Abstract
This paper focused on the relationship between trunk stiffness and postural control during unstable seated balancing. We hypothesized that an increase in trunk stiffness would degrade postural control, and further hypothesized that signal dependent noise (SDN), resulting in increased muscle force variability, was responsible for this impairment. Ten subjects balanced on an unstable seat during four randomized conditions: normal balancing (control condition), trunk muscle co-activation (active stiffness), arm muscle co-activation (attention control), and belt (passive stiffness). Center of pressure (CoP) and EMG data were collected during three 20 s trials. Postural control was quantified by CoP velocity (total path divided by sample time in seconds). Trunk muscle co-activation resulted in significantly higher CoP velocity than the control (P < 0.001) and arm co-activation (P < 0.001) conditions. EMG data confirmed that the trunk co-activation condition had significantly higher muscle activity than the control (P = 0.001) and arm co-activation (P = 0.001) conditions. The belt condition, which increases passive trunk stiffness, showed no degraded postural control, but interestingly produced slightly lower levels of trunk muscle activity than the control condition (P < 0.001). Increased active trunk stiffness from muscle co-activation degraded postural control. Since the arm co-activation condition showed no impairment, attention demands cannot explain this result. Furthermore, since passive trunk stiffness from wearing a belt did not affect performance, it is believed that SDN from increased trunk muscle recruitment, and not an altered postural control strategy from increased joint stiffness, was responsible for the impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human spine is a complex mechanical structure, which is inherently unstable. In vitro experimentation on the lumbar spine has demonstrated that an osteoligamentous spine (spine devoid of muscles) buckles under a 90 N load (Crisco et al. 1992). Clearly, the spine is incapable of supporting any significant load, or for that matter, simply the mass of the upper body. In this sense, the in vivo spine can be defined as being an unstable system. Stability is achieved through the recruitment of trunk muscles and the stiffness they provide (Bergmark 1989; Crisco and Panjabi 1991). Once stability is achieved, the spine system can be made more robust to external perturbations by increasing the trunk muscle antagonistic co-activation (Gardner-Morse and Stokes 2001; Granata et al. 2004).
Following injury, the spine may experience a loss of passive stiffness which may make it less robust to perturbations (Panjabi 1992a, b; Oxland et al. 1992). This is often termed clinical instability. One possible strategy to compensate for altered spinal mechanics would be to encourage patients to increase their level of trunk muscle co-activation to enhance spine stiffness (Stokes et al. 2000). However, with increased muscle activation, there is also an activation dependent increase in force variability or signal dependent noise (SDN) (Slifkin and Newell 2000; Christou et al. 2002; Hamilton et al. 2004). As a result, the spine not only experiences external perturbations but also internal perturbations from noise arising from additional motor unit recruitment. Is it possible for internal noise from increased activation to degrade performance of the trunk musculature? This question has significant implications for the rehabilitation strategy of increasing trunk stiffness through muscle co-activation. Obviously, there are situations in which increased trunk muscle co-activation is advantageous to protect the spine from injury (i.e., before a body check in hockey), but in situations where precise motor control is required (i.e., standing, balance, or gait), perhaps less co-activation and a more supple spine is desirable to improve performance. Consequently, the main goal of this paper was to determine if increased spine stiffness degrades performance for tasks requiring precise motor control. If increased stiffness leads to impaired control, the secondary goal was to determine the source for this degradation.
There were two hypothesis tested in the current paper. First, we hypothesized that increased trunk co-activation leads to degraded postural control during unstable seated balancing. If this is the case, there are a few possible explanations for the degradation: increased joint stiffness may alter postural control strategies (Gruneberg et al. 2004), increased muscle activation may increase SDN (Slifkin and Newell 2000; Christou et al. 2002; Hamilton et al. 2004), or attention demanding trunk muscle co-activation may interfere with the control process (Weeks et al. 2003). We hypothesized that degraded postural control results from SDN, and not from other two sources. To test this hypothesis, subjects’ postural control was compared between conditions where joint stiffness was increased actively through trunk muscles co-activation and passively through a lumbar support provided by a belt to rule out the possibility that altered postural control strategies caused the degradation. Also, postural control was compared between the trunk muscle co-activation condition and the arm co-activation condition to determine if the additional attention demands caused the degradation. If the performance in trunk co-activation condition was significantly worse than in the other two conditions, it could be argued that SDN was the main culprit for this degradation.
Methods
Subjects
Ten subjects, free of any back pain for at least 1 year, volunteered for this study and signed the consent form approved by the Yale University Human Investigation Committee. Anthropometric data are provided in Table 1. No subjects reported having neurological or musculoskeletal problems.
Data collection
EMG signals were recorded from eight trunk muscles using 1 cm diameter, Ag-AgCl, disposable surface electrodes in a bipolar configuration. Following site preparation, the electrodes were placed with a center-to-center spacing of 3 cm over the following muscles on each side of the body: rectus abdominis (RA, 3 cm lateral to the umbilicus), external oblique (EO, medial to the mid auxiliary line at the level of the umbilicus), thoracic erector spinae (TES, 5 cm lateral to T9 spinous process), and lumbar erector spinae (LES, 3 cm lateral to L4 spinous process). A reference electrode was placed laterally over the 10th rib on the right side of the subject. In previous studies, this electrode placement proved to maximize signal-to-noise ratio and demonstrated reduced levels of cross talk (Cholewicki and McGill 1996; Cholewicki et al. 1997). All EMG signals were band-pass filtered between 20 and 420 Hz, differentially amplified (input impedance = 100 GΩ, CMRR > 140 dB) and A/D converted at a sample rate of 1,600 Hz.
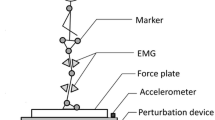
Seated balancing was chosen to localize the effects of trunk muscle control in a system that is inherently unstable. Subjects were placed on a seat equipped with leg and foot supports to prevent any lower body movement (Fig. 1) (Cholewicki et al. 2000). A 30 cm diameter polyester resin hemisphere was attached to the bottom of the seat that was placed on a force plate (Kistler Model 9286AA, Germany) at the edge of a table. Each subject was instructed to maintain his/her balance while sitting on the seat with arms crossed. A safety railing around the force plate provided security in case of loss of balance.
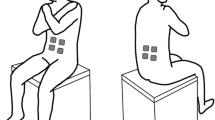
There were four balancing tasks: control, belt, trunk co-activation, and arm co-activation. Each task was performed with the eyes open and closed making a total of eight conditions. The eyes open and eyes closed trials were included to gauge the effect of visual feedback on the relationship between trunk stiffness and postural control during unstable seated balancing. The control condition simply required subjects to balance on an unstable seat. The belt condition produced an increase in passive trunk stiffness and required subjects to balance on an unstable seat while wearing a QUIKDRAW Pro™ lumbosacral brace (Aspen Medical Devices, Irvine, CA) (Fig. 2). For the trunk and arm co-activation conditions, subjects were asked to elevate their muscle co-activation, but not to a set level. The trunk co-activation was designed to increase active trunk stiffness and required subjects to co-activate trunk muscles while balancing. Rectified and filtered EMG muscle activities from the right RA and LES muscles were displayed on an oscilloscope to provide subjects with visual feedback. The arm co-activation condition was used as a control for the trunk co-activation condition to see if the attention required for maintaining increased muscle activity interfered with the performance of the balancing task. Subjects were asked to contract their forearm muscles while making a fist during unstable seated balancing. No visual feedback was given to the subjects when performing arm co-activation, however, we could observe if their arms were co-contracted. Subjects did not appear to have difficulty maintaining some increased level of activation. No trials were removed from the analysis because of insufficient trunk or arm co-activation.
Three trials were performed for each of the eight conditions with a 30 s rest break between each trial. Data collection, lasting 20 s, was initiated after a subject achieved a steady state. Subjects were asked to hold on to the safety railing at all times between the trials to prevent any additional learning. Subjects practiced each task for 1 min both with eyes open and closed. The order of the conditions was randomized between subjects to minimize the effects of learning.
The raw EMG was full-wave rectified, and filtered with a digital, zero-phase lag, low-pass Butterworth filter (4th order, 2 Hz cut-off frequency). The EMG data from each muscle was then normalized to % maximum voluntary contraction (MVC) as described by McGill (1992). Since the requirement for equilibrium requires that flexor and extensor torque be balanced, any increase in extensor activity would most likely need to be balanced by a corresponding increase flexor activity. Consequently, a ratio between flexors and extensors was not used as an index of co-activation. Therefore, EMG data were averaged across all eight trunk muscles.
The center of pressure (CoP) velocity reflected the mean displacement per second of CoP during the 20 s trial. This measure was the most reproducible of all body sway parameters and had the benefit of not being significantly influenced by cognitive demands (Raymakers et al. 2005). Figure 3 shows an example of CoP velocity and EMG for two conditions: eyes open control (EOC) and eyes open trunk co-activation (EOTC).
Data analysis
Preliminary statistics were performed on the data using two repeated measures ANOVAs with the dependent variables being CoP velocity and EMG. The independent variables for each ANOVA were eyes (open and closed) and condition (control, belt, trunk co-activation, arm co-activation). Direction (anterior–posterior and lateral) was also included in the ANOVA to determine if there was a significant difference between the two directions for CoP velocity. Analysis included main effects along with interactions.
Given significant effects from ANOVAs, the following planned pairwise comparisons were performed. To test the hypothesis that increased trunk muscle co-activation leads to degraded postural control, two paired t tests were used that compared the dependent variables CoP velocity and EMG for the control and trunk co-activation conditions. If this hypothesis were true, both CoP velocity and EMG would be significantly higher for the trunk co-activation condition than the control condition.
To test the hypothesis that SDN results in degraded postural control, a planned paired t test was used to compare the dependent variable CoP velocity for the trunk co-activation to the belt condition. If this hypothesis were true, CoP velocity for the belt condition would be significantly lower than the trunk co-activation condition, which would imply that increased joint stiffness alone cannot account for diminished postural control. To ensure that attention demands from increased muscle recruitment did not affect the results, CoP velocity was also compared between the arm and trunk co-activation condition. If CoP velocity for the trunk co-activation condition was significantly higher than the arm co-activation condition, it could be argued that additional attention demands did not degrade postural control.
To minimize the possibility of family-wise type I error, a critical value of P = 0.01 was used for all analyses.
Results
ANOVA results for CoP velocity showed that the main effect of eyes and condition were significant (see Table 2 and Fig. 4), but direction of path and interactions were not. CoP velocity was higher with the eyes closed than with the eyes open (mean CoP velocity, eyes closed 22.6 mm/s (± 6.83), open 13.5 mm/s (± 2.73)). ANOVA results for EMG showed that the condition effect was significant, but not the eyes effect or their interaction (Table 2).
CoP velocity was significantly higher in the trunk co-activation condition than the control (P < 0.001) and the arm co-activation (P < 0.001) conditions (Fig. 4a). As expected, significant differences were observed in EMG between trunk co-activation condition and both control (P = 0.001) and arm co-activation conditions (P = 0.001) with the trunk co-activation EMG being higher than the other two conditions (Fig. 4b).
Results also confirmed that CoP velocity was significantly lower in the belt condition than the trunk co-activation condition (P = 0.002). No differences were found for CoP velocity between belt and control condition (P = 0.282). Interestingly, EMG was significantly lower in the belt condition compared to the control condition (T = 4.36, P < 0.001).
Discussion
There were two main questions being addressed with this study: (1) does increased trunk muscle activity degrade postural control during unstable seated balancing? and (2) is SDN responsible for this impairment? Results clearly support both hypotheses: increased trunk co-activation was shown to degrade postural control, and this degradation was only observed during increased trunk muscle recruitment.
There are a few possible confounders for this study that need to be addressed. First it is possible that the stiffness from the belt is less than the stiffness produced from trunk co-activation. In a previous study, we found that the passive increase in trunk stiffness from a belt is equivalent to, if not more than, the increased stiffness due to trunk muscle co-activation for similar levels found in the current study (Cholewicki et al. 1999). Next, it is also possible that enhanced proprioceptive feedback from wearing a belt could significantly improve balance performance. The degradation from increased joint stiffness could have been mitigated by improvements in body awareness. There are a few reasons why we believe this may not be the case. Our previous work investigating trunk proprioception while wearing a belt showed that changes occur over a period of weeks and that these changes were not consistent (Cholewicki et al. 2006). Since our subjects were not given time to acclimatize to the belts, it appears unlikely that increased proprioception could explain improvements in postural performance. In addition, it would be expected that improvements in postural control from the belts would be more pronounced in the eyes closed condition since the dominant sensory feedback of vision was removed. Our results showed that there were no differences in performance between the control and belt condition when the eyes were closed suggesting no significant proprioceptive advantage was gained. Thus, SDN remains the likely explanation for the poorer postural control under the increased trunk muscle co-activation condition.
CoP velocity obtained in this study match closely those found in previous studies using similar protocol(Cholewicki et al. 2000; Silfies et al. 2003). The CoP velocity in the current study for the control condition was 21.5 mm/s (eyes closed) and 11.8 mm/s (eyes open). Silfies et al. values for similar conditions were approximately 22 mm/s (eyes closed) and 12 mm/s (eyes open), and in the Cholewicki et al. study, values for the eyes open condition were 10.5 mm/s. Our study along with previous work illustrates the importance of visual feedback in postural control. However, visual feedback did not affect the relationship between trunk stiffness and postural control.
In terms of trunk muscle activation comparisons, the average trunk muscle EMG in the control condition was approximately 4% MVC. This is slightly higher than the observed trunk muscle co-activation of approximately 2% MVC found during stable standing (Cholewicki et al. 1997). But this elevated level of activity could be expected since unstable dynamics tend to increase muscle co-activation (Milner and Cloutier 1993; Milner 2002). Interestingly, the reduction in trunk EMG observed with the belt could reflect its stabilizing potential (Cholewicki 2004).
In contrast to our findings, Gruneberg et al. (2004) concluded that passive stiffening of the trunk and hip increases the likelihood of loss of balance during standing (laterally and/or backwards). They showed that increased trunk and hip stiffness resulted in altered movement patterns for maintaining balance after perturbations. They also found that healthy subjects could not rapidly modify movement strategies sufficiently to account for this increased stiffness. This does not appear to be the case for our seated balancing task since the increased stiffness to the trunk via passive bracing did not impair postural control. Differences between the task and amount of passive restraint between the studies may explain these discrepancies. Also in contrast to our findings, other studies have shown improved task accuracy with additional muscle co-activation. Selen et al. (2005) using a modeling approach found that any negative effects from increased SDN were offset by the benefits from increased mechanical impedance during co-activation. Gribble et al. (2003) also showed that accuracy was improved in a reaching task with increased co-activation of muscles crossing the shoulder and elbow joints. However, in these studies, the systems were anchored to stationary objects, whereas in our study, the trunk was supported by the unstable seat and the whole system was free to move. Therefore, increased co-activation would not have the same affect on mechanical impedance of the system. It simply increased the amount of noise in the system without the benefits of resisting movement from perturbation.
In conclusion, this study demonstrated that increased trunk muscle co-activation leads to degraded postural control during unstable seated balancing. However, these conclusions cannot be simply extrapolated to other tasks. Typically, there is a trade-off between a system’s performance and energy cost with more energy being required to minimize performance errors. But increased stiffness of one sub-system will not necessarily result in improved performance of the entire system. First, there is likely an optimum amount of stiffness within each subsystem that is required for stability and performance. As shown with our experiment, the SDN becomes an issue when increased stiffness is achieved via muscle co-activation beyond some optimal level. For example, if subjects increased co-activation beyond their naturally selected level in the experiment of Gribble et al. (2003), it is possible that the accuracy of arm movement could begin to decline. Second, the relationship between stiffness and performance is dependent on the task and the task objectives. For example, the objective for a person with low-back pain (LBP) balancing in our apparatus may be to minimize spine movement. Increased muscle co-activation would be helpful for such a task objective, but not for balance performance (Radebold et al. 2001). Most likely, the central nervous system chooses the appropriate stiffness for all joints to optimize performance of the entire system, metabolic costs, or some other objectives (i.e., tissue loading, pain, etc.) and their combination. And finally, in terms of LBP rehabilitation, exposing patients to various tasks with different objectives should be encouraged during the treatment period to foster optimal muscle co-activation levels.
References
Bergmark A (1989) Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl 230:1–54
Cholewicki J (2004) The effects of lumbosacral orthoses on spine stability: what changes in EMG can be expected? J Orthop Res 22:1150–1115
Cholewicki J, McGill SM (1996) Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech 11:1–15
Cholewicki J, Panjabi MM, Khachatryan A (1997) Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine 22:2207–2212
Cholewicki J, Juluru K, Radebold A, Panjabi MM, McGill SM (1999) Lumbar spine stability can be augmented with an abdominal belt and/or increased intra-abdominal pressure. Eur Spine J 8:388–395
Cholewicki J, Polzhofer GK, Radebold A (2000) Postural control of trunk during unstable sitting. J Biomech 33:1733–1737
Cholewicki J, Shah K, McGill SM (2006) The effects of a three-week use of lumbosacral orthoses on proprioception in the lumbar spine. J Orthop Sports Phys Ther 36:225–231
Christou EA, Grossman M, Carlton LG (2002) Modeling variability of force during isometric contractions of the quadriceps femoris. J Mot Behav 34:67–81
Crisco JJ III, Panjabi MM (1991) The intersegmental and multisegmental muscles of the lumbar spine. A biomechanical model comparing lateral stabilizing potential. Spine 16:793–799
Crisco JJ, Panjabi MM, Yamamoto I, Oxland TR (1992) Euler stability of the human ligamentous lumbar spine: Part II experiment. Clin Biomech 7:27–32
Gardner-Morse MG, Stokes IA (2001) Trunk stiffness increases with steady-state effort. J Biomech 34:457–463
Granata KP, Slota GP, Bennett BC (2004) Paraspinal muscle reflex dynamics. J Biomech 37:241–247
Gribble PL, Mullin LI, Cothros N, Mattar A (2003) Role of cocontraction in arm movement accuracy. J Neurophysiol 89:2396–2405
Gruneberg C, Bloem BR, Honegger F, Allum JH (2004) The influence of artificially increased hip and trunk stiffness on balance control in man. Exp Brain Res 157:472–485
Hamilton AF, Jones KE, Wolpert DM (2004) The scaling of motor noise with muscle strength and motor unit number in humans. Exp Brain Res 157:417–430
McGill SM (1992) A myoelectrically based dynamic three-dimensional model to predict loads on lumbar spine tissues during lateral bending. J Biomech 25:395–414
Milner TE (2002) Adaptation to destabilizing dynamics by means of muscle cocontraction . Exp Brain Res 143:406–416
Milner TE, Cloutier C (1993) Compensation for mechanically unstable loading in voluntary wrist movement. Exp Brain Res 94:522–532
Oxland TR, Crisco JJ 3rd, Panjabi MM, Yamamoto I (1992) The effect of injury on rotational coupling at the lumbosacral joint. A biomechanical investigation. Spine 17:74–80
Panjabi MM (1992a) The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 5:383–389
Panjabi MM (1992b) The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord 5:390–396
Radebold A, Cholewicki J, Polzhofer GK, Greene HS (2001) Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 26:724–730
Raymakers JA, Samson MM, Verhaar HJ (2005) The assessment of body sway and the choice of the stability parameter(s). Gait Posture 21:48–58
Selen LP, Beek PJ, van Dieen JH (2005) Can co-activation reduce kinematic variability? A simulation study. Biol Cybern 93:373–381
Silfies SP, Cholewicki J, Radebold A (2003) The effects of visual input on postural control of the lumbar spine in unstable sitting. Hum Mov Sci 22:237–252
Slifkin AB, Newell KM (2000) Variability and noise in continuous force production. J Mot Behav 32:141–150
Stokes IA, Gardner-Morse M, Henry SM, Badger GJ (2000) Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine 25:1957–1964
Weeks DL, Forget R, Mouchnino L, Gravel D, Bourbonnais D (2003) Interaction between attention demanding motor and cognitive tasks and static postural stability. Gerontology 49:225–232
Acknowledgements
This study was supported by NIH Grant Number 5R01 AR051497 from the National Institute of Arthritis and Musculoskeletal and Skin Disease.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reeves, N.P., Everding, V.Q., Cholewicki, J. et al. The effects of trunk stiffness on postural control during unstable seated balance. Exp Brain Res 174, 694–700 (2006). https://doi.org/10.1007/s00221-006-0516-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0516-5