Abstract
Epidemiological studies have related the consumption of fruits and vegetables to a lower risk of chronic diseases. Phytochemicals are held responsible for these desired effects; vitamin C and phenolic compounds being the most important ones in strawberries and raspberries. With respect to their role in health, it is valuable to study possible changes of these bioactive compounds during processing of fruits. In the present study, total phenolics, anthocyanins and vitamin C were firstly characterised in strawberries, raspberries and their juices. Thermal and high-pressure processes were screened for their effect on the bioactive compounds by treating strawberries and raspberries at different temperature–pressure combinations for one fixed treatment time (20 min). Thermal processing at atmospheric pressure (50–140 °C) had a degradative effect on anthocyanins and vitamin C. High-pressure processing (400, 600, 700 MPa combined with 20, 50, 80, 110 °C) showed no significant effect on the bioactive compounds in function of pressure. Breakdown of anthocyanins and vitamin C did occur at constant elevated pressure as temperature increased. No clear trends were perceptible for the amount of phenolic substances during processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies observed a relation between consumption of fruits and vegetables and a lower risk of chronic diseases like cancer, stroke and heart disease [1–3]. The health-related effect of these plant foods is attributed to bioactive compounds, named phytochemicals. In strawberries and raspberries, the major phytochemicals comprise phenolic compounds and vitamin C [1, 4]. Phenolic compounds are composed of one or more aromatic rings bearing one or more hydroxyl groups and occur often glycosylated in foods. Within the enormous group of phenolics, flavonoids (2/3) and phenolic acids (1/3) are most commonly consumed [5]. The anthocyanins constitute one of the main classes of flavonoids and are natural plant pigments responsible for the red, blue and purple colours of fruits and flowers and thus abundantly present in berries. Vitamin C comprises both ascorbic acid (AA) and dehydroascorbic acid (DHAA). The health-related properties of the studied phytochemicals are mainly attributed to their antioxidant activity [6, 7].
To retain the beneficial health effects, losses of these bioactive compounds in strawberries and raspberries during processing should be minimised. Food processing such as pasteurisation or sterilisation aims at microbial safety and extended shelf life of the food products [8]. Traditional thermal processing, although very efficient in achieving safe food products, can have detrimental effects on product quality. Novel high-pressure pasteurisation applies pressures in the range of 400–600 MPa at ambient temperature and results in products with a prolonged shelf life and fresher characteristics compared to the thermal equivalent [9]. Sterilisation or inactivation of vegetative microorganisms as well as spores using high pressure is currently a popular investigation topic. Inactivation of bacterial spores by pressure alone seems not possible. However, e.g. combination with high temperature, or the use of pressure pulsing are proposed to contribute to the necessary spore inactivation [10–12]. Since residual dormant spores have been detected after pressure pulsed treatments and this method was found to be economically less attractive due to high maintenance costs and decrease of equipment lifetime, high temperature–high pressure processing is the main route for investigating high-pressure sterilisation [13].
In the current study, the influence of temperature and pressure on the concentration of bioactive compounds was investigated. Total phenolic content, anthocyanins and vitamin C in strawberries and raspberries were first determined. A range of temperature–pressure combinations was screened for their influence on the levels of phytochemicals by treating the berries for a constant treatment time. Both a fruit paste and a fruit juice, prepared with and without use of a commercial enzyme preparation, were examined.
Materials and methods
Fruit material
Fresh strawberries (Fragaria x ananassa, cv. Elsanta) and fresh raspberries (Rubus idaeus, cv. Sugana) were purchased at the Belgische Fruitveiling CVBA (Sint-Truiden, Belgium). Plants were grown in the soil under plastic tunnels. Each type of fruit was bought twice, one batch in 2007, the other one in 2009, each time in the same harvesting season (September–October). The fresh fruits were frozen and stored at −40 °C. A batch of at least 500 g of strawberries or 250 g of raspberries was thawed overnight at 4 °C and blended to a paste (Waring blender 7010G, VWR, Heverlee, Belgium) prior to treatment.
Juice preparation
Two ways of juice preparation were applied. The first way (using batch 1) was simply bringing the thawed berries in a domestic juice extractor (Magimix, France), which resulted in a turbid juice. For the second manner (using batch 2), a commercial enzyme preparation (Pectinex BE Colour, Novozymes, Denmark) was used. Berries were blended at first and then treated with the enzymes according to the instructions of the manufacturer. Three systems were compared: (1) HPLC-grade water (milliQ: 18.2 MΩ cm ultra pure water, Millipore, Brussels, Belgium) was added to the fruit paste as a control sample at a concentration of 1 ml per 50 g of fruit paste, (2) Pectinex diluted in 1 ml HPLC-grade water was added in a concentration of 0.3 μl per 50 g strawberry paste and 0.5 μl per 50 g raspberry paste. This is the maximal dosage recommended by the manufacturer and (3) Pectinex diluted in HPLC-grade water was added in a concentration 4 times higher than the maximal recommended dosage. All 3 systems were incubated for 4 h in a shaking water bath at 50 °C, the optimal temperature for enzyme activity. Afterwards, the treated paste was centrifuged at 9,820 g for 15 min at 4 °C (J2-HS centrifuge, Beckman, Analis, Namen, Belgium) to obtain a clear juice. Juice samples were frozen in liquid nitrogen and stored at −40 °C until further analysis. The pellet samples were frozen as well, and the same analyses as for juices were executed as far as possible. Juice preparation was repeated twice for each fruit.
Extraction and quantification of anthocyanins
Since anthocyanins are water-soluble compounds, a simple extraction with cold HPLC-grade water could be performed (fruit paste/water ratio was 1:2 w/w). This mixture was centrifuged at 19,900 g for 15 min at 4 °C (Microfuge 22R, Beckman Coulter, Fullerton, CA, US), and the supernatant was filtered through Chromafil A-45/25 (Macherey–Nagel, Düren, Germany) before HPLC analysis. Each sample was extracted twice.
Anthocyanins were analysed according to the HPLC method adapted from Hong and Wrolstad [14], using a BioLC system (Dionex, Sunnyvale, CA, US), equipped with an AS50 autosampler, GS50 gradient pump and AD25 UV–Vis detector. Separation of the different anthocyanins was achieved by the use of a polymer reversed-phase column (PLRP-S; 250 × 4.6 mm, 5 μm particle size, 100 Å pore size; Varian, Palo Alto, CA, US) at 25 °C and by applying a linear gradient of the two eluents at a flow rate of 1 ml/min. Starting conditions were 90% of eluent A (=4% phosphoric acid in HPLC-grade water) and 10% of eluent B (=60% acetonitrile in HPLC-grade water). Slowly and gradually increasing eluent B established peak separation; for strawberries, % B was increased from 18 to 28% over 45 min, while for raspberries, it was increased from 11.5 to 14.5%. In a final washing step, 100% of B was reached and held for 5 min. Injection volume was 25 μl for all samples, and detection was performed at 520 nm.
To identify and quantify the peaks of pelargonidin-3-glucoside (Pg-3-glu), cyanidin-3-glucoside (Cy-3-glu), cyanidin-3-sophoroside (Cy-3-soph) and cyanidin-3-rutinoside (Cy-3-rut), a standard (Polyphenols, Sandnes, Norway), dissolved in acidified methanol (0.1% HCl), was used. The other peaks were identified based on relative retention times found in literature and were quantified using the standard curve of Pg-3-glu (strawberries) or Cy-3-soph (raspberries).
Determination of vitamin C
Extraction of vitamin C
Vitamin C was extracted from the treated fruit samples by adding extraction buffer (20 mM NaH2PO4 + 1 mM Na2EDTA in HPLC-grade water, pH 2.0) in a ratio of 1:1. This mixture was centrifuged at 20,400 g for 30 min at 4 °C (J2-HS centrifuge), and the supernatant was filtered through Chromafil A-45/25 before freezing and storing at −80 °C. Each sample was extracted twice.
Precolumn reduction by DTT (used for strawberry fruit and turbid juices)
To determine the vitamin C content of strawberry fruit, extracts were divided into 2 parts. The first part was used for direct ascorbic acid analysis; after diluting (1:2) the extract in phosphate buffer (0.1 M K2HPO4 in HPLC-grade water, pH 7.0) and filtering through Chromafil A-45/25, this sample was ready for HPLC analysis. The other part was subjected to a reduction reaction of dehydroascorbic acid (DHAA) to ascorbic acid (AA) by adding dithiothreitol (DTT) solution (2.3 mM DTT in phosphate buffer 0.1 M, pH 7.0) in 1:2 ratio and centrifuging at 19,900 g for 15 min at 24 °C (Microfuge 22R). All DHAA was converted to AA, so that in HPLC analysis, the total vitamin C content was measured in the form of AA concentration. By subtracting the AA concentration of the second part and the first part, the DHAA concentration was calculated.
HPLC analysis was performed on the Dionex BioLC apparatus. Isocratic elution was adopted over a Prevail C18 column (250 × 4.6 mm, 5 μm particle diameter, Grace, Lokeren, Belgium), held at 25 °C. The elution buffer consisted of 1 mM Na2EDTA and 10 mM CH3COONH4 in HPLC-grade water, pH 3.0, and had a flow rate of 0.8 ml/min. Injection volume was 20 μl for all samples, and detection was performed at 245 nm. For quantification, a standard curve was created using a solution of 5 mM ascorbic acid (99%, Acros Organics, Geel, Belgium) prepared in extraction buffer and stored at −80 °C. The exact concentration of the standard solution was checked spectrophotometrically (245 nm, 25 °C, pH 0.69, ε = 10.2 mM−1 cm−1) according to the law of Lambert–Beer.
Precolumn reduction by TCEP (used for raspberry fruit)
The use of DTT as a reductant of DHAA was questioned by Lykkesfeldt [15], and he proposed to apply TCEP (tris (2-carboxyl-ethyl) phosphine). While DTT reduces DHAA to AA efficiently at neutral pH, TCEP can do this at lower pH at which DHAA and AA are more stable. Since adapting the reducing agent could deliver an efficient reduction as well as help to maintain sample stability, the raspberry samples were analysed according to this method. The procedure described for reduction by DTT needed a few adjustments. The extracts were brought to pH 3.5 before being divided into 2 parts. The part for direct ascorbic acid analysis was diluted (1:2) in phosphate buffer (20 mM NaH2PO4 + 1 mM Na2EDTA in HPLC-grade water, pH 3.5). The other part was subjected to reduction by adding 2.5 mM TCEP solution in phosphate buffer (0.1 M, pH 3.5) in 1:2 ratio. The rest of the procedure was analogous to the DTT method.
Postcolumn derivatisation method (used for clear juices)
Using the postcolumn derivatisation method, adapted from Kall and Andersen [16], concentrations of AA and DHAA could be determined in a single run, which was time-saving. AA was quantified using UV detection; DHAA in the same sample was quantified after derivatisation by means of fluorescence detection. Sample preparation before HPLC analysis (on Agilent Technologies 1200 Series with DAD detection, Diegem, Belgium) consisted solely of filtering the extract (Chromafil A-45/25). For quantification of AA, the same column was employed as in the precolumn reduction method; column temperature was maintained at 20 °C. This time, elution buffer consisted of 2.3 mM dodecyltrimethylammonium chloride and 2.5 mM Na2EDTA in a 66 mM phosphate–20 mM acetate buffer adjusted to pH 4.5. Flow rate was 1.0 ml/min. After the sample had passed through the UV detector, it was led through a reaction coil (750 μl, Dionex) held at 55 °C, together with the derivatisation reagent (28 mM o-phenyldiamine in a 12 mM trisodium citrate–55 mM Na2EDTA buffer, pH 3.7). The postcolumn reagent was delivered by an AXP-pump (Dionex) at a flow rate of 0.3 ml/min. DHAA reacted with o-phenyldiamine to form 3(1,2-dihydroxyethyl)furo[3,4-b]quinooxaline-1-one, which could be detected fluorometrically at excitation wavelength 350 nm and emission wavelength 430 nm.
To compose the standard curve of DHAA, half of the standard solution of 5 mM AA was used to convert to DHAA by adding stepwise iodine solution (0.1 M) until a constant light yellow colour appeared. To neutralise the excess of iodine, 2 crystals of Na2S2O3 were added and the standard solution was diluted to a known volume. AA and DHAA standards were brought together, diluted to 0.5 mM AA and 0.025 mM DHAA and stored at −80 °C for maximum 2 months.
Determination of total phenolics
Extraction and quantification of total phenolics
The method employed by Benvenuti et al. [17] was slightly adapted and applied as follows. In a first extraction step, an equal volume of fruit paste and solution of methanol/HCl (2%) (95:5 v/v) was mixed and set to react for 60 min at 4 °C. Afterwards, this reaction mixture was centrifuged at 1,090 g for 15 min at 4 °C. The obtained supernatant was filtered under vacuum (MN615, Macherey–Nagel, Düren, Germany), and the remaining pellet was extracted once again. The whole extraction procedure was repeated twice for each sample. For determination of the total phenolic content, 100 μl of extract, 500 μl of Folin–Ciocalteu reagent and 1 ml of saturated Na2CO3 solution (75 g/L) were brought in a 10-ml volumetric flask. These reagents were mixed, the volume was added up to 10 ml using HPLC-grade water and the sample was put in an oven at 25 °C. After incubating for 2 h, the absorbance of the sample was measured at 750 nm (2100 pro UV–Visible spectrophotometer, Biochrom, Cambridge, UK). Absorbances were converted into concentrations by means of a standard curve of gallic acid (GA), and hence, results were expressed as mg GA per g berries. Quantification was performed in duplicate, and data were therefore the mean of 4 results.
Corrections for the Folin–Ciocalteu method
The Folin–Ciocalteu method is based on an oxidation–reduction reaction; thus, interference of other reducing substances than phenolic compounds can occur [42]. Two sources of interference were tested in this study: ascorbic acid and reducing sugars (glucose and fructose). The sugars did not show any effect on the measurement of total phenolics; the concentrations tested in strawberries were 22 g glucose/l, 50 g glucose/l and 25 g fructose/l; in raspberries, 20 g glucose/l, 20 g fructose/l and 30 g fructose/l. On the other hand, ascorbic acid did have an influence on the measurements, but only for strawberries, not for raspberries (tested for concentrations up to 400 ppm). To correct for this, a standard solution and known dilutions of ascorbic acid were run through the Folin–Ciocalteu procedure and concentrations were plotted against absorbances at 750 nm. The corresponding equation and the previously measured concentration of ascorbic acid for each individual sample were used to calculate a more correct concentration of total phenolics.
Temperature and pressure treatments
In this study, different temperature and pressure levels were screened for their effect on the considered bioactive compounds in strawberries and raspberries (batch 1). Treatment time was fixed at 20 min. High-pressure treatments were divided into 2 classes depending on the temperature they were combined with. Low temperatures in combination with high pressures represented high-pressure pasteurisation conditions, while high temperature–high pressure treatments represented high-pressure sterilisation. Both treatments demanded a different temperature and pressure build-up approach.
High-temperature treatment at atmospheric pressure
Heat treatments at atmospheric pressure were conducted in an oil bath (Grant Instruments, Cambridge, UK), equilibrated in advance at the correct treatment temperature, between 50 and 140 °C. The fruit paste was enclosed in metal tubes (outer diameter = 16 mm; inner diameter = 13 mm, length = 150 mm), avoiding headspace and placed in the oil bath for 20 min. During heat treatment, the temperature of the paste was registered using an Ellab E-val data acquisition system (TM 9616, Ellab, Hilleroed, Denmark) and thermocouples (type T, Thermo Electric Benelux, Balen, Belgium). For all temperatures investigated, a coming-up time of 15 min was observed and temperatures varied ± 2 °C during the isothermal phase. As an example, the temperature profile of 140 °C of raspberry paste is given in Fig. 1. After treatment, the tubes were cooled down in an ice bath to stop further thermal degradation. Berry fruit samples were frozen in liquid nitrogen and stored at −40 °C until further analysis.
Low temperature–high pressure treatment
All high-pressure treatments were performed in a 6-vessel high-pressure equipment (laboratory scale, custom-made, Resato, Roden, The Netherlands). The vessels (outer diameter = 150 mm, height = 207 mm) are constructed of stainless steel and are individually surrounded by a heating coil, connected to an external thermostat (custom-made, with PID controller, TCPS, Rotselaar, Belgium). This equipment provides computer-controlled pressure build-up, temperature control and data logging of both temperature and pressure. The sample chamber has a diameter of 24 mm and a height of 120 mm. Thermocouples (type J) are available in the stopper of the pressure vessel to log the temperature of (the centre of) the sample.
The fruit paste (19 g) was vacuum-packed in a double layer of plastic bags. All samples were placed simultaneously in the vessels, and pressure build-up was started after 1 min. The rate of pressure build-up was rather low, 100 MPa/min, which allowed dissipation of adiabatic heat. After 20 min of isothermal–isobaric treatment, pressure was released instantaneously. Samples were removed from the vessels after 1 min and cooled down in ice water for 10 min. Finally, the fruit samples were frozen in liquid nitrogen and stored at −40 °C until further analysis. Pressures of 400, 600 and 800 MPa were combined with relatively mild process temperatures of 20 and 50 °C. An example of a temperature–pressure profile is presented in Fig. 1. To show the difference in adiabatic heating and heat dissipation, two temperature profiles (20 and 50 °C) at the same pressure (600 MPa) were compared. In the approach of high-pressure pasteurisation, adiabatic heating resulted in a temporary temperature overshoot and a subsequent decrease in the temperature during process time, because the environment is at the (lower) desired process temperature.
High temperature–high pressure treatment
High temperature–high pressure treatments were performed in the same 6-vessel high-pressure equipment as described above. A major difference with low temperature–high pressure treatments was the rate of pressure build-up, 10 MPa/s. Adiabatic heating during pressure build-up contributed in this way to achieve the desired process temperature faster than when heating is only based on conduction/convection. To simulate high-pressure sterilisation conditions, pressures of 400, 600 and 700 MPa combined with temperatures of 80 and 110 °C were chosen. Due to technical restrictions of the high-pressure equipment, the highest pressure possible to combine with the high temperatures (>60 °C) was limited to 700 MPa as opposed to the highest pressure in low temperature–high pressure treatments (800 MPa, <60 °C).
The fruit paste was inserted in polyacetalic cylinders (outer diameter = 18 mm; inner diameter = 12 mm, length = 85 mm) and equilibrated at 10 °C. From the moment these samples were brought into the pressure vessels, they started to heat up due to contact with the pressure medium (propylene glycol based Resato PG fluid) that was already equilibrated at the desired process temperature (e.g. 110 °C). Once the temperature of the samples reached an experimentally validated level (79.7 °C for the example of 110 °C and 400 MPa), pressure build-up was started; immediately pressure increased to 150 MPa followed by a gradual increase at a rate of 10 MPa/s. During pressure build-up, the samples were heated further by adiabatic heating and by heat transfer from the pressure medium to the sample. One minute after the process pressure was reached, isothermal–isobaric conditions were achieved, individual vessels were isolated and zero treatment time was considered. This procedure resulted in variable coming-up times for each temperature–pressure combination tested, from 3.5 to 7 min. The temperature and pressure profiles in the sample holder during the treatment were logged; temperature variations were limited to ±2 °C, pressure variations to ±30 MPa. An example is given in Fig. 1. In this high-pressure sterilisation approach, a temperature overshoot was avoided and isothermal conditions were fulfilled during process time since the environment was kept constant at the desired process temperature. After treatment, samples were removed from the vessels 1 min after pressure release and cooled down in ice water. The fruit samples were finally frozen in liquid nitrogen and stored at −40 °C until further analysis.
Statistical analysis
In order to analyse the characterisation results statistically, the Tukey’s Studentised range test was applied (SAS, v9.2, Cary, US) to search for significant differences among the concentrations of bioactive compounds in the different fruit batches and in the fruit juices. The level of significance was set at p < 0.05.
Results and discussion
Characterisation of bioactive compounds in berry fruit and berry juices
Strawberries
The main anthocyanins in strawberries are identified as pelargonidin-3-glucoside (Pg-3-glu) and cyanidin-3-glucoside (Cy-3-glu) [18, 19]. The chromatograms obtained in the current study for cultivar ‘Elsanta’ showed 3 important peaks. Pg-3-glu and Cy-3-glu could be identified using commercially available standards. Goiffon et al. [19] examined ‘Elsanta’ strawberries as well and determined pelargonidin-3-arabinoside (Pg-3-ara) as the third most important anthocyanin. Since retention times corresponded relatively, the assumption was made that the third peak in the present study was also Pg-3-ara, but the ratios between the different anthocyanins differed between the 2 studies. In this study, Pg-3-glu is the most important (80%), followed by Pg-3-ara (17%) and only 3% of Cy-3-glu.
The absolute concentration of anthocyanins, vitamin C and total phenolics measured in strawberry fruit and juices is given in Table 1. The results for strawberry fruit corresponded well with the results of Patras et al. [20], who examined strawberries of the same cultivar with similar methods of analysis. Anthocyanin levels in the 2 batches of strawberries were significantly different (cf. Table 1). Cy-3-glu was not quantifiable in the second batch, whereas the other anthocyanins were present in higher concentrations compared to the first batch. Vitamin C and total phenolic levels were not significantly different in the 2 batches. Despite different assays being used to measure vitamin C concentrations throughout the experiments, their results can be compared to each other. The vitamin C concentrations of the raw material can be a good reference point to illustrate this. Firstly, different moments of sample taking and different measurements (8 in total for each batch) indicated that the concentrations within a batch were in the same range. Secondly, Tukey tests pointed out that the concentrations between the two batches were not significantly different. Based on these two arguments, it can be concluded that the different assays did not produce significantly different results and that the current results and conclusions were not affected by the use of several assays.
The juice yield obtained in the domestic juice extractor was 79%. Juices were also prepared by making use of a commercial enzyme preparation. Pectinase enzymes can help to clarify juices, to reduce the viscosity and in this way facilitate the pressing of the juice. Not only juice yield, but colour yield can be improved as well, since breakdown of the skin tissue and degradation of structural polysaccharides can help the pigments to diffuse into the juice [21]. In some cases, a negative effect of commercial enzyme preparations on anthocyanins is reported. β-Glycosidase (side-) activity in the enzyme preparation can remove the glycoside substituents of anthocyanins, obtaining unstable aglycons and resulting in colour loss [22, 23]. Following the enzymatic procedure, the control juice yielded only 70% of juice, while the juices treated with Pectinex yielded 84%. Increasing the enzyme dosage above the recommended did not add to the juice yield. Juices prepared in the domestic juice extractor (batch 1) did not show different anthocyanin concentrations compared to the originating strawberry fruit, neither in the juice nor in the pellet. For vitamin C, the concentrations in the juice did not change significantly during juice preparation, but in the pellet, less vitamin C was left and the ratio of AA to DHAA had reduced by a factor of 10. Total phenolics seemed more concentrated in the pellet. Similar observations could be described for the juices prepared in the enzyme-aided procedure (batch 2), with the exception that the ratio of AA to DHAA in the pellet of the juice prepared in the domestic juice extractor did not change as drastically. The incubation step for 4 h at 50 °C could be an explanation for the fact that the concentrations were overall slightly less in the juices compared to the strawberry paste. Although Pectinex contributed to the juice yield, it had no effect on the bioactive compounds during juice preparation. This is in accordance with Versari et al. [24], who observed no changes in total anthocyanin content of strawberry juices prepared using several commercial enzyme preparations, including Pectinex BE 3-L. Literature reports berry juices of black currants and bilberries to benefit from the treatment with Pectinex: It promoted juice extraction and enhanced the release of anthocyanins and other phenolics into the juice [25–27]. Perhaps the different distribution of anthocyanins in the fruits could explain the different response to enzymatic pre-press maceration. Strawberries do not have a pronounced, tough, outer skin that seems to contain the majority of the anthocyanins; the anthocyanins are more distributed throughout the whole fruit. However, black currants for example can really profit from an enzymatic pre-treatment since the skins of black currants are particularly rich in anthocyanins [25, 27].
Raspberries
Anthocyanins found in raspberries are mainly cyanidin glycosides. The chromatograms showed 4 peaks, of which 3 could be identified by means of commercially available standards, as cyanidin-3-sophoroside (Cy-3-soph), cyanidin-3-glucoside (Cy-3-glu) and cyanidin-3-rutinoside (Cy-3-rut). After exploring other studies determining anthocyanin composition of raspberry varieties [28–30] and comparing relative retention times, it was hypothesised that the fourth peak was cyanidin-3-glucorutinoside (Cy-3-glurut).
The results of analysis of the bioactive compounds in raspberries are given in Table 2. Data on the investigated cultivar ‘Sugana’ were not found in literature, but a comparison was made to other cultivars harvested in autumn. Total anthocyanin levels were slightly higher, but in the same range as in the cultivar ‘Autumn Bliss’ [28, 31]. Values for vitamin C were lower than reported by de Ancos et al. [28] for the autumn cultivars but corresponded well with the data of Mullen et al. [30] in cultivar ‘Glen Ample’. For the total phenolic content, de Ancos et al. [28] reported lower values than observed in this study, while Mullen et al. [30] reported a very similar concentration.
As can be seen in Table 2, the total anthocyanin level was the same in the 2 batches, although the ratios between the individual anthocyanins differed. Vitamin C and total phenolic levels did not change over the 2 raspberry batches. In general, raspberries contained more anthocyanins and less vitamin C than strawberries. The total level of phenolic substances was very similar.
The enzyme-aided juice preparation resulted in juice yields for the control juice and the enzyme-treated juices of 55 and 75%, respectively. The juices and pellets showed equal concentrations of anthocyanins and total phenolics compared to the raspberry paste. Only the concentration of cyanidin-3-sophoroside in the pellets seemed to be significantly different from the concentration in the juices. Since Cy-3-soph is the main anthocyanin in raspberries, this affected also the concentration of total anthocyanins in the pellets. As for strawberries, AA and vitamin C concentrations did not vary between raspberry paste and raspberry juices, but in the pellets, the vitamin C content decreased, as did the ratio of AA to DHAA. As in strawberries, the enzyme preparation enhanced the juice yield but did not influence the studied bioactive compounds during juice processing. This is a slightly better result than in literature. Jiang et al. [32] measured a 20% loss of the total anthocyanin pigments when using Pectinex Ultra-SL. Concerning individual anthocyanins, Cy-3-soph was converted into Cy-3-glu and Cy-3-glurut into Cy-3-rut, indicating the hydrolysis of a glucosidic bond and thus β-glucosidase activity. Versari et al. [24] examined several commercial enzyme preparations during raspberry juice processing: Some caused no changes in anthocyanin levels, and others, including Pectinex BE-L, decreased total anthocyanin content by 20%. They attributed the differences between the enzyme preparations to the presence of β-glycosidase activity. Pectinex BE Colour has been produced with red berry juice processing in mind and was assigned the best performance in berry juice preparation with high colour retention [33, 34].
Screening the process stability of anthocyanins in berries
The thermal stability of anthocyanins was studied in the temperature range 80–140 °C in strawberry (Fig. 2) and raspberry paste (Fig. 3). In both fruit pastes, a decrease in concentration in function of temperature is visible, and at 140 °C, almost all anthocyanins were lost. Fruit juices prepared in a domestic juice extractor were submitted to the same thermal treatments, which resulted in a very similar graph (data not shown). Therefore, the rest of the screening experiments were only conducted in fruit pastes. Patras et al. [20] subjected strawberry purée (same cultivar as in the current study) to a thermal treatment at 70 °C equivalent to a pasteurisation process (P70 ≥ 2 min), which caused already a significant reduction in anthocyanin content. The results obtained here correspond more to the results of Sadilova et al. [35]. They measured a decrease in anthocyanin content, purified out of strawberries, of approximately 13% after a treatment of 30 min at 95 °C.
In Fig. 2 (for strawberries) and Fig. 3 (for raspberries), the screening of pressure stability of anthocyanins is shown. Four different temperatures, each at three pressure levels, were investigated. At the temperature levels of 20, 50 and 80 °C, a slight decrease in anthocyanin concentration is visible in function of temperature, while at 110 °C, the anthocyanin concentrations have been influenced to a larger extent. In function of pressure, almost no differences could be distinguished. These results are in accordance with literature data. Pressure treatments at 400, 500 and 600 MPa for 15 min at ambient temperature (~20 °C) did not result in significant different anthocyanin concentrations compared to the untreated strawberry purée [20]. Two other publications discussed the effect of high pressure on anthocyanins, on the one hand in strawberries [36] and on the other hand in raspberries [37], each conducting experiments for 15 min at 20 °C and pressure levels of 200, 400, 600 and 800 MPa. In both fruits, the anthocyanins survived the pressure treatments itself but degraded during subsequent storage.
Screening the process stability of vitamin C in berries
In the study of the thermal stability of vitamin C (Fig. 4), a broader temperature range was investigated compared to the anthocyanins: 50–140 °C. After the treatment at 140 °C, around 30% of vitamin C was left in strawberries, approximately 10% in raspberries. The level of AA decreased in function of temperature. DHAA was formed at first but degraded at higher temperatures, and from 120 °C on, no DHAA could be measured anymore. It is known that AA readily oxidises to DHAA [38]. This oxidation reaction is probably the most important one during the 20-min treatment at 50–90 °C. At higher temperatures, further degradation reactions became significant as well, since both DHAA and AA concentrations decreased. The reactor tubes were closed during the treatment, so that the oxygen concentration in the tubes was limited. This might explain the relatively high AA concentration that remained after 20 min of (non-isothermal) heating.
The same conditions as for anthocyanins were used in the study of the pressure stability of vitamin C in strawberries and raspberries (Fig. 4). For strawberries, a decrease in vitamin C concentration could be distinguished in function of temperature, but not in function of pressure. An exception is 20 °C, where AA as well as DHAA concentrations seemed to slightly decrease as pressure increased. In raspberries, all pressure treatments at 50, 80 and 110 °C yielded more or less equal AA concentrations and nearly no DHAA. At 20 °C, the same trend was visible in raspberries as in strawberries, but these data could not be confirmed by literature. Fifteen minutes at 400, 500 and 600 MPa and ambient temperature yielded the same vitamin C retention in strawberry purée [20]. A pressure treatment for 30 min at 400 MPa and 20 °C resulted in an insignificant vitamin C reduction of 11.32% compared to untreated strawberry coulis and even no reduction in strawberry nectar [39]. Higher temperatures were investigated by Taoukis et al. [40]; 75 °C was combined with pressures of 0.1, 300, 450 and 600 MPa, and the treatment time was 40 min. Only in the model system, the pressure treatment of 600 MPa ended in a lower vitamin C retention compared to the other pressures; in pineapple juice and grapefruit juice, there was no effect of increasing the pressure level at 75 °C.
Screening the process stability of total phenolics in berries
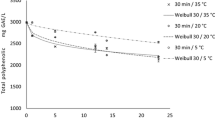
The concentration of total phenolics was determined using the Folin–Ciocalteu method [41]. According to Prior et al. [42], this is a uniformly acceptable method of total phenolic analysis, provided that a few remarks are taken into account. The bottleneck is that the basic mechanism is a general oxidation–reduction reaction. As a consequence, it is often hard to know precisely which compounds are being measured, plus it makes the method susceptible to interfering substances. In the current study, this method was applied and efforts were made to meet the demands postulated by Prior et al. [42] (cf. “Materials and methods”); the results are given in Fig. 5.
In both thermal and high-pressure experiments, the total phenolics seemed to react similarly in strawberries and raspberries. The treatments showed little effect on the total phenolic content; only the thermal data suggested a small increase as temperature increased, but this was not significant. Klopotek et al. [43] performed a thermal treatment of strawberry juice and wine for approximately 5 min at 85 °C and found decreasing levels of phenolic substances. In strawberry purée of the same cultivar as in the present study, a pasteurisation treatment (P70 ≥ 2 min) had no effect on the total phenolic content [20]. A treatment at 600 MPa for 15 min at ambient temperature (~20 °C) resulted in a significantly increased amount of total phenolics compared to the untreated purée. The authors attributed this increase to an increased extractability of some of the phenolic compounds following high-pressure processing. Cao et al. [44] carried out the same thermal experiment as Patras et al. [20], but this ended in a significant increase in total phenolics of the pasteurised strawberry pulp. Their similar high-pressure experiments produced the same results at 500 and 600 MPa as found by Patras et al. [20]. The argument of higher extractability was used to explain both observations.
All these scattered results might be explained by the diversity of the group of phenolics. The reducing capacity is being measured, but there is no information on the type and the amount of specific phenols present. The concentration of total phenolics in the graphs is the final result of several chemical and/or enzymatic reactions occurring during the treatments. Degradation of phenolic substances into other phenols or into non-phenolic reaction products can cause increased, decreased or unchanged concentrations of the phenolic compounds.
Conclusion
Anthocyanins, total phenolics and vitamin C are important phytochemicals in strawberries and raspberries. They were characterised in both fruits and derived juices and screened for their susceptibility to thermal and high-pressure processing. Juice processing using a commercial enzyme preparation delivered higher juice yields but no increase in the amount of bioactive compounds. Thermal processing at atmospheric pressure had a degradative effect on anthocyanins and vitamin C. Anthocyanin concentrations started to decline at temperatures of 90 °C and up and were almost completely lost at 140 °C. Degradation of vitamin C was mainly oxidative: Ascorbic acid was converted into dehydroascorbic acid. Closed tubes limited the oxygen concentration of the fruit samples, which assured only a moderate breakdown of vitamin C. From 100 °C, DHAA was undetectable and probably other (non-oxidative) reactions became relevant. High-pressure processing revealed no significant effect on the bioactive compounds in function of pressure. Degradation of anthocyanins and vitamin C did occur at constant elevated pressure as temperature increased. The results for total phenolics did not show clear trends in function of temperature or pressure. Based on the current data of process stability of anthocyanins and vitamin C, the temperature–pressure ranges valuable for detailed kinetic studies could be marked out.
References
Hannum SM (2004) Potential impact of strawberries on human health: a review of the science. Crit Rev Food Sci Nutr 44:1–17
Ness AR, Powles JW (1997) Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol 26:1–13
Steinmetz KA, Potter JD (1996) Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 96:1027–1039
Gonzalez EM, de Ancos B, Cano MP (2003) Relation between bioactive compounds and free radical-scavenging capacity in berry fruits during frozen storage. J Sci Food Agric 83:722–726
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Croft KD (1998) The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci 854:435–442
Padayatty SJ, Katz A, Wang YH, Eck P, Kwon O, Lee JH, Chen SL, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35
Holdsworth D (2009) In: Simpson R (ed) Engineering aspects of thermal food processing. CRC Press, Boca Raton
Matser AA, Krebbers B, van den Berg RW, Bartels PV (2004) Advantages of high pressure sterilisation on quality of food products. Trends Food Sci Technol 15:79–85
Mujica-Paz H, Valdez-Fragoso A, Samson CT, Welti-Chanes J, Torres JA (2011) High-pressure processing technologies for the pasteurization and sterilization of foods. Food Bioprocess Technol 4:969–985
Rendueles E, Omer MK, Alvseike O, Onso-Calleja C, Capita R, Prieto M (2011) Microbiological food safety assessment of high hydrostatic pressure processing: a review. Lwt Food Sci Technol 44:1251–1260
Wilson DR, Dabrowski L, Stringer S, Moezelaar R, Brocklehurst TF (2008) High pressure in combination with elevated temperature as a method for the sterilisation of food. Trends Food Sci Technol 19:289–299
Barbosa-Canovas GV, Juliano P (2008) In: Gutierrez-Lopez GF, Barbosa-Canovas GV, Welti-Chanes J, Parada-Arias E (eds) Food engineering: integrated approaches. Springer, New York
Hong V, Wrolstad RE (1990) Use of HPLC separation photodiode array detection for characterization of anthocyanins. J Agric Food Chem 38:708–715
Lykkesfeldt J (2000) Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: reliable reduction with tris[2-carboxyethyl] phosphine hydrochloride. Anal Biochem 282:89–93
Kall MA, Andersen C (1999) Improved method for simultaneous determination of ascorbic acid and dehydroascorbic acid, isoascorbic acid and dehydroisoascorbic acid in food and biological samples. J Chromatogr B 730:101–111
Benvenuti S, Pellati F, Melegari M, Bertelli D (2004) Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci 69:C164–C169
da Silva FL, Escribano-Bailon MT, Alonso JJP, Rivas-Gonzalo JC, Santos-Buelga C (2007) Anthocyanin pigments in strawberry. Lwt Food Sci Technol 40:374–382
Goiffon JP, Mouly PP, Gaydou EM (1999) Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal Chim Acta 382:39–50
Patras A, Brunton NP, Da Pieve S, Butler F (2009) Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purees. Innov Food Sci Emerg Technol 10:308–313
Pilnik W (1982) Enzymes in the beverage industry—fruit juices, nectars, wine and beer. Industrie Alimentari 21:585
Wightman JD, Wrolstad RE (1996) beta-Glucosidase activity in juice-processing enzymes based on anthocyanin analysis. J Food Sci 61:544
Wrolstad RE, Wightman JD, Durst RW (1994) Glycosidase activity of enzyme preparations used in fruit juice processing. Food Technol 48:90
Versari A, Biesenbruch S, Barbanti D, Farnell PJ, Galassi S (1997) Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Res Int 30:811–817
Bagger-Jorgensen R, Meyer AS (2004) Effects of different enzymatic pre-press maceration treatments on the release of phenols into blackcurrant juice. Eur Food Res Technol 219:620–629
Buchert J, Koponen JM, Suutarinen M, Mustranta A, Lille M, Torronen R, Poutanen K (2005) Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J Sci Food Agric 85:2548–2556
Landbo AK, Meyer AS (2001) Enzyme-assisted extraction of antioxidative phenols from black current juice press residues (Ribes nigrum). J Agric Food Chem 49:3169–3177
de Ancos B, Gonzalez E, Cano MP (1999) Differentiation of raspberry varieties according to anthocyanin composition. Zeitschrift fur Lebensmittel-Untersuchung Und-Forschung A. Food Res Technol 208:33–38
Garcia-Viguera C, Zafrilla P, Tomas-Barberan FA (1997) Determination of authenticity of fruit jams by HPLC analysis of anthocyanins. J Sci Food Agric 73:207–213
Mullen W, Stewart AJ, Lean MEJ, Gardner P, Duthie GG, Crozier A (2002) Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J Agric Food Chem 50:5197–5201
Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis G (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem 102:777–783
Jiang J, Paterson A, Piggott JR (1990) Effects of pectolytic enzyme treatments on anthocyanins in raspberry juice—short communication. Int J Food Sci Technol 25:596–600
Landbo AK, Kaack K, Meyer AS (2007) Statistically designed two step response surface optimization of enzymatic prepress treatment to increase juice yield and lower turbidity of elderberry juice. Innov Food Sci Emerg Technol 8:135–142
Meyer AS (2005) Enzymatic upgrading of antioxidant phenolics in berry juice and press residues. Fruit Process 6:382–387
Sadilova E, Carle R, Stintzing FC (2007) Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol Nutr Food Res 51:1461–1471
Zabetakis I, Leclerc N, Kajda P (2000) The effect of high hydrostatic pressure on the strawberry anthocyanins. J Agric Food Chem 48:2749–2754
Suthanthangjai W, Kajda P, Zabetakis L (2005) The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus idaeus). Food Chem 90:193–197
Belitz H-D, Grosch W, Schieberle P (2004) Food chemistry. Springer, Berlin
Sancho F, Lambert Y, Demazeau G, Largeteau A, Bouvier JM, Narbonne JF (1999) Effect of ultra-high hydrostatic pressure on hydrosoluble vitamins. J Food Eng 39:247–253
Taoukis PS, Panagiotidis P, Stoforos NG, Butz P, Fister H, Tauscher B (1998) In: Isaacs NS (ed) High pressure food science, bioscience and chemistry. The Royal Society of Chemistry, Cambridge
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Prior RL, Wu XL, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Klopotek Y, Otto K, Bohm V (2005) Processing strawberries to different products alters contents of vitamin C, total phenolics, total anthocyanins, and antioxidant capacity. J Agric Food Chem 53:5640–5646
Cao XM, Zhang Y, Zhang FS, Wang YT, Yi JY, Liao XJ (2011) Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J Sci Food Agric 91:877–885
Acknowledgments
This research has been carried out with the financial support from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verbeyst, L., Hendrickx, M. & Van Loey, A. Characterisation and screening of the process stability of bioactive compounds in red fruit paste and red fruit juice. Eur Food Res Technol 234, 593–605 (2012). https://doi.org/10.1007/s00217-012-1667-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1667-1