Abstract
This study’s objective was to assess the fatty acid composition and oil oxidation traits of hazelnuts that were dried with a drying machine (DM), on concrete ground (CG), and grass ground (GG) during 12-month storage (2014–2016) at 20–25 °C and 70–90% relative humidity. The result showed that monounsaturated was the major fatty acid groups (83.56–85.03%) in hazelnut, followed by polyunsaturated (9.36–11.17%) and saturated (5.61–6.60%) fatty acids. Furthermore, the minor fatty acid contents found were approximately 0.5% that of the total fatty acids. However, none of the following were found at detectable level: caproic acid (C6: 0), caprylic (C8: 0), capric (C10: 0), lauric (C12: 0), eicosadienoic (20: 2), erucic (22: 1), docosadienoic (22: 2) and lignoceric (C24: 0) acids. Consequently, the comparison showed that over 12-month storage, drying hazelnut using DM provides products with a higher oxidative stability than those using sun-dried methods (i.e., CG and GG). Therefore, DM seems to be suitable procedure for drying hazelnut.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hazelnut is one of the most prominent nuts sold at international markets, and Turkey currently is the major world producer and exporter of hazelnut. Unfavorable postharvest processes can influence the quality of hazelnut and handicap the development of its exports for several years. Moreover, postharvest processes such as nut harvesting, blending, drying, and storage condition can variously affect the kernel quality and, by extension, the hazelnut industry and its direct consumption. Therefore, it is important to use suitable postharvest processes to obtain the highest yield and nut quality.
Turkish hazelnut oil’s most abundant fatty acid is oleic acid (C18: 1), followed by linoleic (C18: 2), palmitic (C16: 0), and stearic (C18: 0) acids; furthermore, composition and respective amount of fatty acids maybe affected by several factors such as the nut variety, geography, growing conditions, fertilizer type, seasonality, soil type, climate, latitude, and the postharvest processes [1]. The quality of dried nuts, however, is mainly affected by their drying and storage conditions [2]. In fact, the drying processes play an important role in lipid oxidation during storage.
In Turkey, hazelnuts are conventionally harvested and then sun dried on the grass ground (GG) or concrete ground (CG) and stored at ambient temperatures for a minimum of 1 year [3]. Yet, the conventional drying process is time and labor consuming and ultimately affects the hazelnut quality. Moreover, the weather must also be taken into consideration in hazelnut harvesting, since rains inhibit harvest and postharvest processes, and then it becomes much more difficult to dry hazelnuts [4]. In response to the changes in light and heat, lipid molecules are released to form free fatty acids, which can affect the stability of nut oil [5]. Therefore, it is important to maintain oil stability during the hazelnut-drying process. Moreover, the rapid postharvest processing of hazelnut, particularly drying, is an important parameter in terms of the quality of the final product during the storage phase. In sum, to ensure their long shelf life and to protect them from rancidification processes, hazelnuts must be dried, immediately after harvest [6].
Protecting the overall traits of raw hazelnuts in the drying process and during the first year of storage should be a major concern for the hazelnut industry and market. Unfortunately, in Turkey, little information is available currently in terms of the drying process of raw hazelnut kernels. It is vital to understand the changes in fatty acid composition and oil oxidation of hazelnuts that occur during their drying and storage. Thus, detailed research into this topic will improve our knowledge of Levant hazelnut cultivars and their application to a variety of food and specialty products. To this end, this research sought to determine the influence of three drying processes (CG, GG and DM) and storage period on the fatty acid composition and oil oxidation of Levant hazelnuts grown in the Ordu province of Turkey.
Materials and methods
Samples and drying methods
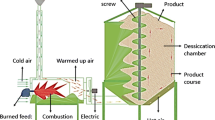
The experiments were conducted on Levant hazelnuts harvested from a single orchard, located in the Bayadi neighborhood (l 40°54ʹ06.99̋N, 37°53ʹ36.07̋E, altitude 300 m) in the Altınordu district, Ordu, Turkey. Levant quality hazelnuts are composed of 44.5% Palaz, 34.0% Tombul and 21.5% Kalinkara cultivars. When the husks had turned yellow, three quarters of the nutshells were brown, and the cluster began to fall, the nuts in the husks are harvested by hand by picking them up from the ground after shaking the branches. The average kernel moisture content was approximately 25% at the time of harvest (August 06–August 10, 2014). The clusters were spread on the GG and dehydrated for 4 days (August 11 to August 14, 2014) to allow moisture loss (22.4%) [3]. Then, the nuts were separated from their husks using huskers (Dinçler Makine, FPHM 2500, Samsun, Turkey), and divided into randomly three groups. The first group was dried in the sun on GG: the grass had been cut using a string trimmer (Oleo-Mac 440T, Italy), and a canvas (TS 4739, TS 1534-2; EN ISO 2286-2, Kale Tente, İstanbul, Turkey) lay on the ground upon which the samples were placed and occasionally mixed. The second group was dried on CG: these nuts were directly placed onto CG and allowed to dry in the sun with occasional mixing. The drying process continued 39 h for CG and GG (Table 1). It is mentioned that CG and GG methods were performed in similar sunshine and environmental conditions (average of wind velocity, ambient air temperature and relative humidity and sunshine duration; 1.4 h km−1, 22.1 °C, 69.8% and 5.24 h, respectively). The hazelnut on CG and GG methods was dried every day from 8:00 a.m. to 8:00 p.m. continuously. After 8:00 p.m., plastic cover (Metroplast, İstanbul, Turkey) was used to prevent the samples from getting wet. The last group was dried in a drying machine (DM): These nuts were directly placed into the machine by conveyor belt (3000 kg), and dried using hot air at 45 °C (FACMA ES 3000, 2013, Italy). Namely, the desiccation was obtained by the forced ventilation of hot air, which the heat-exchanger sends to the ventilator, and at the same time pushes it inside the body of the dryer. The sample, continuously ventilated, was mixed by a central Archimedean screw and it can be ventilated also with non-heated air. The dryer adjusted in temperature was conditioned about 3 h each operation and 1.5 h cease. Meanwhile, the Archimedean screw has continued circulation for 1.5 h in every cycle. The drying process continued until the moisture content was up to 6.8% and lasted for 23 h (Table 1). Additionally, drying parameters and schematic diagram (enthalpy decreases during the drying process due to heat loss) are detailed in Figs. 1 and 2. Drying process was carried out on 15 and 20th day of August 2014 in the Karapınar neighborhood (l 40°58ʹ17.53″N, 37°56ʹ00.41″E, altitude 10 m) in the Altınordu district, Ordu, Turkey (Ordu OSB, Gürsoy Tarımsal Ürünler Gıda Sanayi ve Ticaret A.Ş. Entegre Tesisi). The shell and kernel moisture contents were measured before and after dehydration, and again after drying and before storage and, the drying time (in hours) is shown in Table 1. At the end of drying, the samples were stored under ambient temperature conditions (20–25 °C and 70–90% RH) in jute bags (10 kg) and analyzed every 3 months (Faculty of Agriculture, Ordu University, Ordu, Turkey). Approximately, 1 kg shell (approximately 500 g of kernels) samples were removed and total of 30 kg nuts were used for the analysis.
Storage conditions
The dried nuts were stored in 10-kg jute bags in a store room under conditions of 20–25 °C and 70%–90% relative humidity (RH). The samples were stored for 12 months (2014–2015) and were analyzed every 12 weeks (3 months).
Extraction of hazelnut oil
The hazelnut oil was extracted through a cold press (Pressure force: 10,000 kgf, pressure: 34.7 MPa, temperature: − 5 °C to + 45 °C and capacity; 250 g kernel) method using that used the Ceselsan’s nut oil extraction system (AISI3004, Ceselsan, Giresun, Turkey). The samples of approximately 100 g kernel each were randomly selected and compressed. The recovered oil was separated by centrifugation at 4800 rpm for 5 min and the oil was stored at − 18 °C in freezer until analyzed.
Fatty acid composition
The fatty acid composition of the hazelnut kernel oils was determined by gas chromatography (GC). The methyl esters of fatty acids (FAMEs) were prepared according to the method described by Ficarra et al. [7] with slight modifications. In brief, the oil samples (0.1 g) were placed in a screw-top vial with 10 mL of n-hexane and thoroughly mixed in a dark tube. Additionally, 500 µL potassium hydroxide and methanol mixture was added. The extract was then transferred into a dark glass vial and immediately analyzed by Shimadzu GC-2010 (Shimadzu, Tokyo, Japan). The FAMEs were analyzed using a GC equipped with flame ionization detection. A capillary column DB-23 (30 m × 0.25 mm id × 0.25 µm film thickness; Agilent Technologies, J&W Scientific, USA) was used. The injector temperature and detector temperature were set at 250 °C. The split ratio was set as 1:8, and helium was used as the carrier gas at a flow ratio of 1 mL/min. The column temperature was 90 °C for 7 min, then increased to 240 °C increasing by 5 °C/min; finally, it was held 240 °C for 15 min. The injector and detector were 250 °C. FAMEs were identified in comparison with retention times of authentic standards, and quantified using the Agilent Chem-Station software. The obtained fatty acid composition was used to calculate to the sum of the saturated (∑SFA), monounsaturated (∑MUFA) and polyunsaturated (∑PUFA) fatty acids and their ratio (∑MUFA + PUFA/∑SFA).
Oxidation parameters
To evaluate the oxidative stability of the samples, peroxide value (PV; expressed as meqO2 kg1 oil), the ratio of oleic to linoleic (O/L), and iodine value (IV) were determined. To determine PV, 2–2.5 g of oil was weighed in a glass vial and dissolved in 100 mL of acetic acid/isooctane (3/2, v/v) and supplemented with 0.2 mL of potassium iodide [8]. This mixture was allowed to stand in a dark condition for 5 min, 50 mL of distilled water was later added, and titration was performed. The IV value was determined according to the percentages of fatty acids using the following formula: (palmitoleic acid × 1.901) + (oleic acid × 0.899) + (linoleic acid × 1.814) + (linolenic acid × 2.737) [9].
Statistical analysis
Experiments were performed in triplicates with a randomized block design. Descriptive statistics were obtained with SPSS v. 22.0 (Armok, New York: IBM Corp.). Statistical tests were performed using the SAS-JAMP v. 10.0 (SAS Institute Inc., Cary, North Carolina, USA). A one-way ANOVA was conducted to assess significant differences among levels and the least significance difference (LSD) test was used to compare multiple means. Results were considered to be significantly different at p < 0.05.
Results and discussion
Fatty acids
In the examined hazelnut samples, the statistical test showed a significant effect of drying and storage on the fatty acid composition and the oxidative stability of the nuts. Table 2 shows the results for the effect on the fatty acid composition of hazelnut oils in detail. The FAMEs of the Levant quality hazelnut identified a total 13 fatty acids; of these, oleic acid (C18: 1) was largely present, followed by linoleic (C18: 2), palmitic (C16: 0) and stearic (C18: 0) acids.
Generally, the minor fatty acids were ≤ 1% of the total fatty acid content. However, caproic (C6: 0), caprylic (C8: 0), capric (C10: 0), lauric (C12: 0), eicosadienoic (20: 2), erucic (22: 1), docosadienoic (22: 2) and lignoceric (C24: 0) acids were not present in the samples at detectable levels (< 0.001%). It is known that the fatty acid composition can differ among and within the same hazelnut cultivars due to interacting factors such as variety, geographic origin, growing terms, ripening, manuring, harvest timing, seasonality, soil type, climate, latitude, and storage terms [10]. For example, Tüfekçi and Karataş [11] reported that hazelnut oil from the Central Black Sea region contained high amount of total fatty acids (8.45%), monounsaturated fatty acids (83.54%), but less total polyunsaturated fatty acids (7.85%), whereas that of the Eastern Black Sea region had high contents of linoleic (9.10%) and linolenic (0.096%) acids. In addition, Lane et al. [12] reported that American hazelnut (C. americana Marshall) contained 3.1% palmitic (C16:0), 80.6% oleic (C18:1), and 14.5% linoleic (C18:2) acids.
The major and minor fatty acids were considered in our analysis for any changes in lipid composition as a function of drying and storage time. The hazelnut oil composition is dominated by unsaturated fatty acids (oleic and linoleic), which amounts to > 90% of the total fatty acids present [1, 13], thus making hazelnut highly vulnerable to spoilage-driven lipid oxidation [14].
As shown in Table 2, the samples dried via DM had less oleic acid (C18: 1) and more linoleic acid (C18: 2) compared to those dried via the two sun-dried methods, and there were significant differences among the DM, and sun dried (CG and GG) (p < 0.001). Unlike our study, Fu et al. [5] and Qu et al. [15] reported no significant differences in the fatty acid profiles among their samples treated using different drying methods. In addition, during the storage period, contrary to the findings by Koyuncu et al. [16] and Ghirardello et al. [6], we found that the oleic acid (C18: 1) content of hazelnuts slightly decreased, whereas the linoleic acid (C18: 2) content increased, although with some with fluctuations (p < 0.001).
Regarding the saturated fatty acids, palmitic acid (C16: 0) was the main saturated fatty acid present, followed by stearic acid (C18: 0) [13]. Drying via DM and CG led to a higher palmitic acid (C16: 0) content than that via GG, and there were significant differences among drying methods (p < 0.001); similarly, during storage, we found significant differences among the drying methods in terms of stearic acid (C18: 0) content (p < 0.001; Table 2). During storage, the palmitic acid (C16: 0) content ranged from 3.72 to 4.4.11%, whereas that of stearic acid (C18: 0) ranged from 1.73 to 2.33%. This trend agrees with that mentioned by Koyuncu [13] and Ghirardello et al. [6] who reported that these fatty acids slightly increased during the storage phase of hazelnut postharvest processing.
In our study, the amounts of minor fatty acids were approximately 0.5% of the total fatty acid content (Table 2). These results are consistent with those reported by Turan [3] who showed that the minor acids constituted < 1% of the total fatty acid content. We deliberately chose these minor fatty acids to evaluate any changes in hazelnut oil profiles as a function of the drying methods and storage periods. As shown in Table 2, there were significant differences in the myristic acid (C14: 0) content among the samples treated by the different drying methods (p < 0.01). The highest myristic acid (C14: 0) level was observed in the DM and CG groups (0.03%), whereas the lowest level was observed in the GG group (0.02%). During storage, the drying methods also significantly affected the myristic acid (C14: 0) (p < 0.05).
However, drying methods did not have a significant effect on palmitoleic acid (C16: 1) level (p > 0.05). By contrast, a significant effect of the drying methods was found in term of margaric acid (C17: 0) level (p < 0.01), which had decreased by the end of the storage. Heptadecenoic acid (C17: 1) level was similar among all the methods (p > 0.05; Table 2). However, arachidic acid (C20: 0) content was significant differ in terms of the method used (p < 0.05).
Moreover, the interaction effect of drying and storage time was not found to be significant for eicosenoic (C20: 1), and nervonic (C24: 1) acids (p > 0.05). This result contrasts with that reported by Turan [3], in which an interaction effect of drying and storage time was reportedly found to be significant for the minor fatty acids. Nevertheless, in our study, a significant difference was evident for behenic acid (C20: 0) concerning the drying methods (p < 0.05). These differences possibly developed due to some key factor such as cultivar, drying, storage terms, ecology and cultural practices.
Table 3 shows the data on saturated (SFA), monounsaturated (MUFA) polyunsaturated (PUFA), ratio of unsaturated/saturated (MUFA + PUFA/SFA), ratio of oleic/linoleic (O/L) fatty acids, and the PV of oils. As expected, MUFA was the principal group of fatty acids (83.56–85.03%), followed by PUFA (9.36–11.17%) and SFA (5.61–6.60%). The results agree with those by Alaşalvar et al. [1] and who reported that raw hazelnut kernel oils have a lower proportion of SFA (7.46–9.59%), an intermediate proportion of PUFA (3.92–13.86%), and the highest proportion of MUFA (78.10–87.26%). Similarly, our results are generally in accordance with those reported by Moser [17]; SFA, PUFA and MUFA; 7.4, 13.3 and 79.1%, respectively. In contrast to our results, Amaral et al. [10] reported that although MUFA was the principal group of fatty acids, SFA and PUFA proportions were on par with it for dominance.
In relation to drying, significant differences were found among drying methods (p < 0.001), and such as SFA was more abundant in the DM group (5.92%) than in the CG (5.75%) and GG (5.63%) groups. Likewise, Ozdemir et al. [14] and Delgado et al. [18] reported that drying methods could significantly influence SFA content of nut oils, yet some other studies have reported that the type of drying method does not effect on SFA [5, 15]. During the storage period, the SFA content in our study exhibited fluctuations and variability, but had slightly increased overall (Table 3). This result disagree with that mentioned by Koyuncu [19], Ghirardello et al. [6], and Belviso et al. [20] who reported that the SFA content of hazelnut oil was lower at the end when compared with that at the beginning of the storage period.
MUFA mainly comprised oleic acid (C18: 1), followed by palmitoleic (C16: 1), eicosenoic (C20: 1), heptadecenoic (C17: 1) and nervonic (C24: 01) acids, and it was influenced significantly by drying methods (p < 0.001). These findings agree with those by Delgado et al. [21] who also found varying effects among drying methods in terms of MUFA. As anticipated, MUFA content decreased during the storage period of our study (Table 3), a trend observed by Belviso et al. [20].
Overall, linoleic (C18: 2) and linolenic (C18: 3) acids were the principal acids among the PUFA [18, 22]. The effect of the drying methods was significant for PUFA (p < 0.001), which had a higher content in the DM group (11.17%) than in the CG (9.84%) and GG (9.46%) groups. The results agree with those of Delgado et al. [18] who also found that the drying methods considerably influenced PUFA content. In our study, the PUFA content tended to fluctuate as a function of the storage time (p < 0.001; Table 3). These fluctuations probably developed because of the use of mixed hazelnut cultivars. Moreover, during the storage period, drying via CG and GG groups similarly increased the PUFA content (from 9.84 to 10.17% and from 9.46 to 10.05%, respectively), but it was decreased in the DM group (from 11.17 to 9.44%). These results generally agree with those reported by Belviso et al. [20].
The effects of the drying methods on the unsaturated/saturated fatty acids in the hazelnut samples are shown in Table 3. Unlike Qu et al. [15] and Juhaimi et al. [22], we found a significant influence of drying methods upon the unsaturated/saturated fatty acids. Specifically, the ratio of unsaturated/saturated fatty acids fluctuated but had slightly decreased by the end of the storage time (p < 0.001); a trend is also reported by Koyuncu et al. [16], Ghirardello et al. [6], and Belviso et al. [20]. These changes were significant and probably related to the peroxidation of the unsaturated fatty acids that later lost.
Oxidation of oil
Linolenic acid is more likely to be oxidized than oleic acid [5]. The O/L ratio is considered an acceptable standard to estimate kernel quality [1]. A higher O/L value indicates much better oxidative stability [20]. An interaction effect between the drying and storage period was found in terms of O/L (Table 3). When dried via GG (9.06%) group, the O/L value exceeded that of CG and DM groups (8.71 and 7.62%, respectively), and there were significant differences among drying methods (p < 0.001).
During storage, the ratio of O/L slightly increased from 7.62 to 8.94% for DM, but slightly decreased from 9.06 to 8.36% for the sun dried and all these changes are significant (p < 0.001; Table 3). A similar pattern was reported by Belviso et al. [20] for O/L acid of hazelnut kernels stored at 4 °C for 9 months in vacuum packages. According to Alaşalvar et al. [23], O/L varies among hazelnut cultivars. Moreover, O/L varied from 6.8 to 11.4%, indicating different cultivars provide varying outcome [10].
IV is calculated using the unsaturated fats and oils, and it expresses the amount of absorbed iodine [20, 24]. A higher IV value indicates that the oil is more sensitive, less stable, and more vulnerable to oxidation, whereas low IV value indicates that the oil contains low levels of unsaturated fatty acids. The IV was higher in DM group than that in the CG and GG groups, but these differences were not significant (p > 0.05). During storage, the IV slightly increased from 93.82 to 94.72 for the sun-dried methods. This result agrees with Belviso et al. [20], who reported that the IV increased in Ordu and TGT hazelnut cultivars during a 9 months of storage period. However, we found that the IV slightly decreased in the DM group during the storage period. Naz et al. [25] and Ajith et al. [24] suggested that a decrease of IV is an indicator of oil deterioration.
PV is one of the specifications adopted by the nut industry to assess the storage period of marketable hazelnut [3, 6, 26]. It is also a reliable indicator of walnut oxidative degradation that indicates the stage of oxidation [5]. The differences among the drying methods were significant during the storage period (p < 0.001; Table 3). At the end of the storage period, the highest values were recorded in the GG and CG groups (0.32 and 0.30 meqO2 kg− 1, respectively). This result agrees with that reported by Qu et al. [15] and Fu et al. [5], where the PV value of sun-dried walnut regularly increased, attaining its highest value of 2.35 meqO2 kg− 1 at the end of the drying period. Moreover, the PV value of the direct and intermittent oven-dried specimens was 1.94 and 1.82 meqO2 kg− 1, respectively [5]. It is clear that when hazelnut is subjected to long-term sun-drying and temperatures, its oil deterioration is accelerated. Hence, a drying period conducted over a short time, immediately after the harvest is important for the long-term storage of hazelnuts.
In our study, as anticipated, the PV value dramatically increased, particularly for the sun-dried methods, during the storage (p < 0.001), but such an increase was not constant over time (Table 3). The PV value randomly peaked during the storage period and then decreased [3, 26,27,28]. During the storage period, peroxide generation and disintegration reactions can simultaneously occur; hence, the PV value fluctuates. However, Evren [29], Ghirardello et al. [6] and Raisi et al. [30] reported a steady rise in the PV value during the storage period. This discrepancy is possibly best explained by external factors such as ripening, variety, drying method, storage terms, and ecology and cultural practices.
Conclusions
To the best of our knowledge, in Turkey, this is the first method to study the effect of drying method and storage period on fatty acid composition and lipid oxidation of the Ordu Levant quality hazelnut. In general, during the before- and after-storage period, significant differences were detected among the three drying methods on some major and minor fatty acids in hazelnut oil. Our study shows that the DM method showed higher SFA and PUFA and lower MUFA and MUFA + PUFA/SFA composition, and that MUFA was the predominant group of fatty acids (83.56–85.03%), followed by PUFA (9.36–11.17%) and SFA (5.61–6.60%). Generally, SFA and PUFA were slightly increased, whereas MUFA and MUFA + PUFA/SFA were slightly decreased with evident fluctuations during storage. Regarding O/L, the GG group had a higher O/L ratio compared with that of the CG and DM groups, but, the DM group comparatively had higher IV value. In conclusion, this study shows that drying using DM can provide products with a better oxidative stability over 12 months of storage at an ambient temperature (20–25 °C) and a RH of 70–90%. Overall, based on the results, we recommended the use of DM for drying hazelnut.
References
Alasalvar C, Pelvan E, Topal B (2010) Effect of roasting oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.). Int J Food Sci Nutr 61:630–642
Kashaninejad M, Maghsoudlou Y, Khomeiri M, Tabil G (2010) Resistance to air flow through bulk pistachio nuts (Kalleghochi variety) as affected by moisture content, airflow rate, bed depth and fill method. Powder Technol 2003:359–364
Turan A (2017) Effect of drying methods on nut quality and storage of hazelnut. Ph. D. Thesis, Ordu (in Turkish)
Yıldız T (2016) Labor requirements and work efficiencies of hazelnut harvesting using traditional and mechanical pick-up methods. Turk J Agric For 40:301–310
Fu M, Qu Q, Yang X, Zhang X (2016) Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. Food Sci Technol Leb 65:1126–1132
Ghirardello D, Contessa C, Valentini N, Zeppa G, Rolle R, Gerbi V, Botta R (2013) Effect of storage condition on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol Technol 81:37–43
Ficarra A, Lo Fiego DP, Minelli G, Antonelli A (2010) Ultra fast analysis of subcutaneous pork fat. Food Chem 121:809–814
Anonymous (1990) Oils and fats, 15th edn. Official Methods of Analysis of the Association of Official Analytical Chemists. Washington DC, pp 485–518
Hashempour A, Ghazvini RF, Bakhshi D, Sanam SA (2010) Fatty acids composition and pigments changing of virgin olive oil (Olea europea L.) in five cultivars grown in Iran. Aust J Crop Sci 4:258–263
Amaral JS, Casal S, Citová I, Santos A, Seabra RM, Oliveira BPP (2006) Characterization of several hazelnut (Corylus avellana L.) cultivars based in chemical, fatty acid and sterol composition. Eur Food Res Technol 222:274–280
Tüfekci F, Karataş Ş (2018) Determination of geographical origin Turkish hazelnuts according to fatty acid composition. Food Sci Nutr 00:1–6
Lane JW, Hlina P, Hukriede K, Jersett A, Koirala D, Stewart A, Waxman MA (2012) Probing Wisconsin highbush cranberyry (V. trilobum), dotted horsemint (M. punctata), and American hazelnut (C. americana) as potential biodiesel feedstocks. Ind Crops Prod 36:531–535
Koyuncu MA (2004) Change of fat content and fatty acid composition of Turkish hazelnuts (Corylus avellana L.) during storage. J Food Qual 27:304–309
Ozdemir M, Yıldız M, Gürcan T (2002) Effect of artificial trying air temperature on stability of the major Turkish hazelnut variety Tombul. Gıda 27:35–39
Qu Q, Yang X, Fu M, Chen Q, Zhang X, He Z, Qiao X (2016) Effects of three conventional drying methods on the lipid oxidation, fatty acids composition, and antioxidant activities of walnut (Juglans regia L.). Dry Technol 34:822–829
Koyuncu MA, Islam A, Küçük M (2005) Fat and fatty acid composition of hazelnut kernels in vacuum packages during storage. Grasas y Aceties 56:263–266
Moser BR (2012) Preparation of fatty acid methyl esters from hazelnut, high-oleic peanut and walnut oils and evaluation as biodiesel. Fuel 92:231–238
Delgado T, Pereira JA, Ramalhosa E, Casal S (2017) Comparison of different drying methods on the chemical and sensory properties of chestnut (Castanea sativa M.) slices. Eur Food Res Technol 243:1957–1971
Koyuncu MA, Koyuncu F, Bakır N (2003) Selected drying conditions and storage period and nut quality of walnut selections. J Food Process Preserv 27:87–99
Belviso S, Bell BD, Giacosa S, Bertolino M, Ghirardello D, Giordano M, Rolle L, Gerbi V, Zeppa G (2017) Chemical, mechanical and sensory monitoring of hot air and infrared roasted hazelnuts (Corylus avellana L.) during nine months of storage. Food Chem 217:398–408
Delgado T, Pereira JA, Ramalhosa E, Casal S (2016) Effect of hot air convective drying on the fatty acid and vitamin E composition of chestnut (Castanea sativa Mill.) slices. Eur Food Res Technol 242:1299–1306
Juhaimi FA, Ozcan MM, Uslu N, Ghafoor K (2018) The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus and oils. Eur Food Res Technol 55:190–197
Alasalvar C, Sahidi F, Liyanapanthirana CM, Ohshima T (2003) Turkish Tombul hazelnut (Corylus avellana L.). 1. Compositional characterstics. J Agric Food Chem 51:3790–3796
Ajith S, Pramod S, Kumari CP, Potty VP (2015) Effect of storage temperatures and humidity on proximate composition, peroxide value and iodine of raw cashew nuts. J Food Sci Technol 52:4631–4636
Naz S, Sheikh H, Saddiqi R, Sayeed SA (2004) Oxidative stability of olive, corn and soybean oil under different conditions. Food Chem 88:253–259
Koc Güler S, Bostan SZ, Con AZ (2017) Effects of gamma irradiation on chemical and sensory characteristics of natural hazelnut kernels. Postharvest Biol Technol 123:12–21
Demirci Ercoşkun T (2009) Research on shelf life of processed hazelnut products. PhD Thesis, Ankara (in Turkish)
Turan A, İslam A (2018) Effect of drying methods on some chemical characteristics of hazelnuts (Corylus avellana L.) during storage. J Inst Sci Technol 8:3 (in press)
Evren S (2011) Storage stability of natural hazelnut flour. PhD Thesis, Samsun (in Turkish)
Raisi M, Ghorbani M, Mahoonak AS, Kashani Nejad M (2015) Effect of storage atmosphere and temperature on the oxidative stability of almond kernels during long term storage. J Stored Prod Res 62:16–21
Acknowledgements
This study was supported by Altaş Oil Industry Inc (Ordu, Turkey). The author wishes to thank Assist Prof Fatih ÖNER for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict interest.
Research involving human participants and/or animals
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Turan, A. Effect of drying methods on fatty acid profile and oil oxidation of hazelnut oil during storage. Eur Food Res Technol 244, 2181–2190 (2018). https://doi.org/10.1007/s00217-018-3128-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3128-y