Abstract
This study evaluated the degree to which the disturbance to posture from respiration is compensated for in healthy normals and whether this is different in people with recurrent low back pain (LBP), and to compare the changes when respiratory demand is increased. Angular displacement of the lumbar spine and hips, and motion of the centre of pressure (COP), were recorded with high resolution and respiratory phase was recorded from ribcage motion. With subjects standing in a relaxed posture, recordings were made during quiet breathing, while breathing with increased dead-space to induce hypercapnoea, and while subjects voluntarily increased their respiration to match ribcage expansion that was induced in the hypercapnoea condition. The relationship between respiration and the movement parameters was measured from the coherence between breathing and COP and angular motion at the frequency of respiration, and from averages triggered from the respiratory data. Small angular changes in the lumbopelvic and hip angles were evident at the frequency of respiration in both groups. However, in quiet standing, the LBP subjects had a greater displacement of their COP that was associated with respiration than the control subjects. The LBP group had a trend for less hip motion. There were no changes in the movement parameters when respiratory demand increased involuntarily via hypercapnoea, but when respiration increased voluntarily, the amplitude of motion and the displacement of the COP increased in both groups. The present data suggest that the postural compensation to respiration counteracts at least part of the disturbance to posture caused by respiration and that this compensation may be less effective in people with LBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiration presents a cyclical intrinsic disturbance to balance (Gurfinkel et al. 1971; Gagey and Toupet 1997; Hodges et al. 2002). Recent evidence has confirmed that in a healthy population this disturbance is compensated by small angular displacements of body segments that are phase-locked to respiration. In healthy people, the centre of pressure (COP) at the ground shows little displacement at the frequency of respiration (Gurfinkel et al. 1971; Hodges et al. 2002; Kantor et al. 2001). This compensation, however, does not exist in people with some forms of neurological deficit (Gurfinkel et al. 1971; Gurfinkel and Elner 1988; Kohen-Raz cited in Gagey and Toupet 1997), and preliminary evidence suggests that the displacement of the COP may occur in phase with respiration in people with low back pain (Gagey 1986; Guillemot and Duplan 1995). Further research is required to confirm these findings and evaluate whether this is associated with decreased compensation.
There has been disagreement in the literature regarding the degree to which postural disturbances from respiration may be compensated, although it is agreed that respiration does disturb posture (Gurfinkel et al. 1971; Aggashyan 1974; Boiusset and Duchene 1994; Gagey and Toupet 1997; Kantor et al. 2001; Hodges et al. 2002). Some argue that the peturbations are completely compensated (Gurfinkel et al. 1971), whilst others claim there is no compensation (Hunter and Kearney 1981; Jeong 1991). However, recent evidence has confirmed the presence of phasic trunk and lower limb movements that are in phase with respiration and may act to counteract the respiratory disturbance (Kantor et al. 2001; Hodges et al. 2002). However, the degree of compensation has been shown to vary between individuals (Boiusset and Duchene 1994). Further support for the hypothesis that angular movements compensate for the respiratory disturbance comes from studies that show the disturbance to be greater when segments of the kinematic chain are removed, such as during kneeling (Aggashyan 1974) and sitting (Bouisset and Duchene 1994). Moreover, the compensation is most optimal during quiet breathing and is less efficient when the demand is increased by breathing at higher volumes (Bouisset and Duchene 1994; Hodges et al. 2002; Kantor et al. 2001). When this respiratory volume is increased automatically via hypercapnoea, the respiratory disturbance is better compensated for than when the tidal volume is increased voluntarily (Hodges et al. 2002). This disparity could be attributable to the mechanical differences between the two tasks (Sackner et al. 1984; Gandevia et al. 1999; Hodges et al. 2002), the different frequency and volume of breathing in each condition, or the organisation of the compensatory mechanism; that is, the postural control strategy may be more effective when respiration is under automatic control (Hodges et al. 2002).
Impairments of postural control have been identified in people with low back pain (LBP) in terms of control of both equilibrium and spinal stability (Byl and Sinnot 1988; Alaranta et al. 1994; Alexander and Kinney LaPier 1998; Luoto et al. 1998, 1999; Mientjes and Frank 1999). LBP patients exhibit increased body sway and excursion of COP in quiet standing (Byl and Sinnot 1988; Alexander and Kinney Lapier 1998; Luoto et al. 1998), and have changes in control of spinal muscles (Hodges and Richardson 1996; Brumagne et al. 2000). Although it is impossible to determine the mechanism of these changes from previous studies, these changes in postural control may be due to mechanical aspects or control factors. For instance, mechanically, the postural responses may be altered due to a reduction in the range of spinal motion (Mellin 1986) or to the different resting position of the spine in LBP patients (Byl and Sinnot 1988; Mientjes and Frank 1999). Changes in the control strategy (Hodges et al. 2001), proprioceptive deficits (Alaranta et al. 1994; Brumagne et al. 2000) and impaired functioning of short-term memory (Luoto et al. 1999) as well as altered motor planning (Hodges et al. 2001) may also be implicated. The lumbar spine and pelvis are key elements in the compensatory strategy for respiration (Kantor et al. 2001; Hodges et al. 2002); thus we hypothesised that changes in the normal compensatory strategy may result in increased excursion of the COP with respiration in the LBP population.
The aims of the present study were, firstly, to determine whether there is motion of the centre of pressure with respiration in people with low back pain, secondly, to evaluate the hypothesis that increased motion is due to impairment in the compensatory mechanism which consists of angular displacements of the trunk and lower limbs and, thirdly, we aimed to compare the changes in this compensation with increased respiratory demand.
Materials and methods
Subjects
Recordings were made from ten participants with a history of recurrent LBP and ten healthy control subjects. Subjects in the LBP group were to have had LBP of an episodic nature for at least 18 months (mean duration was 3.54 years) with a minimum of one painful episode per year, but with little (<2 on a visual analogue scale) or no pain at the time of testing and not currently taking any medication for pain relief. Subjects were to have experienced pain that was of sufficient intensity to limit function and for which they had sought medical or allied health treatment and was of insidious onset rather than the result of gross trauma. Control subjects were to have had no history of LBP that had interfered with function. Subjects in both groups were excluded if they had a history of respiratory or neurological disease, lower limb musculoskeletal injuries, uncorrected visual impairment, previous spinal surgery, observable spinal deformity, a history of dizziness or falls, or had undergone intensive abdominal or back muscle training in the preceding three months. Activity level scores, measured with the Baecke Activity Level Questionnaire (Baecke et al. 1982), were 3.0 for the control group and 3.2 for the LBP group. These scores indicate that both groups were of average activity level and, when compared using a t-test for independent samples, no difference was found between the groups (P=0.60). The mean (SD) age, height and weight of the control and LBP subjects were 26 (5.4) years, 1.71 (0.1) m, 66 (15.1) kg and 32 (8.3) years, 1.73 (0.1) m, 69 (14.7) kg, respectively. No difference in any parameter was found (P=0.11, 0.68, and 0.68, respectively). All subjects gave their informed consent prior to inclusion in the study. This study was conducted in a manner consistent with the Declaration of Helsinki and was approved by the Institutional Ethics Committee.
Movement analysis
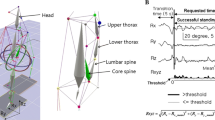
Six sensors (Motionstar Movement Analysis System, Ascension Technology) were placed on the lumbar spine (at L2 and L5), pelvis (anterior and posterior) and lateral thigh (over greater trochanter and 5 cm above the lateral femoral epicondyle) to record kinematic data as shown in Fig. 1. All sensors were on the right side of the subject and fixed using tape. To amplify the pelvic motion, the sensors were attached to the ends of a 1-m lightweight rod that was firmly strapped around the pelvis at the level of the iliac crests (Hodges et al. 2002). Movement data were sampled at 60 Hz using Motion Monitor software (Innovative Sports Training, USA). The resolution of the movement analysis system is reported to be 0.1° within the range used in the present experiment. In additional trials we measured the accuracy of the system to detect angular displacement of the assembly used here and found it to accurately detect angular displacement of less than 0.1° when it was calculated from the linear displacement of the markers. We are confident that these data are not transmitted from the respiratory movements of the abdominal wall as our previous results indicate that the direction of lumbar and pelvic motion varies between individuals and can precede the respiratory movements (Hodges et al. 2002). Movement data were temporally aligned to the other recordings with a trigger.
Forceplate
Vertical reaction force (Fz) and moment around the coronal axis (My) were recorded with a single forceplate (Kistler, USA). Data were recorded at 500 Hz using a CED Power1401 and Spike2 Software (Cambridge Electronic Design, UK).
Respiratory movements
Respiratory phase was recorded with an inductance plethysmograph (Respitrace 200, Non-Invasive Monitoring Systems Inc., USA) placed around the chest. Respiratory data were sampled at 500 Hz and collected with forcepate data.
Procedure
Subjects stood on the forceplate in a relaxed position, with the feet shoulder width apart and arms by their side. A nose clip was worn for all trials. The tasks were as follows:
-
1.
Quiet breathing: The subjects stood for 2 min with the head facing forwards and breathed normally.
-
2.
Hypercapnoea: Subjects breathed through a tube (volume ~1450 ml) to increase the dead-space. Subjects were allowed to accommodate to the dead-space for 20 s, after which 2 min of data were collected.
-
3.
Increased tidal volume: Subjects were given feedback of ribcage motion on a monitor with the target minima and maximas set to match the displacements achieved during hypercapnoea. Two minutes of data were collected.
Tasks were performed in a quasi-randomised manner with the three tasks organised into two sets. These sets consisted of quiet breathing, and hypercapnoea followed by increased tidal volume. It was necessary for hypercapnoea to precede the increased tidal volume condition to allow the target minima and maxima volumes to be set for the increased tidal volume task
Data analysis
Movements of the trunk, pelvis and lower limbs were calculated as the angle between adjacent segments in the sagittal plane to remove the effect of postural sway that would be present if an external reference frame was used (Hodges et al. 2002). The motion of the centre of pressure was approximated from the forceplate data, using the equation COP=My/Fz.
Two different analyses were used to investigate the presence of movements of the trunk, pelvis, lower limbs and the centre of pressure in association with respiration. Firstly, frequency analysis was performed to determine the power of the angular displacement and COP data at the frequency of respiration, and the coherence, a measure of the strength of the association between respiration and movement and COP data, at the frequency of respiration (identified as the dominant peak in the frequency spectrum of the respitrace data). Secondly, averages were triggered from the ribcage motion data to investigate the amplitude of motion time-locked to respiration.
For frequency analysis, the movement and COP data were resampled at 50 Hz so all data were at the same sampling frequency and could thus be compared directly with equal resolution for the Fourier transform analysis. The power spectral densities of the autocorrelations of the motion data were then calculated. Spectral analysis was performed using a Hanning window with no overlap. The frequency of respiration was identified as the largest peak in the power spectrum of respiratory data.
The coherence between the movement parameters and respiration was calculated to evaluate the relationship between the movement and COP data, and respiration. Coherence is a measure of the correlation between two signals in the frequency domain, that is, the extent to which the phase and amplitude of the signals are related at each frequency. A coherence of one indicates that the phase-shift between the waveforms is constant at a particular frequency and the amplitude of the signals at that frequency has a constant ratio. Coherence is independent of the amplitude of the individual signals. Coherence was calculated from the cross-spectral density between two signals which had been normalised by the power spectral density of each waveform. The coherence between the movement parameters and respiration at the frequency of respiration was then identified.
Triggered averages were used to evaluate the amplitude of angular displacement with respiration. The temporal relationship between respiration, angular movement and COP was identified from averages triggered at the peak of inspiration, which were then time-normalised to 100 samples per respiratory cycle and averaged across all respirations. Peak-to-peak displacement was calculated.
Statistical analysis
The principal aim of the experiment was to evaluate whether displacement of the COP was coupled with respiration. Thus the coherence between COP and respiration was compared between groups with a t-test for independent samples.
To evaluate the differences in coherence between movement parameters and respiration, and amplitude of motion from triggered averages, we compared between groups and between respiratory conditions with two-way analyses of variance (ANOVA) and Duncan's multiple range test. The significance level was set at α=0.05. Data are presented as mean (SD) in the text.
Results
When subjects with no history of LBP stood and breathed quietly, small angular displacements in the angles formed between the lumbar spine and pelvis (lumbopelvic angle) and pelvis and thigh (hip angle) were evident in the raw data that were temporally related to the cyclical respiratory movements of the thorax (Fig. 2). It follows that peaks in the power spectral densities of the angle data were present at the frequency of respiration. The coherence between respiratory movement and angular displacements was calculated to quantify the relationship between these parameters. The coherence between respiratory movement and the lumbopelvic and hip angles was ~0.5, indicating relatively consistent temporal and spatial relationships between these parameters. In contrast, the coherence between respiratory movements and the displacement of COP was smaller [0.33 (0.18)]. The absolute amplitude of the respiration-related deviations in movement data was measured from averages triggered from the onset of inspiration. The mean deviation in COP was 2.3 (1.1) mm. Although displacement of the lumbopelvic angle was small, 0.14 (0.05)°, larger amplitude motion was identified at the hip [1.04° (0.90)].
Ribcage movement, COP displacement and angular motion of body segments of a representative control and LBP subject during quiet breathing. Dashed lines are aligned to the inspiratory peaks to facilitate inspection of the respiration-related changes in COP. In the control subject, note the presence of small amplitude changes in the hip and lumbopelvic angle that are in phase with respiratory movement of the chest wall. In the LBP subject, note the larger and more consistent peaks in the COP at the frequency of respiration
When people with recurrent LBP performed the same task (i.e. quiet breathing), they had greater sway than the control subjects (Fig. 3). The increased COP displacement occurring in-phase with the ribcage movement is evident in the raw data of a representative LBP subject presented in Fig. 2. The coherence between respiration and the motion of COP [0.45 (0.21)] was greater than that identified for the control subjects (P=0.03). Furthermore, 78% of the subjects in the LBP group compared with only 44% of the control subjects had a coherence between COP and respiration that was greater than 0.5. There was no difference in the coherence between lumbopelvic/hip motion and respiratory movement of the ribcage at the frequency of respiration (0.45, P=0.86, and 0.40, P=0.89, for lumbopelvic and hip motion, respectively). There was a trend for the range of hip motion time-locked to respiration to be decreased in the LBP group [0.48° (0.25)], although this was not significant (P=0.08). This is likely to be due, at least in part, to the large variation in the control subjects. However, the largest value of hip range of motion that was time-locked with respiration in the LBP group was ~20% smaller than the mean range of hip motion for the control group (see Fig. 3). The absolute amplitude of the lumbopelvic motion was also not different between groups [0.24 (0.28)°, P=0.32]. Furthermore, the range of motion of the COP that was time-locked to respiration was not different between groups, although there was a trend for the amplitude to be increased in the LBP subjects [3.13 (2.78) mm, P=0.36]. Despite this consistent range of motion, the reduced coherence indicates that the COP displacement and respiration were more tightly coupled for LBP subjects.
Range of motion time-locked to respiration and coherence between movement variables and respiration at the frequency of respiration for the control and LBP groups. Means and SD are shown. Note the greater COP motion associated with respiration in people with LBP during quiet breathing and the trend for less hip motion in LBP subjects. Also note the increase in COP displacement in both groups when respiratory demand was increased voluntarily, and the absence of change when the demand is increased automatically via hypercapnoea
When the respiratory demand was increased, subjects in both groups responded in a similar manner. When subjects breathed with an increased dead-space to induce hypercapnoea, the amplitude of ribcage motion was increased involuntarily. Despite this increase in respiratory movement of the ribcage, there was no change in the amplitude of COP motion (measured from the averages triggered from the respiratory movement) (control: P=0.70; LBP: P=0.40) or in the amplitude of the coherence between COP and respiratory movement (P=0.53). The absence of change in COP was associated with an increased coherence between the hip and respiratory motion (P=0.02); yet the absolute amplitude of the hip motion was not changed (P=0.87). There was no change in amplitude of lumbopelvic motion (P=0.73) or coherence between angular motion and respiratory movement (P=0.21). In contrast to the hypercapnoea condition, when subjects voluntarily increased their tidal volume with feedback to match the volumes obtained in the involuntary condition, there were changes in all parameters. Firstly, the amplitude of COP movement was increased compared to both the quiet breathing and hypercapnoea conditions for subjects in both groups (P<0.02). The amplitude of motion time-locked to respiration in the increased tidal volume condition was greater than the other conditions for control subjects [6.7 (2.7) mm, P<0.0001], but was not increased for the LBP subjects [4.8 (1.7) mm, P=0.06]. That is, the amplitude of COP motion increased more for the control subjects than those in the LBP group (P<0.0001). The coherence between hip motion and respiratory movement of the ribcage was increased when tidal volume was increased voluntarily compared to the quiet breathing condition (P=0.03), and there was no difference between groups (P=0.90). However, no change was evident in the coherence between the angular motion of the lumbopelvic angle and respiration (P=0.21). Despite this, the absolute amplitudes of both the lumbopelvic and hip motion were increased with increased tidal volume in the LBP group [0.45 (0.50)° and 1.29 (0.87)°, respectively] and the control group [0.96 (1.26)° and 2.06 (2.00)°, respectively] (P<0.01). Again, this was not different between groups (P=0.48 and P=0.18, respectively).
Discussion
The results of this study show that, unlike pain-free control subjects, people with a history of recurrent LBP have a component of the anteroposterior motion of their COP that is time-locked to respiration. That is, they do not effectively compensate for the respiration-related postural sway. The present data confirmed that in healthy upright subjects, small angular movements of the hip and lumbopelvic angle compensate for the perturbation to posture caused by the movements of respiration. During quiet breathing, it was shown that subjects with LBP exhibited greater sway of their COP in association with respiration and also portrayed a trend for less hip movement. However, when the respiratory demand was increased, there were no differences between subject groups. Taken together, these data argue that, in standing, people with LBP have a less effective postural compensation for respiration when breathing quietly.
Normal response to respiration
There has been a lack of consensus in previous research regarding the extent to which the respiratory perturbations are compensated in healthy subjects. It has been proposed that the periodic disturbance to posture due to respiration is completely counteracted (Gurfinkel et al. 1971) while it has also been argued that no compensation exists (Hunter and Kearney 1981; Jeong 1991). However, the present data are consistent with recent evidence which concluded there is at least a partial compensation (Hodges et al. 2002; Kantor et al. 2001) and confirmed the presence of angular movements of the trunk and lower limbs that were in-phase with respiration. These findings are also in accordance with an earlier study by Gurfinkel and colleagues (1971). It is acknowledged, however, that variation exists between individuals (Boiusset and Duchene 1994; Hodges et al. 2002).
In the present study, when respiratory demand was increased, the findings were congruous with those reported by Hodges et al. (2002). During hypercapnoea, there was no change to the displacement of the COP and this absence of change was associated with an increased coherence between hip and respiratory motion. This is consistent with augmentation of the postural compensation to counteract the greater ribcage displacements. In contrast, when tidal volume was increased voluntarily by matching breath volume to that in hypercapnoea, the postural compensation was least efficient, resulting in greater sway of the COP at the frequency of respiration. This condition is similar to the voluntary-paced respiratory tasks used in the studies by Hunter and Kearny (1981) and Jeong (1991), which both found a significant proportion of body sway to be in-phase with respiration. The difference in results between the hypercapnoea and increased tidal volume conditions may be due to several factors. Firstly, in mechanical terms, it has been reported that hypercapnoea results in a proportional increase in ribcage and abdominal movement (Gandevia et al. 1999), whereas voluntary hyperventilation results in a greater proportion of ribcage expansion (Sackner et al. 1984). However, the results of the present study argue against this alternative as subjects matched ribcage expansion between tasks. In addition, the frequency and volume of respiration have been found to differ between the two tasks (Hodges et al. 2002). These mechanical factors may have an effect on the ability of the central nervous system (CNS) to implement a compensatory strategy. Perhaps more likely is the difference in central regulation of the respiratory movements. That is, in the hypercapnoea task the increase in respiration is involuntary compared to the voluntary nature of the volume-matching task. Thus the respiratory tasks have different control mechanisms and the automatic postural compensatory strategy may be more effective when respiration is under automatic control (Hodges et al. 2002).
Changes in the response of recurrent LBP patients
This study has shown that the normal control of COP is compromised in people with LBP during quiet respiration. This confirms preliminary evidence which suggested that people with LBP have COP displacement that is directly related to respiration (Gagey 1986; Guillemot and Duplan 1995). Gagey (1986) described in a preliminary report the spectral analysis of the COP of LBP patients and identified a peak that was absent in a pain-free population, which coincided with the frequency of respiration. From this, Gagey surmised that functional disorders of the body axis, such as LBP, may result in changes to the postural compensation mechanism for respiration, hence explaining why these patients displayed greater sway of their COP that was synchronous with ventilation. Guillemot and Duplan (1995) confirmed Gagey's preliminary observations of a peak in the spectra of the COP of LBP subjects at the frequency of respiration that was not present in the spectra of healthy subjects. Consistent with these preliminary findings, the present study has shown that LBP subjects portray abnormal COP sway that is in relation to respiration. In addition to LBP, rhythmical disturbance to COP has been observed in people with some forms of CNS pathology (Gurfinkel et al. 1971; Gurfinkel and Elner 1988). However as the mechanisms of such changes are uncertain, it is not known whether the greater COP sway in association with LBP stems from similar origins to CNS pathologies.
There are several possible explanations for the increased COP sway in association with respiration in LBP subjects, which require consideration. It could be hypothesised that the compensation may be affected by a number of mechanisms. For instance biomechanical, sensory, control or organisational aspects could be implicated. Mechanically, LBP has been linked with reduced mobility of the lumbar spine either due to mechanical restriction (McGregor et al. 1995; Mellin 1986), muscle activity (Hodges and Richardson 1996) and/or fear of movement (Watson et al. 1997), which may limit the contribution of the lumbar segment from participation in the compensatory strategy, and hence greater sway of the COP at the frequency of respiration would result. Consistent with this hypothesis, it has been argued that the COP in patients with LBP is situated more posterior than pain-free individual (Byl and Sinnot 1988) and that this altered 'resting position' increases the lumbar lordosis, allows the extensor muscles of the trunk to relax (Byl and Sinnot 1988) and requires less knee extension (Mientjes and Frank 1999), the posture perhaps adopted to avoid pain. The increased lordosis, however, places the lumbar vertebrae in greater extension and thus the potential for further extension on inspiration to counteract the posterior movement of the trunk may then be reduced. Decreased lumbopelvic and hip motion may also arise from pain provocation or fear of pain (Watson et al. 1997), which result in altered movement characteristics and fear-avoidance behaviour. In addition it has been argued that pain may result in hyperactivity of trunk muscles, although the specific muscles involved may vary between individuals (Hodges et al. 2001). Such hyperactivity may act to splint the spine and reduce its contribution to the postural compensation.
Previous research has shown that the ability of the body to produce a counterperturbation to the respiratory disturbances is reduced with removal of segments from the kinetic chain, for example when sitting (Bouisset and Douchene 1994) or kneeling (Aggashyan 1974), in which the pelvis and lower limbs are somewhat constrained in comparison to standing. During such tasks, the compensatory strategy relies substantially on the movements of the spine to counteract the respiratory disturbances and thus it would be interesting to investigate whether LBP would lead to a decreased ability to execute such a compensatory pattern. The present data also suggest that the LBP subjects have a trend for less hip motion. It is not surprising that lumbopelvic and hip motion were not significantly different between groups as multiple different strategies are available; thus it is unlikely that all subjects would change in the same manner.
A restriction in thoracic mobility has also been found in people with LBP (Mellin 1986). Not only could this have an effect on the pattern of breathing, but the stiffness of the thoracic spine influences the potential for movement of this segment of the kinetic chain in terms of its ability to partake in the compensatory strategy. Thus with the decreased contribution of the thorax, the effectiveness of the compensation would be reduced.
The reduction in postural compensation may also be due to changes in afferent input from the lumbar spine and pelvis that have been identified in people with LBP (Alexander and Kinney LaPier 1998). In this patient group proprioceptive deficits have been shown to exist which result in a reduced acuity of lumbosacral position sense and increased error (Alaranta et al. 1994; Brugmagne et al. 2000). In turn, the ability to reposition the spine accurately is also deficient (Brugmagne et al. 2000), thus hampering the control of body posture (Alexander and Kinney LaPier 1998). Coordination of movement based on inaccurate perception of body segment position and inaccurate feedback of the outcome of movement is likely to lead to errors in movement performance and may therefore influence the execution of the compensation strategy.
The decreased postural compensation to respiration in LBP may also be due to changes affecting motor control and planning. Recent studies have reported changes in motor responses and postural control in LBP (Hodges et al. 2002; Alaranta et al. 1994; Alexander and Kinney LaPier 1998) that may be due to changes in higher centres. Although changes in the sensory system cannot be excluded, these authors have argued that the deficits may be due to changes in motor planning due to direct effects of pain on areas of the CNS that are involved in motor planning (see Derbyshire et al. 2002 for review), or factors such as impairment of short term memory (Luoto et al. 1999). These changes may have relevance to the deficits we have identified, but the mechanism of coordination of the compensation for respiration cannot be identified from the present data. The compensation may be organised directly from the pontomedullary respiratory centre; arise from other CNS areas in a feedforward manner; or use a feedback mechanism consisting of afferent information from the periphery. Further research on the organisation of the postural compensation is required in order to draw conclusions as to possible changes in this system of control that may be present in LBP subjects.
A final consideration is that many muscles of the trunk are involved in both respiratory and postural functions and thus the problem may occur in the coordination of these functions. It has been shown that the postural function of several trunk muscles, for example transversus abdominis and the diaphragm, is compromised when respiratory demand is increased (Hodges et al. 2001). The addition of LBP may further compromise the control.
Conclusion
The ability of the CNS to counteract an anticipated disturbance such as respiration, depends upon the static and dynamic equilibrium conditions, body posture, configuration of the base of support and postural chain mobility, several of which have been shown to be inauspicious in LBP. The present data provide further evidence for inadequate postural control in people with LBP, in that they have greater COP sway in association with quiet respiration and have a trend for less hip motion to counteract such disturbance to posture. The data have implications for the management of recurrent LBP patients and suggest that balance training may be required and this may need to involve respiratory challenges.
References
Aggashyan RV (1974) Investigation of dynamics of maintenance of vertical posture by spectral and correlation analysis. Thesis, Academy of Sciences of the USSR, Moscow
Alaranta H, Moffroid M, Elmqvist L, Held J, Pope M, Renstrom P (1994) Postural control of adults with musculoskeletal impairment. Crit Rev Phys Rehab Med 6:337–370
Alexander KM, Kinney LaPier TL (1998) Differences in static balance and weight distribution between normal subjects and subjects with chronic unilateral low back pain. J Sport Phys Ther 28:378–383
Baecke JAH, Burema J, Frijters JER (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. J Clin Nutr 36:936–942
Bouisset S, Duchene JL (1994) Is body balance more perturbed by respiration in seating rather than standing posture? Neuroreport 5:957–960
Brugmagne S, Cordo P, Lysens R, Verschueren S, Swinnen S (2000) The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine 25:989–994
Byl NN, Sinnot PL (1988) Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine 16:325–330
Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL (1997) Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73:431–445
Gagey P (1986) Postural disorders among workers on building sites. In: Bles W, Brandt T (eds) Disorders of posture and gait. Elsevier, Amsterdam, pp 253–268
Gagey PM, Toupet M (1997) The posture ventilation mystery: amplitude of postural sway in the 0.2 hertz frequency band. Retrieved September 17, 2001 from http://perso.club-internet.fr/pmgagey
Gandevia SC, Gorman RB, McKenzie DK, De Troyer A (1999) Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med 160:1598–1603
Guillemot A, Duplan B (1995) Étude de la prévalence des troubles posturaux au sein d'une cohorte de 106 lumbalgiquies. In: Gagey P, Weber B (eds) Entrées du système postural fin. Masson, Paris, pp 71–77
Gurfinkel VS, Elner AM (1988) Contribution of the frontal lobe secondary motor area to organisation of postural components in human voluntary movement. Neirofiziologiya 20:7–15
Gurfinkel VS, Kots YM, Paltsev EI, Feldman AG (1971) The compensation of respiratory disturbances of erect posture of man as an example of the organisation of interarticular interaction. In: Gelfand IM, Gurfinkel VS, Formin SV, Tsetlin ML (eds) Models of the structural functional organisation of certain biological systems. MIT Press, Cambridge, MA, pp 382–395
Hodges PW, Richardson CA (1996) Inefficient muscular stabilisation of the lumbar spine associated with low back pain. Spine 21:2640–2650
Hodges PW, Moseley G, et al. (2003) Acute experimental pain changes postural recruitment of the trunk muscles in pain-free humans. Exp Brain Res (in press)
Hodges PW, Heijnen I, Gandevia SC (2001) Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J Physiol 537:999–1008
Hodges PW, Gurfinkel VS, Brugmagne S, Smith TC, Cordo PC (2002) Coexistence of stability and mobility in postural control: evidence from postural compensation for respiration. Exp Brain Res 144:293–302
Hunter IW, Kearney RE (1981) Respiratory components of human postural sway. Neurosci Lett 25:155–159
Jeong BY (1991) Respiration effect on standing balance. Arch Phys Med Rehabil 72:642–645
Kantor E, Poupard L, Le Bozec S, Bouisset S (2001) Does body stability depend on postural chain mobility or stability area? Neurosci Lett 308:128–132
Luoto S, Aalto T, Taimela S, Hurri H, Pyykko I, Alaranta H (1998) One-footed and externally disturbed two-footed postural control in patients with chronic low back pain and healthy control subjects. Spine 23:2081–2090
Luoto S, Taimela S, Hurri H, Alaranta H (1999) Mechanisms explaining the association between low back trouble and deficits in information processing. Spine 24:255–261
McGregor AH, McCarthy ID, Hughes SPF (1995) Motion characteristics of normal subjects and people with low back pain. Physiotherapy 81:632–637
Mellin G (1986) Correlations of spinal mobility with degree of chronic low back pain after correction for age and anthropometric factors. Spine 12:464–468
Mientjes MIV, Frank JS (1999) Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech 14:710–716
Sackner MA, Gonzalez HF, Jenouri G, Rodriguez M (1984) Effects of abdominal and thoracic breathing on breathing pattern components in normal subjects and in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 130:584–587
Watson PJ, Booker CK, Main CJ (1997) Evidence for the role of psychological factors in abnormal paraspinal activity in patients with chronic low back pain. J Musculoskel Pain 5:41–56
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimstone, S.K., Hodges, P.W. Impaired postural compensation for respiration in people with recurrent low back pain. Exp Brain Res 151, 218–224 (2003). https://doi.org/10.1007/s00221-003-1433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1433-5