Abstract
The aim of the study was to characterize the bioactivity and storability of a steam juicer extract from murta (Ugni molinae T.) berries, comparing with the bioactive quality of the fresh fruit. An extraction assay was performed at three different processing times and quality of the extract was assessed by determining bioactives content, antioxidant activity after processing and during storage at different temperatures (5, 20 and 35 °C). Total polyphenols, total anthocyanins and antioxidant activity in fresh fruits and juice extracts were evaluated as bioaccessible fractions during an in vitro gastric-intestinal digestion process. Weibull distribution was applied to model the storage behavior of the extracts, and fitted well to experimental data on degradation kinetics of bioactive phenolics and antioxidant activity. The best processing times for highest extraction of bioactive phenolics were found to be 28 ± 1 min for frozen-thawed and 34 ± 1 min for fresh fruits. The juice extract can retain around 80% of the polyphenols and anthocyanins after 21 days of storage at 5 °C. The bioaccessibility index of polyphenols in fresh murta berries or in juice achieved a relatively high value of 70% at the end of the small intestine digestive step. However, the bioactives in the bioaccessible fractions of the fruits and juices had significantly different behavior during the gastric-intestinal digestion steps. The juice released the bioaccessible bioactives in the earlier gastric stage, while the fresh fruit unfolded a higher antioxidant activity with increased release of bioactives in the small intestine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is generally agreed that consumption of fruits and vegetables contributes in lowering the risk of degenerative diseases [1,2,3] and protects against cardiovascular and cerebrovascular diseases or even cancers [4, 5]. The antioxidants compounds, like vitamins C and E, carotenoids, polyphenols, flavonoids and phenolic acids, present in fruits and vegetables are considered to be involved in the prevention of damage caused by free radicals [6,7,8,9]. However, these bioactives are exposed to degradation during processing, so that knowledge on the extent of the occurring quality loss is desirable and helpful to achieve a proper handling of biological materials in the production of health-promoting foods.
Murta (Ugni molinae Turcz), also known as murtilla, and other berries from South America have been reported to yield many health benefits [10,11,12] due to the valuable content and diversified composition of bioactive compounds. Murta berries, a fruit with a very pleasant aroma and an important source of vitamins, minerals and fiber, have a high potential for use as an ingredient of functional foods [13]. The berries are consumed locally as fresh fruit, as jam, or mixed with quince cooked in syrup or as beverage in liquefied state or even in liquor preparation. Another commonly prepared home-made recipe consists in processing the berries in a steam juicer to obtain at time of harvest a pasteurized beverage that can be readily bottled for long term storage. This type of processing has been praised for conserving a high quality of the product; however, the effect of processing on the quality of the final extract has not been investigated so far. Before concluding on any potential health effect, an analysis of the bioactives and their stability as affected by the digestion process should be conducted, considering the direct relationship that exists to bioavailability and possible beneficial effects of the bioactives [14]. Nutritional quality of food products is usually assessed by determining the bioaccessibility of bioactives through in vitro digestion procedures that are easier and faster to implement than in vivo or ex vivo assays [15]. Bioaccessibility is defined as the percentage of the bioactives that is released from the food matrix in the gastrointestinal tract to become available for absorption [16]. Bioaccessibility of carotenoids, flavonoids and vitamin C in orange juices has been studied by Aschoff and Col [17]. and it was found that carotenoids and vitamin C would become more bioaccessible, while the bioaccessibility of flavonoids did not change. On the other hand, a reduction of carotenoids bioaccessibility was observed when orange juices were pasteurized [18], which disclosed an effect of processing. Other studies on bioaccessibility of bioactives, like phenolics or anthocyanins, have also been conducted for fruits and vegetables [19, 20].
The bioactives in murta berries are present as phytochemicals entrapped in a solid food matrix and are therefore not bioavailable, unless subjected to the digestive process in the body, when they are released to become first bioaccessible for the absorption process and subsequently available for colonic metabolism. In a steam juicer, saturated water vapor flows upwards through a fixed bed of murta berries, transferring heat to the berries denaturalizing cell wall tissues. As the condensed vapor produced on the berries surfaces flows downwards, a mass transfer process occurs due to an established concentration gradient. The phytochemicals are removed from the plant matrix and migrate into the solvent and flow downwards as juice extract. The advantages of this mass transfer process reside in the intimate contact of the liquid phase with the berries in a surrounding characterized by poor oxygen content and process temperature that would not largely exceed the boiling point of water [21]. This process would depend on concentration gradients, boundary layer and diffusion coefficients that are parameters not easy to determine. Therefore, the aim of this study was to compare the quality of a juice extract with that of the fresh fruit and to evaluate the steam extraction procedure for murta berries based on content of bioactives and process modeling as a function of extraction time and stability of the bioactives during storage at three different temperatures. The quality of the product and its stability during storage were assessed by determining changes in content of selected bioactive compounds and some physico-chemical properties as well as antioxidant activity. An evaluation of the effect on the bioaccessibility of bioactive phenolics and anthocyanin was also performed using an in vitro method to simulate the human digestion system.
Materials and methods
Raw materials
Murta (Ugni molinae Turcz) berries were obtained from local merchants, who sold the hand-plucked berries that grew wildly in the coastal forests in the surroundings of the city of Valdivia, Chile (location 38°48′30″S and 73°14′30″W) during the growing seasons from March to May. Samples were selected to provide a homogeneous group, based on their color, size, and freshness according to visual analysis. Acquired samples were stored at 4 °C in sealed polyethylene bags in a refrigerator for less than 48 h before processing, to maintain the fresh characteristics of the fruits, or kept frozen at − 18 °C in a freezer.
Preparation of murta juice extract with condensed steam
For the extraction process a commercial steam juicer, preferably made of stainless steel, was used. It consisted typically of a water pot to produce steam, a juice collecting container with a steam funnel arranged on the water pot and a fruit container with lid and sieve openings inserted over the juice collecting container. The produced steam flowed upwards through the fixed bed of berries (about 3 kg), where condensation occurred and the extract was collected in the juice container for different processing times (15, 30 and 45 min). The extraction assays were performed in triplicate, whereby timing began when the water in the pot started to boil and the fruit container was then filled with the murta berries. For comparison purposes, a juice was prepared using a mechanical extraction method by blending 25 g berries in 100 mL water. The mixture was filtered and the filtrate centrifuged for 15 min at 4 °C and 3500 rpm to obtain a clarified juice extract.
Modeling procedures
The degradation of total polyphenolics, total anthocyanin as well as antioxidant activity in the extract was modeled using variables, Y (ae)jk , where a corresponds to storage temperature, e to extraction time, j to level of storage time and k to number of replicates. A normal behavior of the response variable μ was assumed (Eq. 1).
The statistical model was derived from a general Weibull distribution as shown in Eq. 2. The values of shape and scale parameters α and β were determined using the maximum likelihood estimator. For a given value of \({\hat {\mu }_{aej}}\) for \({\mu _{aej}}\), the maximum likelihood estimator for \(\sigma\) is given by Eq. 3. The confidence interval was calculated as (mean ± 1.96 s), where s is the standard deviation. The modeling of the Weibull distribution was performed using the free software RStudio.
For iteration purpose, a standardization of experimental data for each parameter was performed to obtain a transformed value, as given in Eq. 4 with the corresponding standard deviation, as given in Eq. 5.
For each extraction process, an initial value k for the corresponding parameter was computed using Eq. 6, after which the adjusted parameter for the Weibull model was obtained from Eq. 7.
Quality characteristics
Proximate analysis, pH, soluble solids and color measurement
To conduct a material balance, initial and final weights were determined for each assay and a proximate analysis of the fresh berries (control) as well as the residual extracted berries was performed according to AOAC methods [22]. The moisture content was determined according to method No. 930.15, using a vacuum oven (JEIO Tech, OV-11, South Korea) and an analytical balance (Mettler Toledo XS205 DU, Schwerzenbach, Switzerland). Crude protein content was determined by method No. 990.03, crude fibre by method No. 978.10 and crude ash by method No. 942.05. All analyses were performed in triplicate and expressed as g per 100 g dry matter (g/100 g dm).
The pH value was measured using a pHmeter (Sanxin, China), and total soluble solids (TSS) content was measured using a refractometer (Atago, 1-T, Tokyo, Japan). Color was evaluated with the CIE Lab System using a spectrophotometer (PCE-CSM 8, PCE Instruments, UK). Three measurements were performed for each sample after instrument´s calibration using a white standard reflectance plate (L* = 96.37, a* = 0.19, b* = 1.68). For comparison purposes, a mechanical juice extract was prepared by blending 25 g murta berries with 100 mL cold distilled water (4 °C), which was filtered and the filtrate centrifuged at 3500 rpm for 15 min to obtain the control sample.
Determination of total phenolic content
Total phenolic content was determined using Folin–Ciocalteu (FC) reagent as described by Chuah and col [23]. with some modifications. Briefly, 50 µL aliquot of the fruit extract were diluted with 3150 µL deionized water and mixed with 200 µL of FC reagent and 600 µL Na2CO3 solution (200 mg/mL) by vortexing for 15–20 s. The reaction proceeded in the dark protected from sunlight for 120 min at ambient temperature. Finally, the absorbance was measured at 765 nm using a spectrophotometer (Spectronic® 20 GenesysTM131, Illinois, USA) and a gallic acid calibration curve. Results were expressed as gallic acid equivalent (mg GAE/100 g or mg GAE/L). All reagents were purchased from Merck (Merck, Darmstadt, Germany). All measurements were performed in triplicate.
Determination of total anthocyanin content
Total anthocyanin content (TAC) was determined by the pH differential method [24]. Absorbance was measured using a Spectronic spectrophotometer (Spectronic® 20 GenesysTM131, Illinois, USA) at 510 and 700 nm in buffers at pH 1.0 and 4.5, using Eq. 8 with molar extinction coefficient of cyanidin-3-glucoside (29600). Results were expressed as cyanidin-3-glucoside (mg cya-3-glu/100 g or mg cya-3-glu/L).
Determination of total chlorophyll and total carotenoids
Photosynthetic pigments such as chlorophyll and carotenoids were determined using official AOAC method N° 942.04 [22]. Briefly, 10 mL murta juice extract were mixed thoroughly with 0.4 g calcium carbonate, 10 mL acetone (85% v/v) and 5 mL petrol ether (0.1N) until pigments were completely separated from fiber. 50 mL of distilled water were then added and mixture was filtered. Absorbance of filtrate was determined at 660, 642 and 470 nm against acetone as blank. Concentration of each of the pigments, total chlorophyll (CHL T ), chlorophyll a and b (CHL a , CHL b ) and total carotenoids, was then calculated in mg/L using Eqs. 9 to 12 respectively.
Determination of DPPH radical-scavenging activity
To determine the free radical scavenging activity of the samples DPPH (2,2,-diphenyl-1-picryl hydrazyl) method [25] was used with some modifications. Dilutions of the extract in different concentrations were prepared in triplicate. An aliquot of 2 mL of 0.1 mM DPPH radical in methanol was added to 1 mL of the sample extract in a test tube and vortex-mixed for 30 s. The mixture was left to react in the dark at room temperature for 30 min. A spectrophotometer (Spectronic® 20 GenesysTM, Illinois, USA) was used to measure absorbance at 515 nm against pure methanol as blank. For each assay, new calibration curves using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were established and the results were expressed as µmoles of Trolox equivalent (TE)/100 g sample.
In vitro gastrointestinal digestion
The in vitro gastrointestinal digestion assay, simulating the physiological state in the upper digestive tract (mouth, stomach and small intestine) was performed according to a procedure described by Tenore and col. [26], with some modifications. The digestion consisted of salivary pre-digestion (MTH), gastric digestion initial (GDI), gastric digestion final (GDF) and small intestinal (duodenal, jejunal and ileal) digestion steps (DDM, JJM and ILM respectively).
For the salivary digestion step, the murta sample (20 g) was mixed with 6 mL of artificial saliva, composed of double-distilled water, 5.21 mg/mL NaHCO3 (Merck, Darmstadt, Germany), 0.88 mg/mL NaCl (Merck, Darmstadt, Germany), 0.48 mg/mL KCl (Merck, Darmstadt, Germany), 0.44 mg/mL CaCl2·2H2O (Merck, Darmstadt, Germany), 1.04 mg/mL K2HPO4 (Merck, Darmstadt, Germany), 2.16 mg/mL of mucin (Sigma, Saint Louis, USA), and 0.1 mg/mL of α-amylase (Sigma, Saint Louis, USA), and adjusted to a pH of 6.8 with 0.1N HCl. The mixture of sample and artificial saliva was homogenized in a plastic bag with 40 mL of pure water in a stomacher (Seward, Worthing, UK) for 3 min to simulate mastication. Sample was withdrawn at the end of simulated mastication (MTH).
To simulate gastric digestion, 5 mg of pepsin (14,800 U) dissolved in 5 mL 0.1 N HCl was added immediately to the simulated bolus of salivary digestion and pH was adjusted to 2.0 with 6 N HCl. The mixture was then incubated at 37 °C using an orbital shaker (Zhicheng ZHWY 110 × 30, China) at 250 rpm for 2 h. Sample was withdrawn after pH adjustment (GDI) and at the end of 2 h digestion period (GDF).
After the gastric digestion, the small intestinal digestion was simulated, adjusting pH to 6.5 with 0.5 N NaHCO3 before 5 mL of a mixture of pancreatin (8.0 mg/mL) and bile salts (50.0 mg/ml) (1:1; v/v), dissolved in 20 mL of water, were added and incubated at 37 °C in the orbital shaker at 250 rpm for 2 h. Sample was withdrawn after adjusting pH to 6.5 (DDM), after first (JJM) and second hour (ILM) of digestion process.
At each step of digestion (at different time intervals), 10 ml aliquots of the obtained extract were withdrawn for analysis, cooling the test tubes in ice for 10 min to stop enzymatic activity of pepsin or intestinal digestion, and centrifuging at 13,000 rpm at 4 °C for 15 min. The supernatants (bioaccessible fractions) were withdrawn and immediately used for the analysis of total polyphenolics, total anthocyanin, antioxidant activity and anthocyanin profile by HPLC-DAD. The bioaccessibility index (BI) was calculated as the percentage of the tested compound remaining in the bioaccessible fraction related to the original non-digested sample (Eq. 13).
C DS is concentration of the bioactive or its antioxidant activity at the end of a digestion step and C FS is the concentration or the antioxidant activity of the same bioactive in the fruit sample as determined with a chemical extraction procedure.
Statistical analysis
All the determinants were performed in three replicates and in each extraction time three measurements were used. Mean values and standard deviations were calculated and results were tested by an analysis of variance using Statgraphics® Plus v. 5.1 (Statistical Graphics Corp., Herndon, VA, USA). Differences among the media were analyzed using the least significant difference (LSD) test with a significance level of α = 0.05 and a confidence interval of 95% (p < 0.05). In addition, the multiple range test included in the statistical program was used to demonstrate the existence of homogeneous groups within each of the parameters.
Results and discussion
Extraction yield and physicochemical analysis
The experimental data for evaluation of extraction yield can be seen in Table 1. Slight differences may be observed in the extraction yield obtained from fresh and frozen-thawed murta berries. In both cases the highest value for total soluble solids occurred at 30 min extraction time, while juice yield was highest at 45 min processing. The process residue diminished continuously with extraction time, which also implied an increase in juice quantity, although at the same time a dilution effect of the juice extract was observed despite the mass loss in extracted berries. Acidity of the extract did not change significantly as can be observed in the non-significant change in pH value with extraction time. In term of quantity, the frozen-thawed berries would yield more juice than the fresh berries after 15 min extraction, but in term of bioactivity and according to developed model, it was found that for the frozen-thawed berries an extraction time of 28 ± 1 min would be sufficient to obtain the highest release of bioactives, while for the fresh fruit extraction time would be slightly longer (34 ± 1 min). This difference may be due to freezing effect that probably caused disruption of the cell walls of the berries, allowing an earlier flow of fluid and its bioactive content. It has been reported that freezing of apples at − 20 °C induced large changes of the cellular structure, leading to cell walls collapse [27]. Effect of low-temperature storage on cell wall metabolism of zucchini fruit has also been studied and microscopical observations have shown that chilling caused cell death and cell collapse [28]. Formation of ice in the intercellular spaces due to the higher freezing point than that in the cytoplasm causes a drop of the water potential outside the cell, leading to cellular dehydration and therefore to cell collapse. This effect has been demonstrated for freeze-damaged oilseed rape leaves, where the proportion of intercellular airspaces increased due to cell contraction and caused severe disruption of the cells’ internal structures [29].
The proximate composition of the murta berries before and after extraction process can be seen in Table 2, showing a significant decrease in carbohydrate content from 77.3 ± 0.1% to 72.4 ± 0.1% with increasing extraction time. This probably occurred due to loss through lixiviation of soluble solids through the damaged internal cell structures. On the other hand, crude fiber in the residue increased significantly from 12.3 to 15.8%, similarly to protein content that also increased from 4.4 to 5.6%, which indicated, as should be expected, that these substances are not carried away in the fluid flow of condensate, but form part of the food matrix that entrapped the crude ash and lipids, which showed in general no significant (p > 0.05) changes in contents during the assayed extraction processes, except for the lipid content after 30 min extraction time that showed a slight but statistically significant difference that may not acquire much relevance for practical purposes. The decrease in lipid content after 30 min extraction time may be due to an intrinsic variation related to raw material of plant origin and cannot be ascribed to a processing trend, since the lipid content after 45 min extraction time was not significantly different (p > 0.05) to initial lipid content in the fresh fruit.
Color characteristics of the juice extract can be seen in Table 3. The juice obtained by mechanical extraction had the highest ΔL-value, indicating highest lightness value of sample, which may be due to the filtration process, when particles in suspension were removed by centrifuging the filtrate. ΔL value (lightness [100 = white, 0 = black]) of the samples increased as extraction time increased, which suggested that most of the suspended particles would be extracted during the first 15 min. Increase in ΔL would be due to a dilution effect with juice extract that contained only soluble solids. The highest value for Δa was measured at 30 min extraction time. This value, which represents in the CIE L* a* b* system the greenness-redness axis (+ a* is the red direction, − a* is the green direction), may be related to the concentration of pigments in the extract that tended to a reddish color. As shown in Table 4, the highest concentration of all analyzed pigments, including anthocyanin, chlorophyll and carotenoids, was reached at an extraction time of 30 min. This correlated significantly with the Δa values that may be used as a characteristic parameter for pigment content. The Δb value represents the yellowness-blueness axis (blue [negative] and yellow [positive]), and is related to color saturation. Similar to ΔC, (Δa 2 + Δb 2)½, that is related to chroma [30], the significant increase in these values suggested a saturation of the perceived red color. ΔE (= ΔL 2 + Δa 2 + Δb 2)½ [30], which is the colorimetric difference between the sample and the white standard reflectance plate, increased with extraction time, congruent with chroma saturation. However, at 15 min extraction the colorimetric difference (ΔE) was less than that of the reference sample obtained after a mechanical extraction, indicating that the extraction process with condensed steam had not reached its maximum. The hue of extract (measured in L*C*h color space) was also achieved early during the process, since ΔH (ratio a*/b*) [30] was highest at 15 min extraction time, so that further extraction contributed mainly to chroma saturation.
Bioactive compounds and antioxidant activity
The bioactive compounds analyzed in the assays were total phenolics, total anthocyanin, total chlorophyll (chlorophyll a and b) and total carotenoids. In Table 4 the contents of the mentioned bioactive compounds determined after extraction times of 15, 30 and 45 min can be seen. DPPH radical scavenging activity is also shown. In all cases the highest values were obtained at an extraction time of 30 min. Total phenolics were the dominant bioactive. Total anthocyanin, total chlorophyll and total carotenoids were found only at a low level. The anthocyanins are found mainly in the skin of the murta berries, which may account for the small concentration obtained in the juice. The occurrence of chlorophyll in the juice extract may be attributed to the presence of pedicel and sepals that typically accompany the fruit but were only a small fraction of the batch of extracted berries. Chlorophyll b predominated and thermal degradation became significant at extraction time over 30 min (Table 4). Total carotenoids were due to the presence of β-carotene that had already been found in fresh murta berries at a level of 44.26 ± 1.17 mg/100 g dm [31], which was reported to be lower than the contents found in carrots or pepper, but higher than those in apricots, cherries, nectarines, peaches and plums [32]. A significant degradation of the total carotenoids was also observed at 45 min extraction time.
The fresh murta berries used for the extraction assays had a TPC in the order of 591 ± 22 mg GAE/100 g (Table 5), which is comparable to values reported for fruits, such as plums, blackberries, cranberries and strawberries [33,34,35]. Under consideration of the extract yield the calculated fractions of extracted polyphenols in the juice were found to be 63, 79 and 61% after 15, 30 and 45 min processing time respectively. Therefore, a significant amount of polyphenols found in the fresh berries would remain in the process residues. Although the juice color showed a significant presence of anthocyanin, extraction yield was relatively poor; only an amount in the order of 10% could be extracted. This low value may be attributed to thermal degradation that occurred during the extraction process [36,37,38]. An analysis of the anthocyanin profile by HPLC-DAD revealed the presence of cyanidin-3-glucoside and peonidin-3-glucoside in the murta berries and juice.
Bioactives and antioxidant activity in bioaccessible fractions
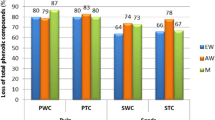
The importance of phenolic compounds that have antioxidant activity lies in their ability as metabolites to neutralize reactive species, contributing to protection against oxidative stress [39]. Total polyphenols, total anthocyanin and antioxidant activity in the bioaccessible fractions of the berries and juice samples analyzed are shown in Table 5. Significant differences in TPC, TAC and antioxidant activity in the berries and the juice samples at the different steps of digestion are noticeable. Total phenolics in the fruits are less bioaccessible in the upper digestive tract but increased in the intestinal step. The juice on the other hand showed full bioaccessibility of the phenolics during salivary and gastric steps, which persisted but decreased in the intestinal steps. At the end of intestinal digestion step (ILM) the BI in the fresh fruit and in the juice, was 69,91 ± 3,92% and 70,54 ± 2,58% respectively, showing no significant difference (p > 0.05). In either case, the determined BI for murta berries was comparatively higher than that found in other fruits, such as peaches (37%), plums (44%), nuts (34%) and tomatoes (61%) [20]. It has been reported that dietary fiber could bind the phenolic compounds, hindering their release from the food matrix [40]. In the freeze-dried murta berries samples total dietary fiber, the soluble and insoluble fractions were determined by AOAC method No. 991.43 [41] and were found in a quantity of 50.8 ± 1.0; 8.3 ± 1.3 and 42.5 ± 2.2 g/100 g dry matter respectively. The presence of fruit pectin with physico-chemical properties and composition similar to that of commercial citric pectin and in high content, has also been reported [42], which may be a probable cause for the lowering of bioaccessibility in the fruit. Similar occurrence was observed for the anthocyanin, although in the intestinal step anthocyanins were not detected, probably due to the presence of the dietary fiber that are macromolecular compounds. The potential of phenolic compounds and their biological effects have been reported in literature [43]. The bioaccessibility index of phenolic compounds in apple was found to be around 55% [44] and it was suggested that the bioaccessibility may be due to linkage of the phenolic compounds to macromolecular compounds that are not dialyzable, or may form complex compounds with minerals, leading to a decrease in solubility.
Antioxidant activity in the bioaccessible fraction showed an increase in the intestinal digestion step for the fresh fruits and a decrease for the juice, which may also be due to the presence of macromolecular dietary fibers, hence stressing the importance of the latter as health promoting compounds. In all analyzed digestive steps, antioxidant activity was observed in the bioaccessible fraction of the juice, although at a lower level compared to the whole fruit. The antioxidant activity in the bioaccessible fraction of the juice also decreased significantly (p < 0.05) in the intestinal digestive steps, which may be attributed to degradation of the released bioactive phenolics and anthocyanin present in the juice. It is noticeable that the murta berries that would be consumed as fresh whole fruit would unfold its health promoting functions more strongly in the intestinal steps, while the fruit juice would achieve this effect already in the earlier steps of digestion, namely in the salivary and gastric stages.
Modeling of degradation kinetics during storage
The juice extract obtained after three different processing times (15, 30 and 45 min) was stored at three different temperatures (5, 20 and 35 °C) and the degradation of total phenolics, total anthocyanin and DPPH radical scavenging activity was monitored. Experimental data were modeled using the general Weibull equation. In Table 6 the values of shape and scale parameters (α and β) of the Weibull distribution model for estimation of total phenolics, total anthocyanin and antioxidant activity of juice extract produced at three different processing times can be seen. The data were standardized for iteration purposes and therefore the auxiliary parameters for initial (k) and minimum (x min) values were also stated in the table. The shape and scale parameters obtained led to theoretical values that are congruent with experimental data. The shape parameter, also known as the Weibull slope, with values approaching unity would define a linear tendency, while values over unity would produce a deviation to an exponential increase. The scale factor would define the amplitude and dispersion of the distribution [45]. The value of 1.96 for the confidence interval was based on the fact that 95% of the area of the normal distribution was within 1.96 standard deviations of the mean. The calculated minimum and maximum values for the confidence interval at 95% showed goodness of fit of experimental data to the Weibull model. Figures 1, 2 and 3 show the degradation kinetic curves for total polyphenols, total anthocyanin and antioxidant activity during storage at three different temperatures for the three different extracts. The juice extracts obtained after 30 min showed the least degradation slope for total polyphenols when stored at 5 °C. In Fig. 4 a 3D graphical representation for the 30-min juice extract from frozen-thawed murta berries can be seen. The best extraction time occurred at 28 min for the frozen-thawed berries and 34 min for the fresh fruits (data not shown). TPC in the juice extract of 45 min was lowest (Table 4), but an increase was observed during the first 5 days of storage at all three temperatures. The lower level of TPC in the juice extract may be ascribed to the observed dilution effect of a prolonged extraction, while the initial tendency to increase at the beginning of the storage period may be the results of formation of reducing substances that were then quantified by the Folin–Ciocalteu assay, which is a methodology based on chemical reduction by a mixture of tungsten and molybdenum oxides [46] and is not exclusive to measure total phenols as reaction with any reducing substances will also occur [47].
It can be seen in Fig. 2 that total anthocyanins content (TAC) in the extracts stored at 5 °C remained almost constant at the initial period of storage and started with a drastic decrease after the 5th and 12th day of storage for the 30 and 45 min juice extracts respectively. The acquired data for these extracts could not be satisfactorily adjusted to the Weibull model, probably due to the small quantity of anthocyanins that was determined for relatively small changes in TAC, which led to large relative differences in TAC that would require a larger number of repetitions for the kinetic modeling. Independent of modeling issues, it is noticeable that storage at 5 °C would slow down anthocyanin degradation in the murta juice extracts.
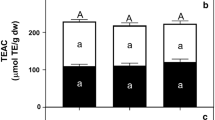
The changes in antioxidant activity can be seen in Fig. 3. Differences among the DPPH values of the three extracts were relatively small. The loss in antioxidant activity at all storage temperatures was fairly similar for all extracts. After 21 days at storage temperatures of 35, 20 and 5 °C residual antioxidant activities of 49 ± 2%; 46 ± 2% and 47 ± 4% were determined respectively. Based on this criterion the juice extracts of murta berries may be considered to have a half-life of 3 weeks.
Conclusions
The experimental assays on murta juice extraction showed that the praised quality of the juice extract may be sustained. The best extraction time to obtain the highest content of bioactives in the juice extract of a steam juicer would be around 28 min for frozen-thawed murta berries and slightly higher at around 34 min for the fresh fruits. Although a high percentage of bioactives would be retained in the process residues that may serve for other product development, the juice extract still retained a significant and diverse quantity of bioactives, like total polyphenols, total anthocyanin and total carotenoids with relatively high antioxidant capacity. The presence of chlorophyll, due to the pedicel and sepal that came together with the fruits, were also valuable compounds that would be found in the juice extracts. It is noticeable that the bioaccessibility index for polyphenols and anthocyanins in the salivary and gastric steps of digestion would be higher in the juice than in the fruits, while in the small intestine digestive steps the same BI would be higher in the fruits. The Weibull model can be used adequately to fit experimental data that described degradation kinetic during storage of the juice extract at different temperatures. The juice extracts would retain more than half of its antioxidant activity after a period of 21 days when stored at 5 °C. The achieved experimental results can serve as a good basic knowledge for a scaling up of the steam extraction process.
References
B.N. Ames, M.K. Shigenaga, T.M. Hagen, Proc. Nat. Acad. Sci. 90, 7915–7922 (1993)
J. Boyer, R. Liu, Nutr. J. (2004). doi:10.1186/1475-2891-3-5
V. López, S. Akerreta, E. Casanova, J.M. García-Mina, R.Y. Cavero, M.I. Calvo, Plant Foods Hum. Nutr. 62, 151–155 (2007)
N. Guthrie, E.M.N. Kurowska, Handbook of Nutraceuticals and Functional Foods (CRC Press, Boca Raton, 2001), pp. 113–126
H. Wang, W. Fan, H. Li, J. Yang, J. Huang, P. Zhang, PLoS ONE (2013). doi:10.1371/journal.pone.0078484
R. du Toit, Y. Volsteedt, Z. Apostolides, Toxicology (2001). doi:10.1016/S0300-483X(01)00446-2
C. Vijaya, D.,M. Raghunath, Food Res. Int. (2010). doi:10.1016/j.foodres.2009.10.006
A. Zulueta, M.J. Esteve, A. Frígola, Food Chem. (2009). doi:10.1016/j.foodchem.2008.09.033
S. Devalaraja, S. Jain, H. Yadav, Food Res. Int. (2011). doi:10.1016/j.foodres.2011.04.008
H. Speisky, A. Peña, M. Gómez, C. Fredes, M. Hurtado, M. Gotteland, O. Brunser, Acta Hortic. (2008). doi:10.17660/ActaHortic.2008.777.74
A. Ruiz, I. Gutierrez, C. Mardones, C. Vergara, E. Herlitz, M. Vega, C. Dorau, P. Winterhalter, D. Von Baer, J. Agric. Food Chem. (2010). doi:10.1021/jf100173x
M. Schreckinger, J. Lotton, M. Lila, E. Gonzalez de Mejia, J. Med. Food (2010). doi:10.1021/jf100975m
E. Scheuermann, I. Seguel, A. Montenegro, R. Bustos, E. Hormazábal, A. Quiroz, J. Sci. Food Agric. (2008). doi:10.1002/jsfa.3111
J.M. Carbonell-Capella, M. Buniowska, F.J. Barba, M.J. Esteve, A. Frígola, Compr. Rev. Food Sci. Food Saf. (2014). doi:10.1111/1541-4337.12049
E. Fernández-García, I. Carvajal-Lerida, A. Pérez-GÁLVEZ, Nutr. Res. (2009). doi:10.1016/j.nutres.2009.09.016
R.P. Heaney, J. Nutr. 131, 1344–1348 (2001)
J.K. Aschoff, C.L. Rolke, N. Breusing, A. Bosy-Westphal, J. Högel, R. Carle, R.M. Schweiggert, Mol. Nutr. Food Res. (2015). doi:10.1002/mnfr.201500327
C.M. Stinco, R. Fernández-Vázquez, M.L. Escudero-Gilete, F.J. Heredia, A.J. Meléndez-Martínez, I.M. Vicario, J Agric. Food Chem. (2012). doi:10.1021/jf2043949
M. Buniowska, J.M. Carbonell-Capella, A. Frigola, M.J. EstEVE, Food Chem. (2017). doi:10.1016/j.foodchem.2016.10.093
D. Tagliazucchi, E. Verzelloni, D. Bertolini, A. Conte, Food Chem. (2010). doi:10.1111/j.1745-4514.2011.00569.x
E. Hackelsberger, Steam Juice Extractor. U.S. Patent No. US4640186 (A) – 1987-02-03 (1987)
AOAC International, Official Methods of Analysis of the Association of Official Analytical Chemists, 16th edn. (AOAC International, Virginia, 1996)
A.M. Chuah, Y.-C. Lee, T. Yamaguchi, H. Takamura, L.-J. Yin, T. Matoba, Food Chem. (2008). doi:10.1016/j.foodchem.2008.03.022
G. Cheng, P. Breen, J. Am. Soc. Hortic. Sci. 116, 865–869 (1991)
W. Brand-Williams, M.E. Cuvelier, C. Berset, LWT Food Sci. Technol. (1995). doi:10.1016/S0023-6438(95)80008-5
G. Tenore, P. Campiglia, A. Ritieni, E. Novellino, Food Chem. (2013). doi:10.1016/j.foodchem.2013.06.051
S. Chassagne-Berces, C. Poirier, M.-F. Devaux, F. Fonseca, M. Lahaye, G. Pigorini, C. Girault, M. Marin, F. Guillon, Food Res. Int. (2009). doi:10.1016/j.foodres.2009.03.001
F. Carvajal, F. Palma, M. Jamilena, D. Garrido, Postharvest Biol. Technol. (2015). doi:10.1016/j.postharvbio.2015.05.013
C. Wei, J. Huang, X. Wang, G.A. Blackburn, Y. Zhang, S. Wang, L.R. Mansaray, Remote Sens. Environ. (2017). doi:10.1016/j.rse.2017.03.042
R.G. McGuire, Hortic. Sci. 27, 1254–1544 (1992)
K. Rodríguez, K. Ah-Hen, A. Vega-Gálvez, J. López, I. Quispe-Fuentes, R. Lemus-Mondaca, L. Gálvez-Ranilla, Int. J. Food Sci. Technol. (2013). doi:10.1111/ijfs.12392
S.Y. Leong, I. Oey, Food Chem. (2012). doi:10.1016/j.foodchem.2012.02.052
N. Balasundram, K. Sundram, S. Samman, Food Chem. (2006). doi:10.1016/j.foodchem.2005.07.042
C. Vasco, J. Ruales, A. Kamal-Eldin, Food Chem. (2008). doi:10.1016/j.foodchem.2008.04.054
L. Fu, B.-T. Xu, X.-R. Xu, R.-Y. Gan, Y. Zhang, E.-Q. Xia, H.-B. Li, Food Chem. (2011). doi:10.1016/j.foodchem.2011.04.079
A. Kirca, B. Cemeroglu, Food Chem. (2003). doi:10.1016/S0308-8146(02)00500-9
A. Kirca, M. Özkan, B. Cemeroglu, Food Chem. (2007). doi:10.1016/j.foodchem.2006.01.019
N. Harbourne, J. Jacquier, D. Morgan, J. Lyng, Food Chem. (2008). doi:10.1016/j.foodchem.2008.03.023
A. Wojdylo, J. Oszmianski, P. Laskowski, J. Agric. Food Chem. (2008). doi:10.1021/jf800510j
J. Parada, J.M. Aguilera, J. Food Sci. (2007). doi:10.1111/j.1750-3841.2007.00274.x
AOAC International, Official methods of analysis of the Association of Official Analytical Chemists, 15th edn. (AOAC International, Virginia, 1990)
E. Taboada, P. Fisher, R. Jara, E. Zuñiga, M. Gidekel, J. Cabrera, E. Pereira, A. Gutiérrez-Moraga, R. Villalonga, G. Cabrera, Food Chem. 123, 669–678 (2010). doi:10.1016/j.foodchem.2010.05.030
L. He, H. Xu, X. Liu, W. He, F. Yuan, Z. Hou, Y. Gao, Food Res. Int. (2011). doi:10.1016/j.foodres.2010.05.023
J. Bouayed, L. Hoffmann, T. Bohn, Food Chem. (2011). doi:10.1016/j.foodchem.2011.02.052
B. Janković, Biopolymers (2013). doi:10.1002/bip.22271
J.D. Everette, Q.M. Bryant, A.M. Green, Y.A. Abbey, G.W. Wangila, R.B. Walker, J. Agric. Food Chem. (2010). doi:10.1021/jf1005935
M. Ikawa, T.D. Schaper, C.A. Dollard, J.J. Sasner, J. Agric. Food Chem. (2003). doi:10.1021/jf021099r
Acknowledgements
The authors gratefully acknowledge financial support of CONICYT-Chile through FONDECYT Project N° 1150451 to conduct this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ah-Hen, K.S., Mathias-Rettig, K., Gómez-Pérez, L.S. et al. Bioaccessibility of bioactive compounds and antioxidant activity in murta (Ugni molinae T.) berries juices. Food Measure 12, 602–615 (2018). https://doi.org/10.1007/s11694-017-9673-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9673-4