Abstract
The probability of accidental or intentional addition of another species of meat in meat products is high. Meat is expensive and in the context of food waste, it is reasonable to reduce loss of this precious material during the production. However, the line between fraud and waste reduction is thin. Minor variations, e.g., up to 1% of unexpected meat content, may be tolerated. But to distinguish between minor variations and severe deviations to the list of ingredients, analytical methods with a low measurement uncertainty are required. The recent introduction of digital PCR indicated that this new method may lead to a reduction of the measurement uncertainty. We present the measurement of proportions of beef, pork, chicken, turkey, sheep and horse meat in a cooked sausage matrix and a procedure to calculate the proportions (w/w) based on target DNA concentrations measured using droplet digital PCR. Six laboratories applied these methods and determined the w/w proportions of 20 sausage samples. It was shown that these methods in conjunction with conversion factors can be used to estimate meat proportions in mixed meat products with superior accuracy and precision compared to results generated by real-time PCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food fraud is a serious problem in the production of meat products. Laws, like the Swiss Food Law, prohibit fraudulent practices [1], but the line between waste reduction and fraud is sometimes unclear. The enforcement of the law relies on the possibility to discover fraud using analytical methods. Effort was made in the past to develop real-time PCR methods for such tasks and many publications present successful quantification methods [2,3,4,5,6,7,8,9]. But, the applied real-time PCR exhibits a typical measurement uncertainty of roughly 30% (best cases, mean of three determinations) or more [10,11,12] within one laboratory and within a certain product type when using an appropriate reference material. This measurement uncertainty is composed of the precision and accuracy. Multiple measurements may improve the precision, but the accuracy may still be biased. Therefore, earlier work tried to ameliorate the accuracy by the use of matrix-adapted reference materials (e.g., boiled sausages [8]). Although these results seemed to correspond better with the true values of the samples, the measurement uncertainty still stayed at about 30% (best cases, one measurement). However, fraudulent practices do not only take place by exchanging expensive meats with cheaper meats but also more discretely by increasing the cheaper proportions. Such minor deviations from recipes or intentional fraudulency cannot be detected by the currently available methods. Previous studies already suggest that digital PCR may be more precise than real-time PCR and therefore measurement uncertainty may be lower [13,14,15,16], making this technique better suited for detecting smaller differences in meat proportions. To convert digital copy number measurements into % (w/w) values, we used conversion factors as described earlier [17] but here using six species at once. To determine the conversion factors, we used previously produced matrix-adapted reference materials. The conversion factors were determined in each of six laboratories participating in a ring trial, to create a data set used to calculate mean conversion factors. These mean conversion factors should be valid for all laboratories using this measurement approach.
Finally, we present three duplex droplet digital PCR (ddPCR) assays for the determination of proportions of pork/beef, horse/sheep and turkey/chicken meat in mixed products and its validation data. The six ring trial laboratories generated this data, allowing the assessment of interlaboratory reproducibility and robustness.
Materials and methods
Reference sausages as calibrators
The sausages used to act as calibrators for the determination of the conversion factors were selected to cover a broad variability of possible meat products: mainly beef/pork, mainly poultry and a sheep/horse combination. They were produced professionally and verified during earlier ring trials. Meat contents and references with the precise description are compiled in Table 1.
Sample sausages
The sample sausages were also taken from previous ring trials. Their meat contents and references with the precise description are compiled in Table 2.
DNA extraction
The DNA extraction was performed by the participants, applying their routine methods. The DNA concentration was determined photometrically and adjusted by dilution to 2 ng/µL. The following DNA extraction methods were applied:
-
Wizard Plus Miniprep DNA purification system (Promega, Madison, USA). 200 mg of starting material eluted into 50 µL elution buffer.
-
Wizard® Magnetic DNA Purification System for Food (Promega, Madison, USA), using the Thermo Scientific Kingfisher Duo Prime automated DNA extraction platform. 200 mg of starting material eluted into 120 µL of TE0.1 buffer (10 mM Tris–HCl, 0.1 mM EDTA, pH 8.0).
-
Relia-Prep-system according to the manufacturers’ manual (Promega, Madison, USA). 300 mg of starting material eluted into 150 µL of elution buffer (10 mM Tris–HCl, pH 8.0).
-
Maxwell instrument in conjunction with Maxwell® RSC PureFood GMO and Authentication Kit (Promega, Madison, USA).
-
CTAB precipitation method [18].
Duplex ddPCR systems
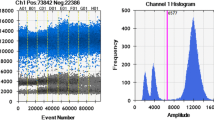
Three duplex ddPCR systems were developed. The systems containing the primers and probes for beef, pork, horse, lamb, chicken, and turkey were already published and described earlier [7, 8]. The optimization of the duplex ddPCR was performed using the recommendations from previous work [19]. All primers/probes, sources and optimal concentrations of the duplex ddPCR systems are compiled in Table 3. Figures 1, 2, 3 and 4 show the populations of the droplets after amplification using the duplex systems for chicken/turkey and horse/sheep developed here. In all systems, the positive population was clearly distinguishable from the negative population and allowed the determination of copies/µL for each species by the QuantaSoft™ software of the QX100/200 system.
Visualization of the ddPCR system for the determination of sheep (Sf) in channel 1 (Fam). The amplitude graph shows two positive and one negative droplet populations. The threshold can be clearly set as indicated in the histogram. We speculate that the double positive population is a result of a different allele sequence within the probe-binding site (Genbank sequence FJ901297.1), leading to a second positive population with different endpoint fluorescence amplitude
Visualization of the dPCR system for the determination of turkey (Trut) in channel 2 (Joe/Hex). The amplitude graph shows one positive and one negative droplet populations. The threshold can be clearly set in between as indicated in the histogram, although the distance between the positive and negative population is close
ddPCR procedure
5 µL DNA extracts were added to 17 µL of reaction mix containing 11 µL Supermix for Probes (Bio-Rad, Cat No. 186-3024) and 6 µL pre-mixed primers and probes. The final concentrations of primers for all applied ddPCR systems are listed in Table 3; probe concentrations were 0.25 µM. 20 µL of these final 22 µL PCRs was mixed with 70 µL of droplet generator oil (Bio-Rad, Cat No. 1863006) to generate a water/oil emulsion. The Bio-Rad microfluidic cartridges for the ddPCR were used to produce this emulsion according to the QX100/200-System manual. After pipetting the 40 µL emulsion into multiwall plates, they were sealed using a Bio-Rad PX1 plate sealer. The emulsion PCR was performed on the following thermocyclers: Mastercycler Nexus (Eppendorf), LifePro Thermocycler (Bioer Technology Co. Ltd.), C1000 Touch Thermal Cycler (Bio-Rad), Biometra T-Gradient 96 cycler (Analytica Jena) or PE 9700 Thermal Cycler (Perkin Elmer). The cycling was performed as follows: initial step of 10 min at 95 °C, followed by 50 cycles of 30 s at 94 °C and 60 s at 55 °C. The ramp rate was fixed to 2.5°C/Sec. After this, a deactivation step of 10 min at 98 °C was applied followed by cooling down to 4 °C. The whole cycling required approximately 2.5 h. The reading of the droplets was then performed using either the QX100/200-System or QX100 droplet reader. All steps were performed according to QX100/200 ddPCR System manual from Bio-Rad Laboratories Inc. USA. Computing was done using the QuantaSoft™ software applying the ABS mode for the Fam and Hex channels.
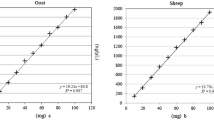
Application of conversion factors was used to calculate % (w/w) proportions. The concentration of DNA (copies per µL) can be measured by digital PCR. And, these values can be taken to calculate meat proportions. But, as previously shown, these directly determined proportions are biased [7, 8, 11, 12] and do not correspond to the % (w/w) proportions. One solution is to use a similar processed reference material with a similar composition. By comparing the directly calculated proportion of DNA copy number concentrations with the known meat proportions (w/w) of this reference material, conversion factors can be calculated for each species according to the following formula:
where Mb is measured beef in copies/µl and Fb is conversion factor for beef. Among the attributes, b: beef (e.g., 858 copies/µl), p: pork (e.g., 1335 copies/µl), c: chicken (e.g., 157 copies/µl), t: turkey (e.g., 115 copies/µl), h: horse, and s: sheep.
The following formula is an example for a sample containing beef, pork, chicken and turkey:
The factors for this example are taken from Table 4.
The conversion factors are determined experimentally using the provided reference sausages with known meat proportions.
Of course, these conversion factors are affected by the precision of the method itself and have therefore to be determined during several independent measurement rounds. Determined in this way, the conversion factors are valid for only the tested matrix and the used PCR systems (real-time PCR and/or digital PCR). Once determined, it makes the use of matrix-adapted reference material obsolete. We have already applied this technique in an earlier study. In this work, we determined the conversion factors for six species. To test if this approach also works in other laboratories, six other laboratories determined conversion factors independently using the same PCR systems and the same reference materials. The final test was performed by an analysis of 20 sample sausages by all 7 laboratories. For digital PCR, all seven laboratories used the ddPCR equipment from Bio-Rad.
Ring trial setup
For this ring trial, the sample sausages from different previous independent ring trials were used. These samples are well-characterized and represent different kinds of sausage products. They may be classified as follows:
-
Products containing mainly beef and pork with contaminations of chicken and turkey.
-
Products containing mainly chicken and turkey with contaminations of pork and beef.
-
Products containing mainly horse and pork with contaminations of lamb and beef.
-
Products containing mainly beef and pork with contaminations of horse and lamb.
-
Products containing mainly lamb and beef with contaminations of horse and pork.
Three of these products were used to generate the conversion factors: one mainly beef/pork product with contaminations of chicken and turkey, one inverse product and one product with equal amounts of horse, pork, beef and lamb.
The DNA from these samples had to be extracted by each laboratory using their own DNA extraction method. All required duplex digital PCR primers and probes were provided pre-mixed and dried. All other materials required to perform digital PCR had to be provided by the participating laboratory.
The results (concentrations of copies per µL) calculated by the QuantaSoft™ software were transferred into the calculation sheet provided. The conversion factors were iteratively optimized by the individual laboratory until the calculated proportions corresponded to the given proportions for the three calibration reference materials. The ddPCR measurements were repeated to deliver three datasets per sample.
Results
Specificity
For the ddPCR systems, specificity is a prerequisite. To test the specificity experimentally, DNA from the following specimens was isolated and tested for unspecific amplification: sheep (Ovis aries), goat (Capra aegagrus), horse (Equus caballus), duck (Anatidae), goose (Anser domesticus), red-legged partridge (Alectoris rufa), turkey (Meleagris gallopavo), rabbit (Oryctolagus cuniculus), buffalo (Bubalis bubalus), hare (Leporidae), roe deer (Capreolus capreolus), red deer (servus elaphus), llama (Lama glama), zebra (Equus grevyi), cat (Felis silvestris catus), dog (Canis lupus familiaris), paprika (Capsicum annuum), black pepper (piper nigrum), onion (Allium cepa), garlic (Allium sativum), nutmeg (Myristica fragrans), celery (Apium graveolens), carrot (Daucus carota sativus), cinnamon (Cinamomum verum), chive (Allium schoenoprasum), beans (Phaseolus vulgaris), parsley (Petroselinum crispum), ginger (Zingiber officinale), black mustard (Brassica nigra), clove (Syzygium aromaticum), wheat (Triticum spp.), rye (Secale cereale), and sage (Salvia fructosa), thyme (Thymus spp.).
The only cross-reactivity above 0.1% emerged with the pork/beef system when using DNA from roe deer and buffalo as a template. These species produce signals similar to beef. This was described already earlier [7] and has to be considered.
Analysis of reference sausages by ddPCR and determination of the conversion factor by all seven laboratories
All participants had to measure the concentration of copies per µL for all expected meat species in the calibration samples (three in total). The conversion factors according to the formula described here, were adjusted until the normalized values (sum in total 100%) were close to the given values (Table 5). The conversion factor for beef was kept to 1 as a fixed point. The resulting conversion factors are compiled in Table 4 and show variations between the laboratories especially for the poultry chicken (RSD 24.8%) and turkey (RSD 24.5%). For chicken, the conversion factor was much higher than for all other species.
Interlaboratory limit of detection (LOD) and limit of quantification (LOQ) within a matrix
The LOD and the LOQ was estimated as the lowest spiking level of all samples which were detected by all laboratories in all assays as positive. At this level, quantification characteristics were determined. The resulting LOD and LOQ were therefore at least 0.5% for chicken, 0.67% for turkey, 0.5% for beef (one negative result at 0.14%), 1% for pork, 1% for horse and 1% for sheep. The exact LOD was not determined, because this approach is being assessed as a quantitative system.
Interlaboratory precision, accuracy and measurement uncertainty within a matrix
Two sample measurements had to be removed from the total 360 sample measurements. They were clear outliers with values far from the values generated by the other laboratories. The reason remained unclear, false setting of the threshold line may be a reason.
The relative standard deviation (RSD) was taken to estimate the precision. The RSD for each sample is compiled in Table 6. The mean RSD over all measurements and laboratories (including DNA isolation) is 18%. Correlation of the RSD to the spiking level is presented in Fig. 5.
Dependence of the % RSD on the spiking level. The line shows the average % RSD at the corresponding spiking level (see also Table 7). Below 5% spiking level, the % RSD increases from 10% or lower to 20% or higher
The relative deviation from the assigned value (spiking) was taken to estimate the accuracy. The deviation for each sample is compiled in Table 6. The mean deviation over all measurements and laboratories (including DNA isolation) is 22%.
Considering the RSD, it is obvious that the MU in the lower spiking levels is significantly higher than in the spiking level above 5%. Therefore, the measurement uncertainties (MU) were calculated by splitting the data into four categories (0–5%, 5–20%, 20–40 and above 40–85% spiking). Quantification below 5% seems to exhibit the highest MU of 38% (see Table 7). In the higher spiking level, the MU is close to 20% (extended MU, assuming four times measured).
Conclusion
The quantification of meat components using ddPCR was shown to be possible. To convert copy number ratios into % (w/w) ratios, conversion factors were determined by each laboratory. With the aid of these conversion factors, it was possible to determine the % (w/w) composition of sample sausages. Average mean values from the seven laboratories corresponded well with the given values. The relative standard deviations were comparable to the best results when using real-time PCR. The final measurement uncertainty (expanded, 95% interval) in the spiking category of 20% or more was below 21% (supposing 4 determinations) which seems to be superior to observed MU using real-time PCR. But, the use of the mainly same instruments and reagents appears to have a positive impact to minimize the MU. In the low meat quantity range (< 5%), the MU was estimated to be 38% or more from four replicate measurements, which is still too high to detect minor variations from the recipe.
For further improvements of the accuracy, the conversion factors should be targeted. We suggest using matrix-specific reference materials to determine matrix-specific conversion factors. Once determined, these conversion factors can be applied for all future analysis without direct analysis of the reference material. This has previously been shown for boiled sausages, resulting in a much better MU compared to results using real-time PCR [17]. Future work therefore should focus on generating such matrix-specific conversion factors. Using the same primers and probes and instrumentation, these factors seem to be transmissible. How far this is transferable to other platforms should also be addressed in future experiments. However, this method seems to give more reproducible and accurate results than comparable real-time PCR systems and is therefore fit for the purpose.
References
Swiss Food Legislation Verordnung über Lebensmittel tierischer Herkunft; 16. Dezember 2016; Art. 9 Abs. 5
Sawyer J, Wood C, Shanahan D, Gout S, McDowell D (2003) Real-time PCR for quantitative meat species testing. Food Control 14:579–583
Brodmann P, Moor D (2003) Sensitive and semi-quantitative TaqMan® real-time polymerase chain reaction systems for the detection of beef (Bos taurus) and the detection of the family Mammalia in food and feed. Meat Sci 65:599–607
Lopez-Andreo M, Lugo L, Garrido-Pertierra A, Prieto I, Puyet A (2005) Identification and quantitation of species in complex DNA mixtures by real-time polymerase chain reaction. Anal Biochem 339:73–82
Laube I, Zagon J, Broll H (2007) Quantitative determination of commercially relevant species in foods by real-time PCR. Int J Food Sci Technol 42:336–341
Eugster A, Ruf J, Rentsch J, Hübner P, Köppel R (2008) Quantification of beef and pork fraction in sausages by real-time PCR analysis: results of an interlaboratory trial. Eur Food Res Technol 227:17–20
Köppel R, Ruf J, Rentsch J (2011) Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, horse and sheep. Eur Food Res Technol 232:151–155
Köppel R, Ruf J, Zimmerli F, Breitenmoser A (2008) Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, chicken and turkey. European. Food Res Technol 227(4):1199–1203
Jonker KM, Tilburg JHC, Hägele GH, De Boer E (2008) Species identification in meat products using real-time. PCR Food Addit Contam 25(5):527–533
Köppel R, Eugster A, Ruf J, Rentsch J (2012), Quantification of meat proportions by measuring DNA contents in raw and boiled sausages using matrix adapted calibrators and multiplex real-time PCR. AOAC Int 95(2):494(6)–499(6)
Uhlig S, Baldauf H, Simon K, Methodenringversuch “All Meat” zum quantitativen Nachweis von DNA der Tierarten Huhn, Pute, Rind und Schwein mittels Tetraplex real-time PCR, Quodata Dresden (in preparation)
Uhlig S, Baldauf H, Simon K, zum quantitativen Nachweis von DNA der Tierarten Pferd, Rind, Schaf und Schwein mittels Multiplex Realtime-PCR Tetraplex-Teil “AllHorse”, Quodata Dresden (in preparation)
Cai Y, Li X, Lv R, Yang J, Li J, He Y, Pan L (2014) Quantitative analysis of pork and chicken products by Droplet Digital PCR BioMed Res Int 2014:6 (article ID 810209)
Floren C, Wiedemann I, Brenig B, Schütz E, Beck J (2015) Species identification and quantification in meat and meat products using Droplet Digital PCR (ddPCR). Food Chem 173:1054–1058
Hanan R, Shehata J, Li S, Chen H, Redda S, Cheng N, Tabujara H, Li K, Warriner R, Hanner (2017) Droplet digital polymerase chain reaction (ddPCR) assays integrated with an internal control for quantification of bovine, porcine, chicken and turkey species in food and feed. PLoS One. https://doi.org/10.1371/journal.pone.018
Qiang Wang Y, Cai Y, He L, Yang J, Li L, Pan (2018) Droplet digital PCR (ddPCR) method for the detection and quantification of goat and sheep derivatives in commercial meat products. Eur Food Res Technol 244:767–774
Köppel R, Ganeshan A, van Velsen F, Weber S, Schmid J, Graf C, Hochegger R (2018) Digital duplex versus real time PCR for the determination of meat proportions from sausages containing pork and beef. Eur Food Res Technol. https://doi.org/10.1007/s00217-018-3147-8
Siegel M, Schnur K, Boernsen B, Pietsch K, Waiblinger HU (2012) First ring trial validation of real-time PCR methods for the quantification of allergenic food ingredients. Eur Food Res Technol 235:619–630
Gerdes L, Iwobi A, Busch U, Pecoraro S (2016) Optimization of digital droplet polymerase chain reaction for quanitifcation of genetically modified organism. Biomol Detect Quantif 7:9–20
Acknowledgements
We thank the cantonal laboratory of Zürich, the Official Food Control Authority of the Canton St. Gallen, Cantonal Office of Consumer Protection Bern. Austrian Agency for Health and Food Safety, Vienna, Austria, Chemisches und Veterinäruntersuchungsamt Freiburg, Germany and National Measurement Institute, Sindey, Australia and Berlin-Brandenburg State Laboratory (LLBB), Germany, for providing the resources for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or living animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Köppel, R., Ganeshan, A., Weber, S. et al. Duplex digital PCR for the determination of meat proportions of sausages containing meat from chicken, turkey, horse, cow, pig and sheep. Eur Food Res Technol 245, 853–862 (2019). https://doi.org/10.1007/s00217-018-3220-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3220-3