Abstract
According to Swiss law, sausages claiming to be made of veal have to contain at least 50% veal. To control the meat proportion of such products, control laboratories use real-time PCR. The measurement uncertainty of this method is at 30%. As a consequence, only extreme fraud can be reliably detected. To analyse sausages for their beef content with lower measurement uncertainty, a duplex droplet digital PCR was developed. Interlaboratory conversion factors were determined to enable weight-to-weight determination using values gained by real-time PCR and droplet digital PCR. Precision and accuracy were investigated examining reference sausages and sausages from the market. Comparing with real-time PCR, results from digital PCR showed a superior interlaboratory measurement uncertainty of 10% and will enable food control laboratories to determine also minor fraud.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Sankt Galler Kalbsbratwurst” is a defined boiled sausage where the origin is certified (Protected Geografical Indication IGP) and the composition is defined. According to Swiss law, at least 50% of the meat has to be veal, the rest pork [1]. As veal is expensive, butchers may modify the basic recipe of 50% veal to their advantage. Since the introduction of PCR, different methods for the quantification of animal DNA were published, mainly real-time PCR methods [2,3,4,5,6,7,8,9,10] and more recently digital PCR [11,12,13,14]. But the actual methods exhibit a typical measurement uncertainty of 30–50%, in some cases even more [8, 10, 15]. Because of this high measurement uncertainty, only major differences are measurable. False composition between 50% and 35% veal proportions cannot be determined with enough certainty to reject such products. Fraud may, therefore, be profitable, with little risk to be discovered. Therefore, a method with lower measurement uncertainty would be favourable for control laboratories.
Recently, digital PCR became available and it was reported that digital PCR may be more precise and accurate than real-time PCR. It was shown that quantification of animal DNA is feasible using digital PCR [11,12,13,14]. However, a direct comparison between real-time PCR and digital PCR is, to our knowledge, not yet published. In addition, earlier experiments showed that weight-to-weight measurement becomes possible when using a correction factor. Such correction factors are needed as the proportions of measured nanogram of DNA (real-time PCR) or in case of digital PCR copy numbers cannot be expected to lead to proportions of weight to weight (recipe). Here we present a duplex pork beef droplet digital PCR system and its validation data in parallel with a multiplex real-time system. Four laboratories generated data applying both systems. The results include interlaboratory measurement uncertainties and individually determined conversion factors leading to values of weight to weight. The results applying droplet digital PCR are promising and the method is suitable to be applied in routine analysis.
Materials and methods
Reference sausages
Reference sausages were produced according to the composition described in Table 1. According to a traditional recipe of “Kalbsbratwurst”, 10 kg of each sausage mixture was produced by the Master Butcher School Spiez, Switzerland. Reference sausages were analysed for the content of water, fat, total protein, connective tissue protein and muscle protein. The principal components were consistent with an average, commercially obtainable “Kalbsbratwurst” already used in an earlier proficiency trial [6].
For the proficiency test, DNA eluates of 66 sausages (50 in duplicate resulting in 116 DNA samples) from the market, mainly type “Kalbsbratwürste”, some were other boiled sausages, were analysed by real-time PCR and digital droplet PCR by all four participating laboratories. In total, 66 eluates of market samples were analysed.
DNA extraction
DNA extraction from all sample matrices was performed using the Wizard Plus Miniprep DNA purification system (Promega, Madison, USA). Usually, 200 mg of ground sample material was extracted and DNA was eluted in 50 µl elution buffer according to the supplier’s manual. The concentration was determined photometrically and adjusted by dilution to 20 ng/µl. For digital PCR, 5 µl of a 2 ng/µl solution of template DNA revealed to be optimal.
Droplet digital PCR
For both applied PCR systems (porcine and bovine), the PCR target sequence for pork and beef has been kept on the same genes as published previously [10]. Both target sequences are coded in the cell nucleus. Optimization steps were performed according to an extensive earlier publication [16, data not shown]. The final optimized concentrations and sequences for the droplet digital PCR are listed in Table 2.
Real-time PCR
The real-time PCR method, called Allmeat, which was applied in this work was published earlier in detail [7]. A ringtrial with this method showed that this method is transferable to different laboratories. Another ringtrial confirmed and extended the earlier findings [15]. Measurement uncertainty of 30% or more was reported for both ringtrials.
Droplet digital PCR procedure (ddPCR)
5 µl DNA extract was added to 17 µl of reaction mix containing 11 µl Supermix for Probes (Cat no.186–3024), and the primers and probes. Primer final concentrations for all applied ddPCR systems were 0.4 µM, probes were at 0.25 µM. 20 µl of these final 22 µl was mixed with 70 µl of oil to generate a water/oil emulsion. The microfluidic cartridges for the ddPCR were used to produce this emulsion according to the QX200-System manual. After pipetting the 40 µl emulsion to the multiwell plates, they were sealed using sealer PX1 from Bio-Rad. The emulsion PCR was performed on a thermoblockcycler (Mastercycler Nexus, Eppendorf). The cycling was done as follows: initial step of 10 min at 95 °C; followed by 39 or 50 cycles of 30 s at 94 °C and 60 s at 60 or 55 °C. The ramp rate was fixed to 2 or 2.5°C/s. After this, a deactivation step of 10 min at 98°c was applied followed by cooling down to 4 °C. The whole cycling required approximately 2.5 h. The reading of the droplets was then performed using the droplet reader of the QX200-System. All steps were performed according to manual of the QX200 Droplet Digital PCR (ddPCR™) System (Bio-Rad Laboratories Inc. USA). Computing was done using the QuantaSoft version 1.6.6.0320 software applying the ABS mode for the Fam and Hex channel. Accepted numbers of droplets were 7000 per reaction.
Application of conversion factors to calculate weight-to-weight proportions
Using real-time PCR, the concentration of DNA can be determined relative to an external standard curve delivering ng/µl DNA. When measuring two analytes such as beef and pork DNA the proportion of each can be calculated. This counts also for digital PCR measuring copy numbers directly. But as previously shown these directly determined proportions often do not correspond to the real proportion in weight to weight [7]. The copy numbers (determined by real-time PCR or digital PCR) per weight is not equal between different species. Many factors can be the reason for this, such as tissue proportion, fat and treatments. One solution is to use a reference material whose composition and production is similar to the matrix of the samples. Comparing the directly calculated proportion and the known properties of the reference material a conversion factor can be calculated according to the following formula (similar for ddPCR and real-time PCR):
where Mb is measured beef in ng/µl or copies/µl (e.g. 1580), Ms is measured pig ng/µl or copies/µl (e.g. 1700), F is the conversion factor (e.g. 1.57).
Example for ddPCR:
The proportion of beef (beef %) by weight to weight (w/w) can be calculated according to the following formula:
Mb is measured beef in ng/µl or copies/µl (e.g. 1580), Ms is measured pig ng/µl or copies/µl (e.g. 1700), F is the conversion factor (e.g. 1.57).
Example for ddPCR:
Of course, this conversion factor is affected by the precision of the method and has, therefore, to be determined during several independent measurement rounds using the known reference material and the external standard curve. Determined in this way, the conversion factor is valid for only the tested matrix (product) and the used PCR systems (real-time PCR and/or digital PCR). Once determined, it makes the use of matrix-adapted reference material obsolete. We applied this technique and determined the conversion factor in this work. To test if this approach works, four other laboratories determined their own conversion factors using the same PCR systems and the same reference material. The final test was done by analysis of 66 market sausages by all 4 laboratories. For digital PCR, all four laboratories used the ddPCR equipment from Biorad (QX200). The primers and probes were provided centralized from one laboratory. Besides the in-house validation of the here proposed duplex digital PCR system, an additional goal was to point out the best quantification strategy to result in the lowest measure uncertainty over more than one laboratory.
Results
Optimization of the ddPCR
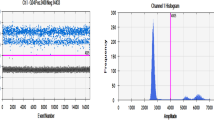
The concentration of the primers of the existing real-time PCR system was titrated. At the optimized concentration, the signal for beef showed a clear upper population of positive droplets (Fig. 1) in channel 1 (Fam). In some cases, the negative population showed a double band (Fig. 1). But this phenomenon did not show up with samples containing only beef. We, therefore, attributed this to a crosstalk from the pork signal to the beef channel (Fam). However, it is clearly distinguishable from the positive population and, therefore, considered as unproblematic. The pork system showed in all cases only two clear populations (Fig. 2) in channel 2 (Hex/Joe).
Visualization of the droplet digital PCR system for the determination of beef (Rd) in channel 1 (Fam). All four reference sausages were analysed (from left: beef: 30%, 40%, 50%, 60%, two dilutions each, total of eight lanes). The amplitude graph shows one positive and two negative droplet populations per sample. The threshold can be clearly set as indicated in the histogram. Experiments showed that the double-negative population is a result of crosstalk from the Hex channel into the Fam channel. This can be seen also in Fig. 1 when comparing the intensities shown in Fig. 2 in parallel
Visualization of the droplet digital PCR system for the determination of pork (Sw) in channel 2 (Joe/Hex). All four reference sausages were analysed (from left: beef: 30%, 40%, 50%, 60%, two dilutions each, total of eight lanes). The amplitude graph shows one positive and one negative droplet population per sample. The threshold can be clearly set in between as indicated in the histogram
Specificity
For the two droplet digital PCR systems, specificity is a prerequisite. To test the experimental specificity, DNA of the following organisms was isolated and tested for unspecific amplification of template DNA of the following species: sheep (Ovis aries), goat (Capra aegagrus), horse (Equus caballus), duck (Anatidae), goose (Anser domesticus), red legged partridge (Alectoris rufa), turkey (Meleagris gallopavo),rabbit (Oryctolagus cuniculus), buffalo (Bubalis bubalus), Hare (Leporidae), roe deer (Capreolus capreolus), red deer (cervus elaphus), lama (Lama glama), zebra (Equus grevyi) cat (Felis silvestris catus), dog (Canis lupus familiaris), paprika (Capsicum annuum), white pepper (piper nigrum), onion (Allium cepa), garlic (Allium sativum), nutmeg (Myristica fragrans), almond (Prunus dulcis), walnut (Juglans regia), hazelnut (Corylus avellana), peanut (arachis hypogaea), celery (Apium graveolens), carrot (Daucus carota sativus), cinnamon (Cinnamomum verum), chive (Allium schoenoprasum), beans (Phaseolus vulgaris), parsley (Petroselinum crispum), ginger (Zingiber officinale), black mustard (Brassica nigra), clove (Syzygium aromaticum), pistachio (Pistachia vera), cashew (Anacardium occidentale), sesame (Sesamum indicum), wheat (Triticum spp), rye (Secale cereale), sage (Salvia fructosa), and thyme (Thymus spp).
The only cross-reactivity emerged with the beef system when using DNA from roe deer and buffalo as a template. These species produce similar signals like beef. This has to be considered when analysing samples which might contain meat from roe deer and buffalo. However, meat from roe deer and buffalo is expensive and is not expected to be common by sausages.
Sensitivity, precision and uncertainty
The sensitivity (LOD) was assayed using a reference sausage containing 1% of pork or 1% of beef meat. All 11 independent runs gave a positive amplification result. We consider, therefore, the LOD at 1% for both analytes.
The LOQ was assayed similarly using reference sausages with 9% pork or beef. The relative standard deviation over all laboratories (RSDR) was 21.4% and accuracy + 0.9% for beef and RSD 15.1%, accuracy + 0.5% for pork.
The target products of this work ranged between 30% and 60% ratio of beef in pork. Precision, accuracy and measurement uncertainty in this range were assessed extensively in this work and are compiled in Table 3.
Analysis of reference sausages by real-time PCR and determination of a conversion factor
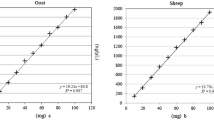
We used a combination of DNA dilution rows for the external calibration of the real-time PCR and a conversion factor to calculate the weight-to-weight proportion. The DNA dilution rows contain the appropriate species and were quantified spectrophotometrically. To calculate the conversion factor as described in “Materials and methods” we analysed reference sausages and adjusted this factor until the normalized proportion of the reference sausage, containing 50% beef and pork, showed 50%. Due to the measurement uncertainty, inherent to real-time PCR, the conversion factor varies from determination to determination. To reduce the variation we determined the factor five times independently. The resulting average factor was 1.5 (Table 4).
Analysis of reference sausages by droplet digital PCR and determination of the conversion factor
Using droplet digital PCR to determine the meat proportions is similar to real-time PCR. One simplification compared to real-time PCR is that no calibration is required to gain quantitative positive droplet values (copies/µl). Again, we measured the concentration of positive droplets for both analytes (beef and pork) of the four reference sausages. We adjusted the conversion factor until the normalized proportion of the reference sausage contained 50% beef and 50% pork. This measurement was repeated 14 times to get an averaged conversion factor with minimal variability (Table 4).
Interlaboratory variation when analysing samples from the market
All four laboratories had to analyse the same DNA extracts by real-time PCR and by digital PCR. In addition, the conversion factors had to be determined several times using the provided 50% reference sausage for each laboratory individually. The results from each method without conversion and with conversion factors were collected and compared (see Table 4 for the conversion factors).
66 sausages (16 samples single, 50 samples in duplicate, resulting in total 116 DNA samples which had to be analysed) from the market, type “Kalbsbratwürste” were analysed by real-time PCR and digital droplet PCR. The following results were obtained.
The four results from each sample (116 in total) conversed by the appropriate factor were averaged and the individual single results were normalized to this mean value. The results were graphically compiled for all real-time results (Fig. 3) and for all droplet digital PCR results (Fig. 4). The relative standard deviation (RSD) was calculated for the results of all four laboratories. All these RSD values were averaged. This was done for the results of real-time PCR with and without conversion factor and also for the results using droplet digital PCR with and without conversion factor (Table 5).
Four laboratories using real-time PCR measured all 116 samples. Each value was normalized to the mean values over the values of all four labs of this sample. These normalized values are compiled here (y-axis). The x-axis represents the sample number. The variation is obviously significant. Additionally, a bimodal effect can be seen from sample 60 to 116. The conversion factors do not correlate with this effect. The reason for this bimodal effect could not be revealed
Four laboratories using digital droplet PCR measured all 116 samples. Each value was normalized to the mean values over the values of all four labs of this sample. These normalized values are compiled here (y-axis). The x-axis represents the sample number. Compared to the compilation of the real-time values the variations are moderate and close to the mean values (100%). The outliers seem to be random, they are not linked to a, e.g. laboratory
Conclusion
The results revealed for real-time PCR a RSD of almost 40% without and 24% with applying the conversion factor. Using ddPCR, the averaged RSD over all four laboratories was only 4.3% without conversion factor and 10.2% with conversion factor. As the conversion factor corrects for accuracy, it is not possible to calculate true values without applying a conversion factor. However, the factors vary (Table 5) reflecting the variation of the applied methods. Comparing the RSD of the factors and the RSD of the results it seems that the factors have a crucial impact on the RSD of the results between the laboratories. It should be addressed in future experiments if the use of the same factors in all laboratories, leads to better reproducibility, specially, in combination with droplet digital PCR.
The here-presented droplet digital PCR method exhibited a better precision and the results seem to be more reproducible between different laboratories than results generated with real-time PCR. In consequence, smaller deviations from the legal requirements can be detected applying ddPCR. These findings apply to sausages containing around 50–50% veal/pork. Other combinations have to be investigated in future experiments.
References
Swiss Food Legislation Verordnung über Lebensmittel tierischer Herkunft; 16. Dezember 2016; Art. 9 Abs. 5
Sawyer J, Wood C, Shanahan D, Gout S, McDowell D (2003) Real-time PCR for quantitative meat species testing. Food Control 14:579–583
Brodmann P, Moor D (2003) Sensitive and semi-quantitative TaqMan® real-time polymerase chain reaction systems for the detection of beef (Bos taurus) and the detection of the family Mammalia in food and feed. Meat Sci 65:599–607
Lopez-Andreo M, Garrido-Pertierra LL, Prieto A, Puyet I A (2005) Identification and quantitation of species in complex DNA mixtures by real-time polymerase chain reaction. Anal Biochem 339:73–82
Laube I, Zagon J, Broll H (2007) Quantitative determination of commercially relevant species in foods by real-time PCR. International Journal of Food Science Technology 42:336–341
Eugster A, Ruf J, Rentsch J, Hübner P, Köppel R (2008) Quantification of beef and pork fraction in sausages by real-time PCR analysis: results of an interlaboratory trial. European Food Research Technology 227(1):17–20
Köppel R, Ruf J, Zimmerli F, Breitenmoser A (2008) Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, chicken and turkey. European Food Research Technology 227(4):1199–1203
Eugster A, Ruf J, Rentsch J, Köppel R (2009) Quantification of beef, pork, chicken and turkey proportions in sausages: use of matrix-adapted standards and comparison of single versus multiplex PCR in a interlaboratory trial. European Food Research Technology 230:55–61
Jonker KM, Tilburg JHC, Hägele GH, De Boer E (2008) Species identification in meat products using real-time. PCR Food Additives Contaminants 25(5):527–533
Köppel R, Ruf J, Rentsch J (2011) Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, horse and sheep. Eur Food Res Technol 232:151–155
Cai Y, Li X, Lv R, Yang J, Li J, He Y, Pan L (2014) Quantitative Analysis of Pork and Chicken Products by Droplet Digital PCR. BioMed Research International Volume 2014 (2014), Article ID 810209, 6 pages
Floren C, Wiedemann I, Brenig B, Schütz E, Beck J (2015) Species identification and quantification in meat and meat products using Droplet. Digital PCR (ddPCR. Food Chem 173:1054–1058
Shehata R, Li RH,J, Chen S, Helen Redda H, Cheng S, Li TN,H, Hanner WK R (2017) Droplet digital polymerase chain reaction (ddPCR) assays integrated with an internal control for quantification of bovine, porcine, chicken and turkey species in food and feed. PLOS one. doi.https://doi.org/10.1371/journal.pone.0182872
Wang Q, Cai Y, He Y, Yang L, Li J, Pan L (2018) Droplet digital PCR (ddPCR) method for the detection an quantification of goat and sheep derivatives in commercial meat products. Eur Food Res Technol 244:767–774
Uhlig S, Baldauf H, Simon K, Methodenringversuch “All Meat” zum quantitativen Nachweis von DNA der Tierarten Huhn, Pute, Rind und Schwein mittels Tetraplex real-time PCR, Quodata, in preparation
Gerdes L, Iwobi A, Busch U, Pecoraro S (2016) Optimization of digital droplet polymerase chain reaction for quanitifcation of genetically modified organism. Biomolecular detection Quantification 7:9–20
Acknowledgements
We thank the cantonal laboratory of Zürich, the Official Food Control Authority of the Canton St.Gallen, Cantonal Office of Consumer Protection Bern, Switzerland and AGES CC Biochemie Vienna, Austria, for providing sample material and resources for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or living animal subjects.
Rights and permissions
About this article
Cite this article
Köppel, R., Ganeshan, A., van Velsen, F. et al. Digital duplex versus real-time PCR for the determination of meat proportions from sausages containing pork and beef. Eur Food Res Technol 245, 151–157 (2019). https://doi.org/10.1007/s00217-018-3147-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3147-8