.43) (Fig. 3c).
Discussion
The results of this study indicate that restriction of lumbar motion leads to compromised quality of postural recovery after unexpected support surface translations. This finding supports the hypothesis that movement of the lumbar spine is important for maintenance of standing balance and that compromised spine motion in LBP is likely to contribute to the balance deficits consistently reported in this group.
Limitation to lumbar movement is associated with poor balance
This study is the first to specifically investigate the effect of restricted lumbar spine movement on maintenance of postural equilibrium following support surface translation. The data extend the previous observation that the postural response to perturbation in the roll or pitch planes is compromised by limitation of motion over multiple segments including either the hip and lumbopelvic region or the entire region extending between the hip and thorax (Gruneberg et al. 2004). In that study, the authors concluded that extensive limitation of movement of the trunk and hip complex adversely affects postural reaction as evidenced by increased compensatory head and arm movements and reduced leg muscle activity. The present data extend this finding to suggest that restriction of the lumbar spine alone is sufficient to compromise balance recovery, and the availability of other segments, such as the hip, cannot compensate for this restriction.
Although most argue that balance in quiet stance and the response to small postural perturbations are maintained predominately by movement and moments at the ankle joint (ankle strategy) (Horak and Nashner 1986), others argue that moments and movements at the trunk contribute even during quiet standing. Evidence for this has come from studies of respiration (Gurfinkel et al. 1971; Hodges et al. 2002). In those studies, small movements of the spine and pelvis have been reported in phase with breathing and are thought to counteract the perturbation to balance from the displacement of centre of mass with breathing. The results of the present study provide further evidence for a role of the trunk in tasks with minor disturbance to balance. Although the corset did not lead to increase COPapV in quiet standing prior to the perturbation, postural recovery was compromised following the small perturbation amplitude by the restriction of spine movement. Previous work has shown that small-amplitude flexion (~3°) at the lumbar spine was adequate to contribute to balance in response to a perturbation induced by a 1 kg weight dropping into a hand-held box (Mok et al. 2011a) and motion of the spine of less than 1° in anticipation of the perturbation to the trunk from arm movement (Hodges et al. 1999). Taken together, these observations indicate that lumbar spine movement, although small in amplitude, plays a critical role in postural control and contributes even when perturbations are small.
Several studies have highlighted the direction-specific control of trunk muscles to maintain balance in standing in response to support surface translations (Henry et al. 1998; Jones et al. 2012). These data clearly show that motion of the spine and tightly controlled trunk muscle activation, rather than simple trunk stiffening, are involved in control of balance in standing. Other work has shown that when trunk muscle activity is increased (Reeves et al. 2006), the quality of postural control in sitting is impaired. Besides muscle activation, suboptimal control of lumbar movement and impaired postural sway during seated balancing task has been evident in people with LBP (Radebold et al. 2001; van Dieen et al. 2010; Willigenburg et al. 2013). The result suggested that either restricted (Radebold et al. 2001; van Dieen et al. 2010) or increased (Willigenburg et al. 2013) lumbar movement is associated with poor balance while sitting on an unstable base, although differentiation of passive versus active involvement of the spine is not feasible with the seated balancing paradigm (van Dieen et al. 2010; Willigenburg et al. 2013). However, that work excludes the contribution of the legs and the current work indicates that restriction of spine in standing, without impact on the legs, compromises balance quality.
Previous studies have investigated the effect of soft lumbar corset to sitting on an unstable base in healthy individuals (Reeves et al. 2006; Cholewicki et al. 2007). The results indicate reduced activity of the lumbar erector spinae muscles, but no change in the COPV from trials with no brace (Reeves et al. 2006; Cholewicki et al. 2007). The latter finding concurs with our finding that baseline COPV was not changed by the application of lumbar corset. The effect of a lumbar brace that maintains the lumbar spine in a fixed lordotic position in patients with chronic discogenic LBP was recently reported (Munoz et al. 2010). During quiet standing in that study, the application of the brace induced a significant backward displacement of CoP and a more frequent change in CoP direction. Although it is not possible to determine whether the latter effect is the result of changes in stiffness (faster oscillation of a stiffer structure) or compromised active control (less accurate coordination of COP displacement), it does infer a more complex control problem akin to the greater number of postural corrections in the present study after the perturbation. While the authors deduced this to imply improved balance performance with lumbar brace, the results of the present study provide an alternative interpretation. Increased frequency of postural corrections has been suggested as a manifestation of compromised accuracy of the postural correction mechanisms (Rougier and Garin 2006). Further, in the present study, greater number of postural corrections was associated with a longer time to postural recovery. Taken together, we argued the findings of Munoz et al. (2010) might indicate that during quiet standing, reduced lumbar movement compromised the quality of postural control. Although increased frequency of postural correction was evident, postural steadiness (COP sway area) remained unchanged with the application of the brace in the LBP group (Munoz et al. 2010). However, this difference to the present study is not surprising as postural steadiness in quiet stance does not correlate with postural recovery from perturbation and should be treated as a separate measure of postural control (Mackey and Robinovitch 2005).
Application of a lumbar corset did not change postural reaction latency
Postural latency represents the time required for initiation of the postural recovery action. Accurate and rapid onset is important for maintenance of upright balance (Shumway-Cook and Woollacott 2007). Postural latency is a sensitive indicator of balance performance. Postural latency is increased in elderly participants with poor balance (Woollacott et al. 1986) and in healthy individuals during jaw clenching (Hosoda et al. 2007). Postural latency did not change with application of a rigid lumbar corset in the present study. This implies that a response could be initiated with a similar latency, but this is likely to involve moments generated at segments other than the spine, such as hip and ankle. Despite the preservation of response latency, the organization of the postural response was sufficiently compromised to reduce the quality of balance recovery. Although balance is controlled by coordination of the multiple segments in the kinetic chain from feet to head, the reduced contribution of the lumbar spine could not be completely compensated by motion at adjacent segments.
There may be differences in the ability of people with and without LBP to redistribute motion between segments to compensate for reduced spinal motion. Although greater displacement of CoP associated with breathing has been shown in people with LBP in association with reduced lumbopelvic movement (Grimstone and Hodges 2003), this is not observed when healthy individuals are given LBP experimentally (Smith et al. 2005). The authors argued that people with no history of LBP were able to use movement from other joints (such as hip and knee) to compensate for the reduced lumbar spine movement and maintain postural stability. Although participants in the present study may have adopted a similar strategy when wearing a brace, this was insufficient to completely compensate for the reduced spinal motion. If so, the present data would underestimate the effect that may be apparent if no compensation was used. The effect of a brace in people with LBP may have a greater impact on balance than for a group of pain-free control participants. This warrants further investigation with measurement of joint kinematics in addition to force plate measures.
Methodological considerations
There are some methodological issues to consider. First, the movement of the lumbar spine is unlikely to be completely restricted by the corset used here. Although the corsets were tailor-made and individually moulded to each participant, small movement at the lumbar spine might still be possible. This could not be measured as the tight fitting of the corset meant placement of sensors on the lumbar spine for movement detection was not feasible. Nevertheless, the present results show that restriction of the lumbar spine movement, even if not completely eliminated, impairs postural recovery. Second, compensatory movements at adjacent segments were not recorded but would be worthy of consideration in future work.
Previous work has highlighted the important role of spinal and lower limb movement in postural compensation for the perturbation to balance caused by breathing (Gurfinkel et al. 1971; Kantor et al. 2001; Hodges et al. 2002). The corset used in the present study will have modified breathing as a result of reduced abdominal displacement and spinal motion. Although this may have had a subtle effect on the postural strategy, we do not believe this affects our results as there was no difference in variables at baseline prior to the perturbation, despite the application of the corset. This could be further investigated in future work, particularly for corsets that extend to the rib cage and that would have a greater impact on breathing (Puckree et al. 2005).
Conclusion
The results of this study suggest that movement of the lumbar spine plays a critical role in postural recovery following unexpected support surface translation. Time of onset of postural reaction was not affected by the brace, which suggests the response remains triggered as quickly in this condition as trials without the brace. However, despite the maintained onset of response, the reduced contribution of lumbar movement to the postural adjustment compromised its quality. Poor balance control in people with LBP could be explained by reduced lumbar movement.
References
Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Brauer SG, Woollacott M, Shumway-Cook A (2001) The interacting effects of cognitive demand and recovery of postural stability in balance-impaired elderly persons. J Gerontol A Biol Sci Med Sci 56:M489–M496
Cholewicki J, Reeves NP, Everding VQ, Morrisette DC (2007) Lumbosacral orthoses reduce trunk muscle activity in a postural control task. J Biomech 40:1731–1736
Grimstone SK, Hodges PW (2003) Impaired postural compensation for respiration in people with recurrent low back pain. Exp Brain Res 151:218–224
Gruneberg C, Bloem BR, Honegger F, Allum JH (2004) The influence of artificially increased hip and trunk stiffness on balance control in man. Exp Brain Res 157:472–485
Gurfinkel VS, Kots YM, Paltsev EI, Feldman AG (eds) (1971) The compensation of respiratory disturbances of erect posture of man as an example of the organisation of interarticular interaction. MIT Press, Cambridge
Henry SM, Fung J, Horak FB (1998) EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol 80:1939–1950
Hodges P, Cresswell A, Thorstensson A (1999) Preparatory trunk motion accompanies rapid upper limb movement. Exp Brain Res 124:69–79
Hodges PW, Gurfinkel VS, Brumagne S, Smith TC, Cordo PC (2002) Coexistence of stability and mobility in postural control: evidence from postural compensation for respiration. Exp Brain Res 144:293–302
Hodges PW, Coppieters MW, Macdonald D, Cholewicki J (2013) New insight into motor adaptation to pain revealed by a combination of modelling and empirical approaches. Eur J Pain 138:1138–1146
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55:1369–1381
Hosoda M, Masuda T, Isozaki K, Takayanagi K, Sakata K, Takakuda K, Nitta O, Morita S (2007) Effect of occlusion status on the time required for initiation of recovery in response to external disturbances in the standing position. Clin Biomech (Bristol, Avon) 22:369–373
Jones SL, Hitt JR, Desarno MJ, Henry SM (2012) Individuals with non-specific low back pain in an active episode demonstrate temporally altered torque responses and direction-specific enhanced muscle activity following unexpected balance perturbations. Exp Brain Res 221:413–426
Kantor E, Poupard L, Le Bozec S, Bouisset S (2001) Does body stability depend on postural chain mobility or stability area? Neurosci Lett 308:128–132
Mackey DC, Robinovitch SN (2005) Postural steadiness during quiet stance does not associate with ability to recover balance in older women. Clin Biomech (Bristol, Avon) 20:776–783
Mok NW, Brauer SG, Hodges PW (2007) Failure to use movement in postural strategies leads to increased spinal displacement in low back pain. Spine 32:E537–E543
Mok NW, Brauer SG, Hodges PW (2011a) Changes in lumbar movement in people with low back pain are related to compromised balance. Spine 36:E45–E52
Mok NW, Brauer SG, Hodges PW (2011b) Postural recovery following voluntary arm movement is impaired in people with chronic low back pain. Gait Posture 34:97–102
Munoz F, Salmochi JF, Faouen P, Rougier P (2010) Low back pain sufferers: is standing postural balance facilitated by a lordotic lumbar brace? Orthop Traumatol Surg Res 96:362–366
Puckree T, Lauten VA, Moodley S, Naidoo J, Ramsammy K (2005) Thoracolumbar corsets alter breathing pattern in normal individuals. Int J Rehabil Res 28:81–85
Radebold A, Cholewicki J, Polzhofer GK, Greene HS (2001) Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 26:724–730
Reeves NP, Everding VQ, Cholewicki J, Morrisette DC (2006) The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res 174:694–700
Rougier P, Garin M (2006) Performing saccadic eye movements modifies postural control organisation. Neurophysiol Clin 36:235–243
Sheeran L, Sparkes V, Caterson B, Busse-Morris M, van Deursen R (2012) Spinal position sense and trunk muscle activity during sitting and standing in nonspecific chronic low back pain: classification analysis. Spine 37:E486–E495
Shumway-Cook A, Woollacott MH (2007) Motor control: translating research into clinical practice. Lippincott Williams & Wilkins, Philadelphia
Smith M, Coppieters MW, Hodges PW (2005) Effect of experimentally induced low back pain on postural sway with breathing. Exp Brain Res 166:109–117
van Dieen JH, Koppes LL, Twisk JW (2010) Low back pain history and postural sway in unstable sitting. Spine 35:812–817
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Willigenburg NW, Kingma I, van Dieen JH (2012) Precision control of an upright trunk posture in low back pain patients. Clin Biomech (Bristol, Avon) 27:866–871
Willigenburg NW, Kingma I, van Dieen JH (2013) Center of pressure trajectories, trunk kinematics and trunk muscle activation during unstable sitting in low back pain patients. Gait Posture. doi:10.1016/j.gaitpost.2013.02.010
Woollacott MH, Shumway-Cook A, Nashner LM (1986) Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev 23:97–114
Acknowledgments
We thank Peggy Chan, Nelson Cheng, Shirley Le, and Kopey Yuen for assistance with data collection. NM was supported by the Competitive Research Grant for Junior Researchers, The Hong Kong Polytechnic University. PH was support by the National Health and Medical Research Council of Australia (APP1002190).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mok, N.W., Hodges, P.W. Movement of the lumbar spine is critical for maintenance of postural recovery following support surface perturbation. Exp Brain Res 231, 305–313 (2013). https://doi.org/10.1007/s00221-013-3692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3692-0