Abstract
The aim of the present work was to study the effect of hot-air drying on the quality of chestnut slices, regarding fatty acid and vitamin E composition. Chestnut slices of two varieties, Longal and Judia, were dried in a tray dryer at 50 °C, for 1, 2, 4, 6, 8 and 10 h. Concerning fatty acids, at beginning both varieties presented significant different contents in C16:0, C18:0, C18:1, C18:2cc, C20:0 and C20:1, being C18:2cc the most abundant. Concerning vitamin E, both varieties had similar γ- and δ-tocopherol contents; however, after 10 h of drying their concentrations decreased in Longal (19.2 and 14.4 %, respectively). It was also in slices of Longal variety that a significant decrease was observed in C18:0 (15.0 %), C18:1 (19.4 %), C20:0 (14.3 %) and C20:1 (11.1 %) after 10 h of drying, suggesting this variety to be a little more heat sensible than Judia. Even though some variation on lipid composition was observed along drying of chestnuts, the variety showed to have a higher effect than the drying process itself. Thus, hot air convective drying seems to be an interesting process to apply in the future to this nut in order to produce a healthy snack, not causing potential losses from a nutritional point of view.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chestnut tree production is of great importance to several European countries such as Portugal and Italy; however, chestnut fruits are perishable, requiring efficient preservation methods, being cold storage the most commonly used technologies. However, during this process two major problems can be observed: weight loss due to water evaporation and mold development due to the high moisture content of the fruits [1]. Thus, to minimize losses it is important to find alternative solutions for chestnuts preservation. These processes should be economic and permit industrial scale-up, while providing shelf life extension without quality loss. Drying under controlled conditions could be a possible technological alternative, having Delgado et al. [2] already stated that after 10 h of drying the proximate composition of chestnuts was almost unaffected by the thermal process.
From the nutritional point of view, besides being a gluten-free nut, chestnut is quite interesting [3] because it is a good source of fibre, starch, protein, aminoacids, minerals, fatty acids and vitamin E. Although presenting a low crude fat content, chestnut fat is constituted by high unsaturated fatty acids (USFA) [4], namely monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids. This fatty acid profile plays an important role in diverse physiological processes affecting health and chronic diseases such as the regulation of plasma lipid levels, cardiovascular and immune functions, insulin action and neuronal development and visual function [5]. Moreover, its fat also contains some vitamin E, with several benefits on human health, namely by minimizing the adverse effects of inflammatory diseases (e.g. rheumatoid arthritis or hepatitis) [6], strengthen the immune system and reduce the risk of cancer [7], or aid in the treatment of Parkinson’s syndrome [8]. However, most of the published studies on chestnut technological preservation have neglected the effects on the fat composition.
Until now, only two studies evaluated the effect of industrial processing on chestnut composition. The effect of chestnuts storage during 3 months at around 0 °C (relative humidity of 90 %), after industrial peeling by flame or fire (“brûlage”) and after freezing in a tunnel with a CO2 flow were studied by de Vasconcelos et al. [9] and de Vasconcelos et al. [10]. On those works the fat content and vitamin E profile were included together with several other components. Furthermore, Künsch et al. [11] and Krist et al. [12] evaluated the effect of roasting at 210 and 190 °C on weight loss, starch, sucrose and fructose contents, total fatty acids and sensory properties as well as volatiles of chestnuts.
Chestnuts drying at lower temperatures than those applied during roasting may be an interesting option. Several works on chestnut hot air drying have already been performed, including drying kinetics [13–16]; energetic requirements [17]; proximate composition [2]; temperature effect on morphological, chemical (reducing sugars, starch, amylose, sugar and damaged starch) and rheological properties of chestnut flours [18–20]; and effect of drying followed by rehydration on chestnuts properties [21–23]. Until now no studies have been performed on the effect of drying on chestnuts lipid composition.
Nowadays consumers are looking for healthy snacks, being the market share of dried fruits increasing. In this way the production of dried chestnut slices might have great potential, due to low caloric value, excellent lipid composition and gluten-free properties of chestnuts. Thus, the aim of our work was to evaluate for the first time the effect of hot air convective drying at 50 °C on the quality of chestnut slices, regarding their fatty acid and vitamin E composition, in order to evaluate the possible induction of oxidation and the occurrence of potential losses from a nutritional point of view.
Materials and methods
Reagents and standards
HPLC grade n-hexane was purchased from Merck (Darmstadt, Germany) and 1,4-dioxane from Sigma (Madrid, Spain). Methanol and KOH were acquired from Panreac (Barcelona, Spain). Boron trifluoride in methanol (14 %), butylated hydroxytoluene (BHT), and ascorbic acid were obtained from Sigma. All other chemicals were of analytical grade from diversified suppliers.
Concerning standards, α-, γ-, δ-tocopherols were purchased from Sigma or Matreya (State College, USA). 5 mg mL−1 standard solutions were prepared in ethanol and kept at −20 °C. Their accurate concentrations were evaluated by UV spectrophotometry according to their molar absorptivity values in ethanol (3056, 3810 and 3673 M−1 cm−1; [24, 25]). Dilutions in n-hexane were performed as required for calibration or other purposes. The internal standard for vitamin E quantification was tocol (2-methyl-2-(4,8,12-trimethyltridecyl) chroman-6-ol), obtained from Matreya Inc. A 100 μg mL−1 solution was prepared in n-hexane and kept at −20 °C. Triundecanoin was used as the internal standard for fat estimation, based on the total fatty acid amounts, and was purchased from Sigma. A 10 mg mL−1 solution was prepared in n-hexane. A commercial standard solution with 37 fatty acid methyl esters (FAME) was used for the calibration of the FID signals (Supelco 37 FAME mix, Sigma, Bellefonte, USA).
Plant material
The chestnuts used in this study were acquired in Bragança (NE Portugal) in November 2012 and stored in cold chambers (4 ± 1 °C) until the drying experiments were carried out. Two varieties were used in this study, Longal and Judia, acquired directly to chestnut producers of Macedo de Cavaleiros and Vinhais (Bragança, Portugal), respectively.
Drying experiments
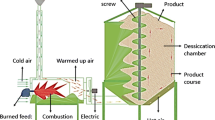
The exterior shells of chestnuts were carefully removed with a knife and the fruits sliced with approximately 4 mm of thickness. Around 150 g of chestnut slices were dried in a tray dryer (Armfield, Ringwood, England) at 50 °C, with an air velocity at 1.2 ± 0.1 m s−1, along different times. After drying for 1, 2, 4, 6, 8 and 10 h, samples were frozen and freeze-dried, as well as the control (fresh chestnuts). For each drying time all drying experiments were performed in triplicate.
Lipid extraction
The total fat was determined on 5 g of sample, using petroleum ether for 24 h in a Soxhlet apparatus (P Selecta, Barcelona, Spain). The lipid extraction was performed according to the method described by Cruz et al. [26], with some modifications. An accurate 300 mg portion of lyophilized sample was weighted in a plastic tube, followed by the addition of two internal standards solutions: tocol (15 µL; 10 mg mL−1) for vitamin E quantification, and triundecanoin (45 µL; 20 mg mL−1), for total fatty acids estimation, followed by two antioxidants—BHT (50 µL, 5 mg mL−1 in methanol), and ascorbic acid (50 mg), and three glass pearls. Propan-2-ol (1.58 mL) and ciclohexane (2.04 mL) were added for lipid extraction. The mixture was agitated briefly by vortexing and kept overnight under refrigeration. The non-lipid material was removed by washing with 2.28 mL of 0.9 % NaCl solution, with agitation by vortexing and centrifugation (5000 rpm, 5 min; Heraeus Sepatech, Am Kalkberg, Germany). The top layer was transferred to a second another tube, and a second extraction was performed with cyclohexane (2.04 mL), and the upper layers combined.
An extract volume, corresponding to approximately two thirds of the total, was transferred to Pyrex derivatization tubes. The solvent was evaporated under a nitrogen stream (60 °C) and resuspended in about 0.5 mL of dichloromethane. Hydrolysis was performed with 1.5 mL of KOH (0.5 M in methanol) at 100 °C (10 min). After reaching room temperature, methylation was completed by the addition of 1.5 mL of BF3 (14 % in methanol) and heated for further 30 min at 100 °C. After cooling, 0.9 % NaCl (2.5 mL) and n-hexane (3 mL) were added and the mixture was mixed by vortexing, followed by centrifugation (3000 rpm, 5 min). The supernatant was transferred to a tube with anhydrous sodium sulphate to remove the residual water, followed by centrifugation at 5000 rpm for 5 min. A supernatant portion (2 mL) was directly transferred to a clear glass vial (Supelco, Sigma, Bellfonte, USA), the solvent was evaporated under a nitrogen stream (60 °C), resuspended with heptane (1 mL) and the solution mixed in a vortex. This vial was positioned in the gas chromatograph autosampler for fatty acid analysis.
To the remaining lipid extract (1/3) anhydrous sodium sulphate was added followed by centrifugation (5 min, 13,000 rpm, Heraeus Sepatech Biofuge Pico, Am Kalkberg, Germany). The supernatant was transferred to an amber glass vial. The solvent was evaporated under a gentle nitrogen stream (40 °C), resuspended in the same volume of n-hexane (HPLC grade) and placed in the HPLC autosampler for vitamin E quantification.
Fatty acids analysis
Fatty acids analysis was performed on a Chrompack CP 9001 chromatograph (Chrompack, Middelburg, Netherlands) equipped with a split-splitless injector, a Chrompack CP-9050 autosampler and a flame ionization detector (FID). The temperatures of the injector and detector were 250 and 270 °C, respectively. Separation was achieved on a 50-m × 0.25-nm i.d. CP-Sil 88 column (0.19-µm film; Chrompack-Varian). Helium was used as carrier gas at an internal pressure of 120 kPa. The column temperature was 140 °C, for a 5 min hold, and then programmed to increase to 220 °C at a rate of 5 °C min−1 and then held for 15 min. The total analysis time was 35 min, but a further 5-min elution was applied for complete elution of possible interference compounds. The slip ratio was 1:50 and the injected volume was 1.2 µL.
Fatty acids identification (from C11:0 to C22:6 n-6) was accomplished by comparing the relative retention times of FAME peaks with standards from diversified suppliers and from literature data. Peaks were corrected using empirical response factors obtained by the standard FAME solution. The fatty acids results were calculated initially on a relative percentage basis and then expressed in mg 100 g−1 dry matter. Total fat was estimated after performing a Soxhlet extraction using petroleum ether for 24 h.
HPLC chromatographic conditions for vitamin E
The liquid chromatograph consisted of a Jasco integrated system (Easton, USA) equipped with an autosampler (AS-2057 Plus), a PU-980 intelligent pump, connected to a fluorescence detector (FD) (FP-2020 Plus; λ excitation = 290 nm and λ emission = 330 nm). The chromatographic separation was achieved on a Supelcosil™ LC-SI column (75 × 3.0 mm, 3 µm; Supelco, Bellefonte, USA), operating at constant room temperature (23 °C). A mixture of n-hexane and 1,4-dioxane (97.5:2.5, v/v) was used as eluent at a flow rate of 0.7 mL min−1. The compounds were identified by chromatographic comparisons with authentic standards by co-elution and by their UV spectra. Tocopherols were evaluated by the internal standard method based on the fluorescence data.
Statistical methods
The statistical analysis was performed on SPSS software (Version No. 20.0). The effect of drying time and the influence of cultivar over fatty acid and vitamin E contents and profiles were evaluated by the two-way analysis of variance (ANOVA) (p < 0.05), followed by the Tukey HSD Post-hoc test because normality was observed and the variances of the groups were identical. The normality and variance homogeneity were evaluated by Kolmogorov–Smirnov and Levene’s tests, respectively. Comparisons were carried out at 95 % confidence. Principal component analysis (PCA) was also performed for the fatty acids and tocopherols results of the two chestnut cultivars along drying time. The PCA score plot was used to differentiate cultivars and verify the effect of drying time on chestnut lipid profile.
Results and discussion
Fat content and fatty acid composition
The fat contents in chestnuts were low (Table 1). Even though significantly higher values were determined at the beginning in Longal (3.26 ± 0.11 g fat 100 g−1 dry weight) than Judia (2.77 ± 0.45 g fat 100 g−1 dry weight), along drying the two varieties presented similar fat contents. Moreover, the fat contents determined at the beginning of the drying process and after 10 h were not significantly different, indicating that this parameter was almost unaffected by the thermal process, as stated by Delgado et al. [2].
Regarding the fatty acids contents of the two chestnut cultivars along drying (Table 1), the results showed that chestnut variety × time interaction was a significant source of variation for all of fatty acids (p ≤ 0.001). At the beginning, the fresh chestnuts (t = 0 h) of Longal variety contained 18.8 % saturated fatty acids (SFA) and 80.8 % unsaturated fatty acids (USFA), consisting of 36.2 % monounsaturated fatty acids (MUFA) and 44.6 % polyunsaturated fatty acids (PUFA), while Judia variety presented 18.6 % SFA and 81.1 % USFA, consisting of 28.1 % MUFA and 53.0 % PUFA. These results were in agreement with Borges et al. [4] (SFA 14.1–18.6 %; MUFA 22.5–39.3 %; PUFA 42.0–60.1 %), España et al. [27] (SFA 19 %; MUFA 31 %; PUFA 41.5–56.7 %) and Barreira et al. [28] (SFA 16–19 %; MUFA 36–38 %; PUFA 43–48 %).
Concerning fatty acids individually, linoleic acid followed by oleic acid were the predominant in both chestnut varieties. At the beginning (t = 0 h), significant differences were observed between the two varieties in most of the fatty acids, with the exception for palmitoleic acid (C16:1n9) and α-linolenic acid (C18:3n3). These results were similar to Barreira et al. [29] who also found significant differences between chestnut varieties for several fatty acids.
Regarding fatty acid composition along drying, Longal variety showed a significant decrease in some fatty acids contents, particularly after 10 h of drying. For C18:0, C18:1n9, C20:0 and C20:1n9 the following losses (%) were observed: 14.9, 19.4, 13.9 and 11.1 %, respectively. In opposition, Judia variety maintained or even increased the contents of some fatty acids after 10 h of drying. This small effect of drying at 50 °C on chestnut fatty acid composition of Judia might be associated to variations in the sample itself, as a result of intra genotype diversity and influence of environmental and growing conditions. These results are in line to those of Künsch et al. [11] after studying the effect of roasting. These authors had also observed differences between varieties and along the roasting time with regard to the analysed fatty acids, being these differences more pronounced in some varieties such as Lüna and Marigoule.
At the beginning (t = 0 h), significant differences were found between both varieties on the total SFA content (Table 1), with 601 mg fatty acids 100 g−1 dry matter and 488 mg fatty acids 100 g−1 dry matter for Longal and Judia varieties, respectively. During the drying process small variations were found in SFA contents, being Longal values almost always higher than Judia, but these differences were not found at the end of the drying process (10 h) (572 and 566 mg 100 g−1 dry matter for Longal and Judia, respectively). Palmitic acid (C16:0) was the major SFA. At the beginning (0 h), Longal and Judia presented 518 mg 100 g−1 dry matter and 429 mg 100 g−1 dry matter of palmitic acid, corresponding to 15.9 and 16.3 % of the total fatty acids, respectively. These values were in agreement with those reported by Borges et al. [4] (12.5–16.8 %), Fernandes et al. [30] (14.6 %), Fernandes et al. [31] (18 %) and Barreira et al. [28] (16–17 %). Significant differences between varieties were only found at the beginning (0 h), 1 and 4 h. Stearic and arachidic acids (C18:0 and C20:0, respectively) were two fatty acids that also contributed to the SFA, with small variations on their contents along drying.
In relation to MUFA, significant differences were always found between the two varieties, presenting Judia lower values than Longal. Concerning drying, some variation was observed along time but without any decreasing trend. The MUFA majority was constituted by oleic acid (C18:1), with 1136 and 705 mg 100 g−1 dry matter for Longal and Judia varieties at zero hours, corresponding to 34.9 and 26.8 %, respectively. These values were also in agreement with Borges et al. [4] (20.7–37.6 %), Fernandes et al. [30] (20.3 %), Fernandes et al. [31] (28 %) and Barreira et al. [28] (35–37 %). Along drying, both varieties presented significant differences on the oleic acid contents. In addition, palmitoleic acid (C16:1n9) and eicosenoic acid (C20:1n9) were also important to MUFA, with small variations along drying. A significant decrease was only observed on eicosenoic acid content for Longal variety after 10 h of drying.
In relation to PUFA, significant differences were found between the two varieties at the beginning (1451 and 1396 mg 100 g−1 dry matter for Longal and Judia, respectively); however, after 10 h no effect and a slight increase (around 10 %) was detected on both varieties when comparing with the beginning, respectively, showing the negligible effect of drying at 50 °C on chestnut fatty acid composition. Linoleic acid (C18:2n6) and α-linolenic acid (C18:3n3) were the most representative PUFAs. At zero hours, linoleic acid contents of 1285 and 1212 mg 100 g−1 dry matter were quantified in Longal and Judia varieties, respectively, being these values significantly different. In opposition, similar α-linolenic acid contents were obtained for both varieties at 0 h (159 and 176 mg fatty acids 100 g−1 dry matter). The linoleic and α-linolenic acids corresponded to 39.5 and 46.1 %, and to 4.9 and 6.7 % for Longal and Judia varieties, respectively. These values were in agreement with Borges et al. [4] (37.6–50.9 % for C18:2n6 and 4.5–10.0 % for C18:3n3), Fernandes et al. [30] (53.2 % for C18:2n6 and 7.7 % for C18:3n3), Fernandes et al. [31] (44 % for C18:2n6 and 7 % for C18:3n3) and Barreira et al. [28] (38–40 % for C18:2n6 and 4–5 % for C18:3n3). Along drying, small variations were observed.
With respect to trans fatty acids isomers (indicatives of the thermal effect on the fatty acid composition) negligible values were obtained at the beginning (7.6 and 5.8 mg 100 g−1 dry matter for Longal and Judia, respectively), as expected. It was interesting to notice that despite remaining very low even after 10 h of processing, a significant increase (to 10.1 mg fatty acid 100 g−1 dry matter) was observed only for Longal. Although being recognized as detrimental for the human health if ingested in significant amounts, particularly for the cardiovascular system [32, 33], our results were negligible from the bioactive point of view due to the small contents. However, our data reinforced that Longal variety is slightly more sensible to drying than Judia.
Vitamin E
The results obtained for the vitamin E composition for the two chestnut cultivars along the drying time are shown in Table 2. The major component was γ-tocopherol with 15.5 and 14.6 mg 100 g−1 dry matter in Longal and Judia varieties, respectively, at the time of zero hours, without significant differences between them. However, lower γ-tocopherol contents were observed in Longal variety after 10 h of drying whereas in Judia variety no significant differences were detected, showing again Longal to be more heat sensitive than Judia. For α-tocopherol, the second most abundant tocopherol, at the beginning (t = 0 h) significant differences were found between the two varieties (0.87 and 1.50 mg 100 g−1 dry matter for Longal and Judia, respectively), showing Judia always higher amounts than Longal along drying. Nevertheless a significant decrease in α-tocopherol content was observed for the former variety after 10 h of drying (20.0 %). In terms of δ-tocopherol, no significant differences were found between the two varieties at the beginning (t = 0 h), with 0.40 and 0.43 mg 100 g−1 dry matter for Longal and Judia, respectively. Again a significant decrease was observed after 10 h of drying but only for Longal variety (15.0 %).
Until now, there were no studies that had evaluated the effect of thermal processing on vitamin E composition. On general, the results of our study showed that the drying temperature of 50 °C only caused minor losses in some vitamin E vitamers.
Principal component analysis (PCA)
After performing a PCA to the fatty acid and vitamin E composition, two principal components were extracted (PC1 and PC2) that accounted for 71.2 % of the total variation (Fig. 1). The main variables associated with each principal component were: PC1—C18:1, C20:0, C20:1, MUFA and γ-tocopherol with positive component loadings, as well as α-tocopherol with a negative component loading; PC2—C16:1, C18:0, C18:2 and PUFA, all with positive component loadings. PC1 and PC2 explained 46.1 and 25.1 % of the variation, respectively. According to the PCA the two varieties were clearly differentiated whereas their lipid profiles along drying time were not so well differentiated. These results indicated that chestnut variety had a more important role than drying at 50 °C on fatty acid and vitamin E composition, even though some effect was detected in some specific compounds mainly in Longal variety.
Conclusions
For the first time the effect of hot air convective drying on quality and particularly on fatty acid and vitamin E composition of slices of two chestnut varieties, Longal and Judia, was studied. At the beginning significant differences were detected between varieties on some major fatty acids. After 10 h of drying only Longal variety had significant reductions on some fatty acid contents (C18:0, C18:1n9, C20:0 and C20:1n9), suggesting this variety to be more heat sensitive than Judia. Nevertheless, global MUFA and PUFA contents on both varieties did not decrease after 10 h of drying. From the results obtained it is possible to conclude that, despite being exposed to hot air drying after being sliced, no significant differences were observed on the fatty acid composition of Longal and Judia varieties. Concerning vitamin E, a decrease on γ- and δ-tocopherol contents was observed in Longal but not in Judia, this last variety with a decrease observed at α-tocopherol level. After performing a PCA, it was concluded that variety had a greater role than drying time on fatty acid and vitamin E composition. Therefore, although hot air convective drying could in some situations modify the lipid composition of this nut, the composition of dried sliced chestnuts at 50 °C was not much different from fresh chestnut. Thus, low temperature hot-air drying can be efficiently applied to this healthy and gluten free nut, from which other products can be produced such as snacks.
References
Rodrigues P, Venâncio A, Lima N (2012) Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res Int 48:76–90
Delgado T, Pereira JA, Casal S, Ramalhosa E (2015) Effect of drying on color, proximate composition and drying kinetics of sliced chestnuts. J Food Process Eng. doi:10.1111/jfpe.12244/epdf
Goulão L, Valdiviesso T, Santana C, Oliveira CM (2001) Comparison between phenetic characterisation using RAPD and ISSR markers and phenotypic data of cultivated chestnut (Castanea sativa Miller). Genet Resour Crop Evol 48:329–338
Borges OP, Carvalho JS, Correia PR, Silva AP (2007) Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. J Food Compos Anal 20:80–89
Benatti P, Peluso G, Nicolai R, Calvani M (2004) Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr 23:281–302
Venkatraman JT, Chu W (1999) Effects of dietary ω-3 and ω-6 lipids and vitamin E on serum cytokines, lipid mediators and anti-DNA antibodies in a mouse model for rheumatoid arthritis. J Am Coll Nutr 80:602–613
Lee C-YJ, Wan JM-F (2000) Vitamin E supplementation improves cell-mediated immunity and oxidative stress of Asian men and women. J Nutr 130:2932–2937
Itoh N, Masuo Y, Yoshida Y, Cynshi O, Jishage K, Niki E (2006) γ-Tocopherol attenuates MPTP-induced dopamine loss more efficiently than α-tocopherol in mouse brain. Neurosci Lett 403:136–140
de Vasconcelos MCBM, Bennett RN, Rosa EAS, Ferreira-Cardoso JV (2009) Industrial processing effects on chestnut fruits (Castanea sativa Mill.). 2. Crude protein, free amino acids and phenolic phytochemicals. Int J Food Sci Technol 44:2613–2619
de Vasconcelos MCBM, Nunes F, Viguera CG, Bennett RN, Rosa EAS, Ferreira-Cardoso JV (2010) Industrial processing effects on chestnut fruits (Castanea sativa Mill.). 3. Minerals, free sugars, carotenoids and antioxidant vitamins. Int J Food Sci Technol 45:496–505
Künsch U, Schärer H, Patrian B, Höhn E, Conedera M, Sassella A, Jermini M, Jelmini G (2001) Effects of roasting on chemical composition and quality of different chestnut (Castanea sativa Mill) varieties. J Sci Food Agric 81:1106–1112
Krist S, Unterweger H, Bandion F, Buchbauer G (2004) Volatile compound analysis of SPME headspace and extract samples from roasted Italian chestnuts (Castanea sativa Mill.) using GC-MS. Eur Food Res Technol 219:470–473
Cletus AB, Carson JK (2008) Drying curves and apparent diffusivity of New Zealand chestnut variety “1015”. J Food Eng 85:381–386
Delgado T, Pereira JA, Baptista P, Casal S, Ramalhosa E (2014) Shell’s influence on drying kinetics, color and volumetric shrinkage of Castanea sativa Mill. fruits. Food Res Int 55:426–435
Guiné RPF, Fernandes RMC (2006) Analysis of the drying kinetics of chestnuts. J Food Eng 76:460–467
Moreira R, Chenlo F, Chaguri L, Vázquez G (2005) Mathematical modelling of the drying kinetics of chestnut (Castanea sativa Mill.). Influence of the natural shells. Food Bioprod Process 83:306–314
Koyuncu T, Serdar U, Tosun I (2004) Drying characteristics and energy requirement for dehydration of chestnuts (Castanea sativa Mill.). J Food Eng 62:165–168
Correia P, Leitão A, Beirão-da-Costa ML (2009) The effect of drying temperatures on morphological and chemical properties of dried chestnuts flours. J Food Eng 90:325–332
Correia P, Beirão-da-Costa ML (2012) Effect of drying temperatures on starch-related functional and thermal properties of chestnut flours. Food Bioprod Process 90:284–294
Moreira R, Chenlo F, Torres MD, Rama B (2013) Influence of the chestnuts drying temperature on the rheological properties of their doughs. Food Bioprod Process 91:7–13
Attanasio G, Cinquanta L, Albanese D, Di Matteo M (2004) Effects of drying temperatures on physico-chemical properties of dried and rehydrated chestnuts (Castanea sativa). Food Chem 88:583–590
Moreira R, Chenlo F, Chaguri L, Fernandes C (2008) Water absorption, texture, and color kinetics of air-dried chestnuts during rehydration. J Food Eng 86:584–594
Moreira R, Chenlo F, Chaguri L, Mayor L (2011) Analysis of chestnut cellular tissue during osmotic dehydration, air drying, and rehydration processes. Drying Technol 29:10–18
Nesaretnam K, Yew WW, Wahid MB (2007) Tocotrienols and cancer: beyond antioxidant activity. Eur J Lipid Sci Technol 109:445–452
Podda M, Weber C, Traber MG, Packer L (1996) Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 37:893–901
Cruz R, Casal S, Mendes E, Costa A, Santos C, Morais S (2013) Validation of a single-extraction procedure for sequential analysis of vitamin E, cholesterol, fatty acids, and total fat in seafood. Food Anal Methods 6:1196–1204
España MSA, Galdón BR, Romero CD, Rodríguez ER (2011) Fatty acid profile in varieties of chestnut fruits from Tenerife (Spain). CyTA J Food 9:77–81
Barreira JCM, Casal S, Ferreira ICFR, Peres AM, Pereira JA, Oliveira MBPP (2012) Chemical characterization of chestnut cultivars from three consecutive years: chemometrics and contribution for authentication. Food Chem Toxicol 50:2311–2317
Barreira JCM, Casal S, Ferreira ICFR, Oliveira MBPP, Pereira JA (2009) Nutritional, fatty acid and triacylglycerol profiles of Castanea sativa Mill. cultivars: a compositional and chemometric approach. J Agric Food Chem 57:2836–2842
Fernandes Â, Antonio AL, Barros L, Barreira JCM, Bento A, Botelho ML, Ferreira ICFR (2011) Low dose γ-irradiation as a suitable solution for chestnut (Castanea sativa Miller) conservation: effects on sugars, fatty acids, and tocopherols. J Agric Food Chem 59:10028–10033
Fernandes Â, Barreira JCM, Antonio AL, Bento A, Botelho ML, Ferreira ICFR (2011) Assessing the effects of gamma irradiation and storage time in energetic value and in major individual nutrients of chestnuts. Food Chem Toxicol 49:2429–2432
Ascherio A, Katan MB, Zock PL, Stampfer MJ, Willett WC (1999) Trans fatty acids and coronary heart disease. New Engl J Med 340:1994–1998
Katan MB, Zock PL, Mensink RP (1995) Trans fatty acids and their effects on lipoproteins in humans. Annu Rev Nutr 15:473–493
Acknowledgments
Teresa Delgado acknowledges the Fundação para a Ciência e Tecnologia (FCT) for the financial support through the PhD Grant—SFRH/BD/82285/2011, CIMO through the PEst-OE/AGR/UI0690/2014 Project and REQUIMTE through the UID/QUI/50006/2013 and NORTE-07-0124-FEDER-000069 projects, as well as POCTEP—Programa de Cooperação Transfronteiriça Espanha-Portugal through the RED/AGROTEC—Experimentation network and transfer for development of agricultural and agro industrial sectors between Spain and Portugal Project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Delgado, T., Pereira, J.A., Ramalhosa, E. et al. Effect of hot air convective drying on the fatty acid and vitamin E composition of chestnut (Castanea sativa Mill.) slices. Eur Food Res Technol 242, 1299–1306 (2016). https://doi.org/10.1007/s00217-015-2633-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2633-5