Abstract
Impact of wheat flour replacement at 34% by ternary blends of 20% teff (T), 7% chestnut (CN) and 7% chickpea flours (CP) used native and submitted to heat moisture treatment (HMT) on in vitro starch digestibility were investigated in breads thereof. During the early stages of hydrolysis (0–60 min), HMT breads were hydrolyzed to a smaller extent than their native counterparts depending on the flour. All samples practically reached the plateau after 120 min and approached the equilibrium percentage of starch hydrolysed C∞ to an extent higher than 99.5% in all cases. Higher and delayed resistance towards the action of digestive enzymes was provided by CP flour on HMT when incorporated to bread formulations. The lowest value for hydrolysis index corresponded to samples with thermally treated T and CP flours that reached the lowest equilibrium percentage of starch hydrolyzed C∞, and hence leading to the lowest expected glycaemic index. Maximum formation of slowly digestible starch was achieved in breads with thermally treated T and native CP flours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blending grains constitute a simple and useful strategy to maximize food values, provided material-processing property relationships are well known. Grains are basic, ubiquitous and healthy raw materials that complement one another in multigrain products to enhance desirable functional and nutritional properties, as reported for ancient crops [1], minor cereals [2], pseudocereals [3], and legumes [4] in blended wheat-based matrices.
Processing leads to an alteration in the food structure and influences the nutritional characteristics of the food including starch digestibility. Endogenous factors of the food matrix and the macroscopic structure of the food influence the catalytic efficiency of the enzymes responsible during in vitro starch hydrolysis [5]. The presence of protein in the food matrix influences the rate of starch digestion by creating a stronger network, that may act as a barrier towards starch digestibility [6]. The presence of dietary fibre can impede enzymatic attack by increasing viscosity [7], and thus, they may act to slow down starch hydrolysis by restricting the movement of enzymes, and overall slowing digestion. Cooking or processing may sometimes reduce the starch digestibility as the conformational changes in proteins may occur that could facilitate the formation of disulfide-linked polymers [8]. The high concentration of anti-nutrients such as phytic acid, lectins, enzyme inhibitors in legumes may also play a role in starch digestibility.
A suitable slow release and absorption of glucose may be generated in a food matrix according to the processing conditions and surrounding ingredients [9]. The ingestion of foods, rich in both slowly digestible starch (SDS) and resistant starch (RS), promote the improvement of the intestinal microbial flora, prevention of diabetes, reduction of chronic diseases, among other benefits [10]. In foods with a high Rapidly Digestible Starch (RDS) content such as bread, starch digestibility can be altered through the modification of the chemical structure or molecular organization of starch by physical methods considered more natural, non-toxic and highly safe like heat moisture treatment (HMT) which is free of by-products of chemical reagents [11]. HMT allows the amylose and amylopectin fractions to assume a rubbery state, allowing them to interact to form double helices and to increase the overall stability of the granule to disruption [12], resulting in increased RS. The creation of amylose–lipid complexes helps to hinder granular swelling, as well as to develop further entanglement between the starch polymers. Together these factors aid in the formation of RS by restricting the ability of digestive enzymes to breakdown starch [13]. HMT caused the clumping of starch granules and the aggregation of denatured protein [14], affecting starch digestibility in higher extent in wheat flours than in wheat starch attributed to the higher protein and lipids contents of flour than starch [14].
In author’s previous studies, HMT effects of non-wheat teff, chestnut, and chickpea flours on dough viscoelastic and thermal parameters and on the structural pattern of breads were investigated in associated wheat-based matrices. Suitable trends for the enhancement of the physical characteristics of breads in terms of larger specific volume, higher viscoelastic and textural profiles, with lower and slower staling kinetics on ageing were achieved, in breads.
However, despite the functional and nutritional benefits of HMT blended matrices, as a wholegrain multigrain initiative, extensive studies of the effect of the thermal treatment of flour blends on starch digestibility of breads were not found in the reported literature. The current paper is aiming at investigating how HMT influenced in vitro starch hydrolysis kinetics and formation of relevant starch nutritional fractions in mixed grain matrices.
Materials and methods
Flours
Commercial flours from refined common wheat Triticum aestivum (WT), teff Eragrostis tef (T), chestnut Castanea sativa (CN), and whole chickpea Cicer arietinum (CP) were obtained from the Spanish market. Refined WT (70% extraction rate) of 195 × 10−4 J energy of deformation W, 0.57 curve configuration ratio P/L, and 58.8% water absorption in Brabender Farinograph, was used. Carboxymethylcellulose Aquasorb® A-500 (CMC) was bought from Copenhagen Pectin (Denmark), and commercial wheat sour dough Pie was kindly supplied by Ireks (Spain). Two replicates were made for each analysis. Moisture, protein, dietary fibre and fat contents (% flour, moisture basis) determined following the ICC methods [15], were 14.30, 12.10, 2.19%, 1.34 (WT); 12.62, 12.30, 10.76%, 4.10 (T); 6.90, 6.00, 9.00, 3.82% (CN), and 11.88, 16.58, 22.17, 6.13% (CP), respectively.
Heat-moisture treatment (HMT)
HMT conditions (15% moisture content, 1 h and 120 °C) were selected based on previous experiments [16], in which maximization of viscometric profile and minimization of loss of hydration properties of flour samples were applied as criteria. In gluten-poor matrices’ starch plays a key role as structuring biopolymer. A high viscosity profile during pasting and gelling of hydrated flour blends is necessary to hold CO2 during fermentation and to fix a porous aerated structure after baking. Single T, CN and CP flour samples were placed into screw-capped glass containers. Small amount of distilled water was added slowly with frequent stirring until moisture levels (w/w) of the total mixture reached 15%, and equilibrated for 24 h at room temperature. Hydrated samples were kept for 1 h at 120 °C in a convection oven (P-Selecta, Barcelona, Spain). Untreated native flours were used as controls. Untreated (−) and HMT (+) single flours were used in quaternary blends (T:CN:CP:WT) in the presence of WT- for dough-making.
Bread making of wheat and wheat-based blended flours
Specific flour composition was set after a prospective study on the compositional and functional characteristics of non-wheat flours (native and HMT) was performed (unpublished results). Results pointed out that besides the superior nutritional value as compared to wheat, teff, chestnut and chickpea individual flours were sensitive to HMT in terms of increased water absorption, viscosity after heating–cooling cycles, increased consistency (forward-extrusion test), and acceptable dough handling ability during processing. This behaviour made flours interesting candidates to be integrated in wheat diluted systems with good prediction as dough strengtheners. Percentages of replacement resulted from experimental studies aimed at knowing maximum amount of each flour without significant deleterious effect on dough machinability. Binary doughs from WT flour replaced by increasing amounts of T (10, 20, 30, 40%), CN (4, 7, 10%) and CP (4, 7, 10%) flours were made, respectively, and dough stickiness measurements were performed. Doughs characterized as non-sticky (< 100 g force) were selected, and the respective maximum percentage of wheat flour replacement was used to make the quaternary blends. In accordance, doughs and breads were prepared from wheat-based blended flours (T, CN, CP) by WT replacement at 34%, and incorporation of ternary blends of T (20%, flour basis), CN (7%, flour basis), and CP (7%, flour basis) flours according to a Multilevel Factorial Design with the following attributes: 3 experimental factors (T, CN and CP flours) at 2 levels, coded 0 (untreated) and 1 (HMT), and 5 error degrees of freedom. The model resulted in 8 randomized runs in 1 block. A 3 digit bread sample code was set referring to no HMT (0) and HMT (1) T (1st digit), CN (2nd digit), and CP (3rd digit) flours in sample formulation, as it follows: 110, 101,100, 000, 001, 111, 010, 011. Blended flours (100 g), water (100%, flour basis), commercial compressed yeast (3%, flour basis), salt (2%, flour basis), commercial sour dough Pie (5%, flour basis), and CMC (3%, flour basis) were mixed in a 10 kg mixer at 60 revolutions min-1 for 10 min up to optimum dough development. Preliminary tests were performed to know the amount of water necessary to avoid stickiness and deleterious effects on dough machinability, and 100% of water absorption was enough for all the formulations to assure dough handling ability during processing. CMC was added to dough formulations to help dough structuring ability in weakened wheat-based systems where gluten is diluted because of wheat flour replacement by gluten-free flours [4]. Fermented doughs were obtained after bulk fermentation (10 min at 28 °C), dividing (300 g), rounding, molding, panning and proofing up to maximum volume increment (50 min at 28 °C), and were baked at 225 °C for 25 min to make blended breads. Two baking trials were conducted per formulation.
Enzymatic determinations
In vitro starch hydrolysis kinetics and relevant starch fractions in blended breads was determined following the AACC (2005) method 32–40 [17], adapted as previously described [18]. RDS and SDS were measured after incubation for 20 and 120 min, respectively [17]. Each bread sample (100 mg) was incubated with pancreatic -amylase (10 mg) and amyloglucosidase (12 U) in 4 mL of 0.1 mol/L sodium maleate buffer (pH 6.0) in a shaking water bath (200 strokes/min) at 37 °C. Seven tubes were prepared per sample formulation to take aliquots at 0, 20, 60, 90, 120, 180, and 960 min, respectively. After incubation, samples were heated at 100 °C for 5 min, and ethanol
In vitro starch hydrolysis kinetics and relevant starch fractions in blended breads was determined following the AACC (2005) method 32–40 [17], adapted as previously described [18]. RDS and SDS were measured after incubation for 20 min and 120 min, respectively [17]. Each bread sample (100 mg) was incubated with pancreatic α-amylase (10 mg) and amyloglucosidase (12 U) in 4 mL of 0.1 mol/L sodium maleate buffer (pH 6.0) in a shaking water bath (200 strokes/min) at 37 °C. Seven tubes were prepared per sample formulation to take aliquots at 0, 20, 60, 90, 120, 180, and 960 min, respectively. After incubation, samples were heated at 100 °C for 5 min, and ethanol: water (95:5, v:v) was added for enzyme inactivation, prior to centrifugation at 720 g for 10 min. Total digestible starch (DS) was determined in the supernatant after 16 h of incubation while RS was determined in the pellet as the starch remaining after 16 h incubation. The digestion kinetics and expected glycaemic index (eGI) of bread were calculated [18, 19]. A first order kinetic equation [C = C∞ (1 − e−kt)] was applied to describe the kinetics of starch hydrolysis, where C, C∞ and k were the hydrolysis degree at each time, the maximum hydrolysis extent and the kinetic constant, respectively. The hydrolysis index (HI) was calculated as the relation between the area under the hydrolysis curve (0–16 h) of blended bread samples and the area of standard material from white bread (control) [20]. The expected glycaemic index (eGI) was calculated using the equation eGIwb = 8.198 + 0.862 HI [21] using white bread as the reference, and the conversion to eGIglucose using glucose as the reference food: eGIglucose = 0.71⋅eGIwb [22, 23].
Statistical analysis
Statistical package Statgraphics Plus V 5.1 (Statpoint Technologies, Warrenton, Virginia, USA) was used to perform univariate (One-way analysis of variance ANOVA) and multivariate (two-way analysis of variance MANOVA, Pearson correlation matrix, non-linear regression analysis and factor analysis FA) data analysis. Results were presented as the mean value ± standard deviation of at least duplicate determinations. Significant differences within pairs of means were assessed by Fisher’s least significant differences test LSD at 95% confidence interval (p < 0.05) in all cases. FA was carried out using a matrix of normalized correlation to calculate the eigenvalues (loadings), eigenvectors and related components with the original variables. The first two factors using principal components as factoring type were plotted to show factor scores in scatter plots for variables and samples.
Results and discussion
Starch hydrolysis kinetics
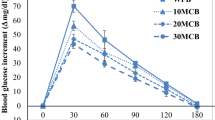
In starch, increased, decreased or unchanged susceptibilities to enzyme hydrolysis were observed as a result of HMT ascribed to variations in starch source as well as to differences in treatment conditions [24, 25]. Some authors reported that supramolecular structural disorganizations and the formation of densely packed starch fractions caused by HMT facilitated enzymatic accessibility to starch granules [24]. Other authors reported higher amylose content and crystallinity in HMT than in native starch samples, resulting in samples with a lower hydrolysis rate [25]. Starch hydrolysis that follows first order kinetics (99.23 < R2 < 99.87), proceeded at different rate and extent for HMT blended samples (Table 1). The steady state kinetic constant (k, min−1) of amylolysis ranged from 0.0491 (110) to 0.0623 (011) in treated samples vs. 0.0527 in native breads (000), evidencing from slightly slower to slightly faster hydrolysis kinetics, respectively, depending on the thermally treated flour in bread formulation. C∞ that corresponds to the equilibrium percentage of starch hydrolyzed after 16 h, varied from 83% (101, 111) to 88% (100) vs 87% (000), so that all the HMT samples showed a lower/equal extent of starch hydrolysis than native untreated samples. During the early stages of hydrolysis (0–60 min), HMT breads were hydrolyzed to a smaller extent than their native counterparts (Fig. 1a). After 20 min, starch hydrolysis took place from 50.6% (100) to 59.9% (011), after 60 min from 80.0% (111) to 83.4% (000) of total starch was digested, and after 90 min from 82.5% (111) to 86.3% (000) of starch was enzymatically hydrolyzed (Fig. 1a; Table 1). All samples practically reached the plateau after 120 min and approached the equilibrium percentage of starch hydrolyzed C∞ to an extent higher than 99.5% in all cases (Fig. 1a). Calculation of the samples hydrolysis indices (HI %), the proportion of flour starch that is theoretically digestible, by dividing the area under the hydrolysis curve of each blended sample by the corresponding area of the control sample (Table 1) pointed out the lowest value in samples 101 and 111 in good accordance with the lowest equilibrium percentage of starch hydrolyzed C∞, and hence leading to the lowest eGI (91–92). The glycemic index (GI), which characterizes the carbohydrate in different foods, is ranked on the basis of the postprandial increase in blood glucose [26]. An increased intake of low GI foods is recommended with emphasis on diabetics and subjects with impaired glucose tolerance [12].
Multiple analysis of variance (data not shown) provided information on the significant (p < 0.05) single and/or interactive effects of HMT of non-wheat flours T, CN and GP in blended breads on starch hydrolysis kinetics. CP flour submitted to HMT (1) compared to native (0) flour provided lower (C∞: 83 vs 87%) and slower (H90: 84 vs 86%) hydrolysis kinetics, encompassing lower AUC (18,334 vs. 19,053), HI (98% vs 102%), and subsequent eGI referred to either white bread (eGIwb: 93 vs. 96) or glucose (eGIg: 66 vs. 68). Simultaneous presence of T and CN affected the rate of hydrolysis k depending on HMT of the associated blend: when both flours are native (00) or thermally treated (11), hydrolysis kinetics gave the lowest k value (0.0545 min−1); whereas, with one of the flours thermally treated (01, 10), hydrolysis proceeded faster (k 0.0613 min−1). In complex systems like breads, non-starch components play an important role on starch hydrolysis kinetics. HMT, may cause the starch granules to clump together, forming small lumps, denatured protein may spread over and adhere to the surfaces of the starch granules clumps, and amylose–lipid complex formation can take place modifying starch hydrolysis kinetics in complex systems [5]. Non-wheat flours used in this study are rich in protein (12.30-16.58%) and lipids (3.80–6.13%), particularly CP (16.58, 6.13%), favouring the interactions between starch and non-starch components on HMT, and thus, causing delayed resistance towards the action of digestive enzymes. In addition, the high amount of dietary fibres in CP (22.17%) can impede enzymatic attack by either increasing viscosity (soluble fibres) or providing sterical hindrance (insoluble fibres), and they may act to slow down starch hydrolysis by restricting enzyme mobility and interfering enzyme attack, respectively.
Relevant starch nutritional fractions
Categorized starch fractions based on its rate of digestion and the location at which it is metabolized include RDS, SDS and RS, defined as the three consecutive nutritional fractions divided by reaction time when “in vitro” starch digestion takes place (Fig. 1b). Differences in susceptibility of starch to the α-amylase resulted in the different amounts of relevant starch nutritional fractions found in the native and HMT blended matrices (Table 2). In the current research, values for RDS and RS (g/ 100 g bread, as is) averaged 27.1 and 1.6, respectively (Table 2), irrespective of the thermal treatment of any of the compositional flours used either singly or in association. From studies of in vitro digestion, it has been observed that there is a transition in the smoothness of the progress curves of reducing sugar production from RDS to SDS [27] in good agreement with profiles in Fig. 1a HMT blended breads explicited a moderate range of SDS values (g/ 100 g bread, as is) ranging from 12.0% (101) to 17.9% (100), vs. untreated control breads (000) that averaged 13.7% (Table 2). HMT of CP flour significantly (p < 0.05) decreased SDS formation (from 15.6 to 13.2%). Among the flours used, CP flour exhibits the lowest digestible starch content (49%) and the higher amount of non-starch components: dietary fibre (22%), protein (17%) and lipids (6%). Upon HMT, increased molecular associations between starch and dietary fibre, protein and/or lipids may take place, and resulting structures can act as a barrier towards enzyme attack. Beside this, HMT may induce depolymerization of constituents in variable extent, mainly fibre, and hence may favour bread accessibility to solvents, acids and hydrolyzing enzymes, as the main reason for the SDS drop in thermally treated CP samples. Maximum SDS values 14.9–17.9% were achieved in breads 110, 100, 010 (Table 2; Fig. 1b). The addition of hydrolyzed pea protein significantly reduced wheat starch amylolysis at the first 40 min of digestion, but no inhibitory effect was observed at later digestion times [28]. In the majority of reports, HMT results in slight to moderate increases in thermostable RS and/or SDS contents [11] in starch systems. Interactions between competing structural changes within granules (e.g., crystallite disruption, increased molecular associations, polymorphic conversion, and cracks at granule surfaces) on HMT are reported to be the basis for the observed differences [29]. In flour systems, additional active components such as protein, fibres, and lipids can modify the starch molecular structure on hydrothermal treatments, particularly in presence of high moisture content (27%), and high temperatures (170 °C) as reported for superheated steam processing treatment of wheat flours [30]. Only under these conditions induced higher mobility of the molecules facilitates interactions between starch, protein and lipids during processing, thereby partly restricting accessibility of starch chains to be hydrolyzed by enzymes, and leading to the formation of SDS and RS. Present HMT conditions (15% moisture, 120 °C) are milder than those observed to provoke significant formation of starch RS and SDS fractions, so that more discreet changes were observed.
Relationships between nutritional parameters and sample classification
Using Pearson correlation analysis, a range of correlation coefficients (r) (from − 0.8098 to 0.9537) were obtained for the relationships within starch digestibility kinetics and relevant starch nutritional fractions of HMT blended matrices (Table 3). Significant (p < 0.05) interdependences between RDS and SDS with AUC (− 0.7103, 0.7705) and HI (− 0.7596, 0.7875), were found, respectively, in good accordance with the shape of the hydrolysis curves (Fig. 1a). Since all the curves have reached the plateau at 120 min of reaction, higher SDS values mean higher AUC, and consequently larger HI. In addition, RDS and SDS negatively correlated (r − 0.8098), result compatible with the nature of the breads having the same quali and quantitative compositional flours and similar amount of total starch (41–44%).
Factorial analysis (Fig. 2) classified analytical variables into two different factors explaining 80% of the variability of the results (VE). Factor 1 (65% VE) grouped all the starch digestion kinetic parameters and starch nutritional fractions with the exception of RS which belonged to factor 2 (15% VE) (Fig. 2a). Scores of Factor 1 and Factor 2 clearly differentiated breads with untreated (0) and HMT (1) CP flour in formulation (Fig. 2b). Untreated CP breads (110, 000, 100, 010) vs. HMT CP breads (011, 101, 111, 001) were characterized by higher moisture content (42–44% vs. 41–42%), greater SDS (14–18% vs. 12–15%), C∞ (86–88% vs. 83–87%) and eGI (95–97 vs. 91–94), moderate RS (1.3–1.6% vs. 1.6–1.9%) and lower k (0.0491–0.0569 vs. 0.0514–0.0623 min−1) and RDS (25–27% vs 27–30%).
Scatterplots from factor analysis (Factor 1 vs. Factor 2) of starch digestibility parameters (a) and classification of blended wheat-based breads (b) formulated with teff (T), chestnut (CN), and chickpea (CP) flours. Three digit code refers to untreated (0) and heat-moisture treated (1) T:CN:CP flours replacing wheat flour in sample formulation
Conclusions
Dilution of wheat flour matrices at 34% by incorporation of ternary blends of T, CN and CP flours submitted to HMT of the individual, binary or ternary mixtures of non-wheat compositional flours, provided changes in starch digestibility kinetics of the resulting HMT breads. During the early stages of hydrolysis (0–60 min), HMT breads were hydrolyzed to a smaller extent than their native counterparts. All samples practically reached the plateau after 120 min and approached the equilibrium percentage of starch hydrolysed C∞ to an extent higher than 99.5% in all cases. CP flour provided major changes on HMT leading to lower and slower hydrolysis kinetics, lower eGI and decreased SDS formation. The lowest value for HI corresponded to samples with thermally treated T and CP flours that reached the lowest equilibrium percentage of starch hydrolyzed C∞, and hence leading to the lowest eGI. Maximum SDS values were achieved in breads with thermally treated T and native CP flours. Non-wheat flours used in this study are rich in protein and lipids, particularly CP (16.58, 6.13%), favouring the interactions between starch and non-starch components on HMT, and thus, causing delayed resistance towards the action of digestive enzymes. In addition, the high amount of dietary fibres in CP (22.17%) can impede enzymatic attack by either increasing viscosity (soluble fibres) or providing sterical hindrance (insoluble fibres), and they may act to slow down starch hydrolysis by restricting enzyme mobility and interfering enzyme attack, respectively.
References
Angioloni A, Collar C (2011) Nutritional and functional added value of oat, Kamut, spelt, rye and buckwheat versus common wheat in breadmaking. J Sci Food Agric 91:1283–1292. https://doi.org/10.1002/jsfa.4314
Angioloni A, Collar C (2013) Suitability of oat, millet and sorghum in breadmaking. Food Bioprocess Technol 6:1486–1493. https://doi.org/10.1007/s11947-012-0786-9
Collar C, Angioloni A (2014) Pseudocereals and teff in complex breadmak-ing matrices: impact of lipid dynamics on the bread functional and nutritional profiles. J Cereal Sci 59:145–154. https://doi.org/10.1016/j.jcs.2013.12.008
Collar C, Angioloni A (2017) High-legume wheat-based matrices: impact of high-pressure on starch hydrolysis and firming kinetics of composite breads. Food Bioprocess Technol 10:1103–1112. https://doi.org/10.1007/s11947-017-1883-6
Singh J, Dartois A, Kaur L (2010) Starch digestibility in food matrix: a review. Trends Food Sci Technol 21:168–180. https://doi.org/10.1016/j.tifs.2009.12.001
Kim EHJ, Petrie JR, Motoi L, Morgenstern MP, Sutton KH, Mishra S, Simmons LD (2008) Effect of structural and physicochemical characteristics of the protein matrix in pasta on in-vitro starch digestibility. Food Biophys 3:229–234. https://doi.org/10.1007/s11483-008-9066-7
Sasaki T, Kohyama K (2012) Influence of non-starch polysaccharides on the in vitro digestibility and viscosity of starch suspensions. Food Chem 133:1420–1426. https://doi.org/10.1016/j.foodchem.2012.02.029
Oria M, Hamaker B, Schull J (1995) In vitro protein digestibility of developing and mature sorghum grain in relation to α-, β-, and γ-kafirin disulfide crosslinking. J Cereal Sci 22(1):85–93. https://doi.org/10.1016/S0733-5210(05)80010-4
Lehmann U, Robin F (2007) Slowly digestible starch—its structure and health implications: a review. Trends Food Sci Technol 18:346–355. https://doi.org/10.1016/j.tifs.2007.02.009
Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA (2010) Resistant starch as functional ingredient: a review. Food Res Int 43:931–942. https://doi.org/10.1016/j.foodres.2010.02.004
BeMiller JN, Huber KC (2015) Physical modification of food starch functionalities. Annu Rev Food Sci Technol 6:19–69. https://doi.org/10.1146/annurev-food-022814-015552
Chung HJ, Liu Q, Hoover R (2009) Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr Polym 75:436–447. https://doi.org/10.1016/j.carbpol.2008.08.006
Hasjim J, Jane JL (2009) Production of resistant starch by extrusion cooking of acid-modified normal-maize starch. J Food Sci 74:C556–C562. https://doi.org/10.1111/j.1750-3841.2009.01285.x
Chen X, He X, Fu X, Huang Q (2015) In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture treatment. J Cereal Sci 63:109–115. https://doi.org/10.1016/j.jcs.2015.03.003
ICC (1976–1996) ICC standard methods of the International Association for Cereal Chemistry, 104/1, 105/2, 110/1, 115/1, 136, 162, 166. The Association, Vienna
Collar C, Armero E (2018) Impact of heat moisture treatment and hydration level on physico-chemical and viscoelastic properties of doughs from wheat-barley composite flours. Eur Food Res Technol 244:355–366. https://doi.org/10.1007/s00217-017-2961-8
AACC (2005). Approved methods of the American Association of Cereal Chemists, AA CC, 10th edn. St. Paul, Minn
Angioloni A, Collar C (2011) Physicochemical and nutritional properties of reduced-caloric density high-fibre breads. LWT Food Sci Technol 44:747–758. https://doi.org/10.1016/j.lwt.2010.09.008
Englyst KN, Vinory S, Englyst H, Lang V (2003) Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br J Nutr 89:329–339. https://doi.org/10.1079/BJN2002786
Chung HJ, Liu Q, Pauls PK, Fan MZ, Yada R (2008) In vitro starch digestibility, expected glycaemic index and some physicochemical properties of starch and flour from common bean. (Phaseolus vulgaris L.) varieties grown in Canada. Food Res Int 41:869–875. https://doi.org/10.1016/j.foodres.2008.03.013
Granfeldt Y, Björck I, Drews A, Tovar J (1992) An in vitro procedure based on chewing to predict metabolic responses to starch in cereal and legume products. Eur J Clin Nutr 46:649–660
Fernandes G, Velangi A, Thomas MS (2005) Glycemic index of potatoes commonly consumed in north American. J Am Diet Assoc 105:557–562. https://doi.org/10.1016/j.jada.2005.01.003
Ferng LH, Liou CM, Yeh R, Chen SH (2016) Physicochemical property and glycemic response of chiffon cakes with different rice flours. Food Hydrocoll 53:172–179. https://doi.org/10.1016/j.foodhyd.2015.02.020
Wang H, Zhang B, Chen L, Li X (2016) Understanding the structure and digestibility of heat-moisture treated starch. Int J Biol Macromol 88:1–8. https://doi.org/10.1016/j.ijbiomac.2016.03.046
Liu YF, Laohasongkram K, Chaiwanichsiri S (2016) Effects of heat-moisture treatment on molecular interactions and physicochemical properties of tapioca starch. MOJ Food process Technol 3:00072. https://doi.org/10.15406/mojfpt.2016.03.00072
Jenkins AL (2007) The glycemic index: looking back 25 years. Cereal Foods World 52:50–53. https://doi.org/10.1094/CFW-52-1-0050
Dona AC, Pages G, Gilbert RG, Kuchel PW (2010) Digestion of starch. In vivo and in vitro kinetic models used to characterize oligosaccharide or glucose release. Carbohydr Polym 80:599–617. https://doi.org/10.1016/j.carbpol.2010.01.002
López Barón N, Sagnelli D, Blennow A, Holse M, Gao J, Saaby L, Müllertz A, Jespersen B, Thava Vasanthan T (2018) Hydrolyzed pea proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll 79:117–126. https://doi.org/10.1016/j.foodhyd.2017.12.009
Hoover R (2010) The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit Rev Food Sci Nutr 50:835–847. https://doi.org/10.1080/10408390903001735
Hu Y, Wang L, Zhu H, Li Z (2017) Modification of physicochemical properties and in vitro digestibility of wheat flour through superheated steam processing. J Cereal Sci 74:231–237. https://doi.org/10.1016/j.jcs.2017.02.012
Acknowledgements
The authors acknowledge the Institutions Ministerio de Economía y Competitividad (MINECO) and Federación Europea de Desarrollo Regional (FEDER) for funding the Project AGL2015-63849-C2-1-R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Collar, C., Armero, E. Kinetics of in vitro starch hydrolysis and relevant starch nutritional fractions in heat-moisture treated blended wheat-based bread matrices: impact of treatment of non-wheat flours. Eur Food Res Technol 244, 1977–1984 (2018). https://doi.org/10.1007/s00217-018-3109-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3109-1