Abstract
The effects of high intensity pulsed electric fields (HIPEF) processing (35 kV/cm for 1,500 μs using bipolar 4-μs pulses at 100 Hz) on color parameters and viscosity, as well as peroxidase (POD), pectin methylesterase (PME) and polygalacturonase (PG), were evaluated during 77 days of storage at 4 °C and compared to thermal treatments at 90 °C for 1 min or 30 s for unprocessed tomato juice. HIPEF-treated tomato juice showed higher values of lightness than the thermally processed and the untreated juice throughout storage time (P < 0.05). Viscosity of HIPEF-treated tomato juice was also greater than both thermally treated and untreated for the first 35 days of storage. POD of HIPEF-treated tomato juice was inactivated by 97% whereas in the case of the thermally treated, 90 and 79% inactivation was achieved after 1 min and 30 s, respectively. The highest PME inactivation in tomato juice was obtained by PEF (82%) and heat treatment at 90 °C for 1 min (96%). PG of PEF-treated tomato juice was inactivated by 12% whereas thermal treatments at 90 °C for 1 min or 30 s achieved 44 and 22%, respectively. Despite the low rates of PG inactivation obtained, the pattern followed in the residual activity along the storage time was similar in the tomato juice treated by HIPEF than the thermally processed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increase in the demand for minimally processed fresh-products has raised an interest in the development and implementation of new techniques for food processing such as high-intensity pulsed electric fields (HIPEF). Heat processing is traditionally used to extend the shelf life of tomato juice by inactivating microorganisms and enzymes, although undesirable changes are induced [1]. Pulsed electric fields (PEF) are commonly used in food processing in low or moderate intensity (1–5 kV/cm), applied usefully for food extraction [2, 3]. On the other hand, HIPEF is used at high intensity (>20 kV/cm) as an alternative for conventional pasteurization and a method of stabilizing food [4, 5]. In addition, HIPEF as a non-thermal technique has proved to increase the shelf life of various fruit juices without greatly affecting their nutritional and sensory properties [6]. Color and viscosity are the most important quality attributes of tomato juice that influence the consumer’s choice; thus, they are important criteria for tomato processors [7]. Some of the enzymes involved in the changes in these quality parameters are peroxidase (POD), pectin methylesterase (PME) and polygalacturonase (PG). Although polyphenol oxidase (PPO) is one of the main enzymes involved in color changes, tomato juice is not as sensitive as apple juice to enzymatic browning catalysed by PPOs. In addition, POD in grape juice has been reported to be less HIPEF labile than PPO [8]. POD catalyzes the oxidation of a wide range of natural compounds, especially those containing aromatic groups [9]. On the other hand, the ability of tomato juice to hold its solid portion in suspension over the shelf life of the product is mainly dependent upon the total amount and quality of pectin material present in the system [10, 11]. PME and PG are involved in the breakdown of these pectins, causing cloud and viscosity losses, in addition to gelation of commercial juices [12].
There is more information currently available on microbial than on enzymatic aspects affected by commercial scale HIPEF processing. A tomato juice treated at 40 kV/cm for 57 μs showed the same microbiological stability stored at 4 °C for 112 days than a thermally processed tomato juice at 92 °C for 90 s [13]. However, there are few studies on POD, PME and PG activity changes during commercial shelf life of HIPEF-treated tomato juice. Elez-Martínez et al. [14] reported better results for HIPEF-processed orange juice compared to heat-treated juice in terms of enzymatic inactivation and stabilization. They observed that PME kept the residual activity constant around 20% and no activation of POD during 56 days as observed, stored either at 4 or 22 °C.
The objective of this research was to compare the effects of HIPEF processing and thermal treatments on POD, PME and PG activities and on related physical parameters, such as color and viscosity, during the storage of tomato juice.
Materials and methods
Tomato juice preparation
Fresh ripened tomatoes (Lycopersicon esculentum var. Flandia Prince) were washed, sorted, chopped and crushed. The juice to be treated was obtained by filtering the ground mash through a screen size of 1.27 mm to remove peel and seeds and it was immediately treated.
HIPEF treatment
Pulse treatments were carried out using a laboratory scale pulse generator (OSU-4F, The Ohio State University, Columbus) that provides squared-wave pulses within eight co-field flow chambers in series. The gap distance between electrodes and treatment chamber volume was 0.29 and 0.012 cm3, respectively. The flow rate of the process was adjusted to 60 ml/min and controlled with a variable speed pump (model 75210-25, Cole Palmer, Vernon Hills, IL, USA). The treatment temperature was kept below 35 °C using a cooling coil, which was connected before and after each pair of chambers and submerged in an ice-water shaking bath. HIPEF treatment was set up at 35 kV/cm for 1,500 μs, using bipolar squared-wave pulses of 4 μs and a pulse frequency of 100 Hz. These HIPEF conditions were settled down to obtain five decimal logarithms as minimal reduction in Lactobacillus brevis as the reference microorganism of tomato juice deterioration (data not shown).
Thermal treatments
In order to compare the effectiveness of the HIPEF treatment to that of a conventional thermal treatment, tomato juice samples were heat pasteurized at 90 °C for 1 min or 30 s. These conditions were selected based on literature [13]. Tomato juice was processed in a tubular stainless steel heat exchange coil immersed in a hot water shaking bath using a gear pump to maintain the desired flow rate (Universidad de Lleida, Lleida, Spain). Once processed, the juice was immediately cooled in a heat exchange coil immersed in an ice-water bath.
Packaging and storage
The HIPEF fluid handling system was sanitized, first with 4% (w/v) NaOH and then with 10% (w/v) chlorine and 20% (v/v) ethanol solutions prior to processing. The first 200 ml of treated liquid were discarded to ensure stationary treatment conditions. Polypropylene sterilized bottles of 100 ml were directly filled with tomato juice from the treatment system. After that, the container was tightly closed and stored at 4 °C for 77 days.
Color measurements
The color of the juice was measured using a Macbeth Color-Eye 3,000 colorimeter (Macbeth-Kollmorgen Inst Corp., Newburgh, NY, USA) at room temperature. Equipment was set up for illuminant D75 and 10° observer angle. The equipment provided CIE-Lab values of L* (lightness), a* (redness) and b* (yellowness). Hue angle (h°) was calculated using the following Eq. 1 [15, 16]:
Determination of viscosity
Viscosity was measured from approximately 30 ml of tomato juice using a rotatory viscometer (model DV-I, Brookfield, Stoughton, MA, USA) with a UL adapter. Tomato juice viscosity was determined at 60 rpm by placing in the UL adapter.
Enzyme activity measurements
Peroxidase (POD)
POD activity in tomato juice was measured using the method described by Elez-Martínez et al. [17]. All chemicals were purchased from Scharlab Chemie, SA (Barcelona, Spain). The enzyme extracts for the determination of POD activity were obtained by homogenization of 10 ml tomato juice with 20 ml of 0.2 mol/L sodium phosphate buffer (pH = 6.5). The homogenate was centrifuged (24,000g, 15 min) at 4 °C (Centrifuge AVANTI™ J-25, Beckman Instruments Inc., Fullerton, CA, USA). The supernatant was filtered through a Whatman no. 1 filter and the resulting liquid constituted the enzymatic extract, which was immediately used for the POD activity determination. POD activity was assayed spectrophotometrically by placing 2.7 ml of 0.05 mol/L sodium phosphate buffer (pH = 6.5), 0.2 ml p-phenylenediamine (10 g/kg) as H-donor, 0.1 ml hydrogen peroxide (15 g/kg) as oxidant and 0.1 ml of enzymatic extract in a 1 cm path cuvette. The oxidation of p-phenylenediamine was measured at 509 nm and 25 °C using a CECIL CE 2021 spectrophotometer (Cecil Instruments Ltd, Cambridge, UK). POD activity was determined by measuring the initial rate of the reaction, which was computed from the linear portion of the plotted curve. One unit of POD activity was defined as a change in absorbance at 509 nm/min per ml of enzymatic extract.
Pectin methylesterase (PME)
PME activity was measured using the method described by Kimball [18]. Pectin, sodium chloride and NaOH were purchased from Acros Organics (NJ, USA), Rectapur (Fontenay, France) and Panreac Quimica (Barcelona, Spain), respectively.
A 10 ml aliquot of tomato juice tempered at 30 °C was mixed with 40 ml of 1% pectin-salt substrate (also at 30 °C) and incubated at 30 °C. The solution was adjusted to pH 7.0 with 2.0 N NaOH, and then the pH of the solution was readjusted to pH 7.7 with 0.05 N NaOH. After the pH reached 7.7, 0.10 ml of 0.05 N NaOH was added. The time required for the solution’s pH to return to 7.7 was measured. Blank (without PME added) was taken into account. PME activity, expressed as pectin esterase units (PEU) was calculated by Eq. 2:
where [NaOH] is the NaOH concentration (0.05 N), V NaOH is the volume of NaOH used (0.10 ml), V juice is the volume of juice used (10 ml), and t′ is the time needed in minutes for the pH to return to 7.7 after the addition of NaOH.
Polygalacturonase (PG)
A sample of 2.5 ml of tomato juice was transferred to a 50 ml centrifuge tube and centrifuged at 7,500g for 10 min. The supernatant was decanted and replaced with cold distilled water. Then, the pH of the mixture was adjusted at pH 3.0 with 0.1 M HCl. After that, the sample was centrifuged at 9,000g for 15 min. The supernatant was again decanted and 1.2 M NaCl (1:1) was added to the pellet and left for 1 h. After this time, a centrifugation at 18,200g for 10 min was carried out, and the supernatant was assayed for PG activity. All steps were performed at 4 °C.
The polygalacturonase activity assay was based on the release of reducing groups produced by PG and measured by spectrophotometry [19]. A portion of 100 μl of the enzyme extract was mixed with 300 μl of 0.2% polygalacturonic acid and incubated at 35 °C for 10 min. To stop the reaction, 2 ml of 0.1 M borate buffer, pH 9.0 and 400 μl of 1% cyanoacetamide were added to the reaction mixture and boiled for 10 min. After cooling down, the absorbance was measured at 276 nm and 22 °C. A blank was determined in the same way without addition of enzyme.
Percentage of residual enzyme activity was calculated and related to that of the untreated juice. The relative residual activity for POD, PME and PG activity, RA (%), was defined as indicated by Eq. 3:
where A t and A 0 were the enzyme activities of the treated and untreated samples, respectively.
Statistical analysis
The experiments were conducted three times and triplicate measurements were performed for each sample. Analysis of variance (ANOVA) and Tukey’s multiple-comparison method at the 5% significance level was performed for the determination of significant differences between thermally processed, HIPEF processed and non-treated tomato juices using Statgraphics Plus v 5.1 for Windows (Statistical Graphics Co., Rockville, MD, USA). Moreover, principal component analysis (PCA) was conducted to observe correlations between the measured variables. As a result of the PCA, data can be reduced to a set of new variables called principal components (PCs). The loadings of the PC define the direction of greatest variability and the score values represent the projection of each variable onto the PC.
Results and discussion
Effects of HIPEF and thermal treatments on the color of tomato juice
The effects of HIPEF and heat treatments on lightness (L*) and hue angle (h°) of tomato juice and the changes during storage are shown in Tables 1 and 2. Just after treatment (day 0), there were differences (P < 0.05) in the amount of L* of the tomato juice but not in the amount of h° value of the product.
Generally, PEF treatments have been reported as a method for better preserving the color in juices compared to heat processing [14, 20]. Although L* values of tomato juice increased after every treatment applied, HIPEF-treated tomato juice showed significantly higher L* compared to thermally processed and untreated tomato juices (P < 0.05) during 77 days of storage. A decrease in L* values is associated with the formation of dark colour compounds in the juice due to the nonenzymatic browning reactions, thus reducing the acceptance of the juices [21].
The kind of treatment applied led to significant (P < 0.05) but slight differences on hue angle (h°) during storage (Table 2). Changes in tomato juice color could be influenced by the tomato cultivar, the preparation of the juice and the treatment applied, as suggested by other authors [22]. In general, in spite of obtaining statistical differences between treatments on h°, the differences between HIPEF and thermal treatment were small. Thus, further research would be needed to know more about the effects of HIPEF on color compounds.
Effects of HIPEF and thermal treatments on viscosity
Greater final viscosity in tomato juice is desired, mainly because clarified tomato juice does not have any commercial value nowadays. The viscosity loss during the storage time could be attributed to the degradation of pectic substances by endogenous enzymes such as PME and PG. Thus, the different inactivation levels of each enzyme could explain changes in viscosity as well as in insoluble solids content during storage of tomato juice.
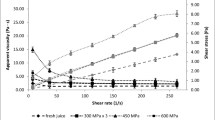
The effects of HIPEF and heat treatments on tomato juice viscosity are shown in Fig. 1. Significant differences (P < 0.05) were detected in the viscosity of juices just after processing. Tomato juice treated by heat (90 °C for 1 min) and by HIPEF had the highest values of viscosity (204.6 ± 0.6 cP and 201.9 ± 0.4 cP), showing no significant differences between them. Tomato juice treated at 90 °C for 30 s (120.1 ± 1.3 cP) and the untreated (114.2 ± 1.0 cP) had the lowest viscosity values. There are few studies about the influence of HIPEF on the viscosity of juices. Min et al. [13] observed that the viscosity of tomato juice (381 cP) did not significantly change after either thermal (92 °C for 90 s) or HIPEF processing (40 kV/cm for 57 μs), which was related to the fact that PG and PME were effectively inactivated. Similar results were obtained by Cserhalmi et al. [23] who studied the effects of the application of HIPEF on the viscosity of different citrus juices. They did not observe changes in viscosity between untreated juices and juices processed by 50 pulses at 28 kV/cm. In contrast, in this study an enhancement of the viscosity of the treated tomato juices was observed. There are other factors involving viscosity of tomato juice, such as the variety of the tomato used or the maturity at the moment of processing [7]. In addition, insoluble solids generally contribute to increased consistency of tomato-products. Pectin is especially important because it acts for holding insoluble solids in suspension and thus reducing the tendency for serum separation [24].
In general, viscosity of all the samples followed a decrease during storage. A stronger decay in the viscosity of all the samples was observed with the exception of the viscosity of the tomato juice heated at 90 °C for 30 s which showed a more constant trend than the others (Fig. 1). For the first 35 days of storage, the viscosity of HIPEF-treated tomato juice was above the heat viscosity values. From 42 days on, the viscosity of the HIPEF-treated tomato juice was lower than heat-treated at 90 °C for 1 min, but above the heated for 30 s. From day 56 on, HIPEF-processed tomato juice viscosity kept constant (66.3 ± 0.8 cP) and below the thermal sample viscosity until the end of storage.
Effects of HIPEF and thermal treatments on peroxidase activity
HIPEF treatment at 35 kV/cm for 1,500 μs using 4-μs bipolar pulses at 100 Hz depleted 97% of POD activity in the fresh juice (Fig. 2). In contrast, thermal treatments at 90 °C for 1 min or 30 s inactivated 90 and 79% of the initial tomato juice POD, respectively. These results are in agreement with the reduction obtained in commercial heat-pasteurized juices [25]. Elez-Martínez et al. [14] obtained a complete POD inactivation applying a HIPEF treatment at 35 kV/cm for 1,500 μs using 4-μs bipolar pulses at 100 Hz in orange juice. No increase in POD activity was observed in orange juice after 56 days at 4 °C. After that time, slight increase in the POD was produced in HIPEF-treated tomato juice. Some studies indicate that several enzymes in aqueous solutions are not inactivated by HIPEF treatments at low temperatures [26]. However, oxidative enzymes such as POD or PPO suspended in liquid foods have been successfully reduced after HIPEF processing keeping the temperature below 40 °C [27]. In the present study, the temperature did not increase above 35 °C during HIPEF-treatment while other authors present results obtained when temperature attained 50 °C during HIPEF treatment [28].
According to Fig. 2, the POD residual activity in the HIPEF-treated tomato juice was kept constant during 56 days of storage. Non-treated tomato juice showed a decrease in the residual POD activity values for 14 days. The shelf life of non-treated tomato juice was around 15 days of storage due to the rapid growth of spoilage microorganisms. On the other hand, POD activity of thermally treated juice presented a decay during the first 28 days until its complete inactivation. Peroxidase is one of the most thermally stable enzymes found in fruits and vegetables. Although the role of POD in quality changes is not well established, it is a commonly used indicator for the inactivation of endogenous enzymes during heating because in addition to its stability the assay is simple and rapid [29]. Moreover, the process of inactivation is likely to be induced through denaturation of the enzyme by modifying its conformational state. However, it has been demonstrated that HIPEF probably produced greater changes in the enzyme structure with a strong polarization of the protein molecules and ending with the formation of hydrophobic interactions or covalent bonds and subsequent formation of aggregates [30]. Zhong et al. [31] attributed changes in the secondary structure, demonstrating that a-helix relative content decreased after the treatment, as the main reason of the POD activity loss in HIPEF-treated horseradish (25 kV/cm for 1,740 μs).
Effects of HIPEF and thermal treatments on pectin methylesterase and polygalacturonase activities
PME inactivation is desired when cloudy juices are prepared because it acts on the pectin material present in the food matrix resulting in cloud destabilization and loss of turbidity of the tomato juice [32]. Besides, the PME action on tomato juice leads to a substrate for the PG, which is also present in the tomato fruit. PG inactivation should be attained because it is responsible for the decrease in viscosity, as a direct consequence of pectin solubilization [33].
PME inactivation by a thermal treatment of 90 °C for 1 min (96%) was higher (P < 0.05) than that achieved by the HIPEF-treated tomato juice (82%). In contrast, a thermal treatment at 90 °C for 30 s inactivated only 71% of PME activity. Similar levels of PME inactivation (87%) in tomato were observed by Espachs-Barroso et al. [34] who applied HIPEF at 0.5 Hz for 1,600 μs of total treatment time at 16.8 kV/cm. However, applying 0.02 ms HIPEF pulses at 24 kV/cm for a total treatment time of 8 ms, Giner et al. [35] reached 93.8% inactivation of tomato PME. On the other hand, Elez-Martínez et al. [14] obtained 81.6% reduction of PME activity in orange juice on applying 4-μs bipolar pulses at 35 kV/cm for 1,000 μs and 200 Hz. The differences in the results reported could be related to the resistance of PMEs from different sources to the HIPEF treatments, and the characteristics of the system used [34].
Residual PME activity of HIPEF-treated tomato juice remained significantly constant around 14% during 77 days of storage at 4 °C (Fig. 3). According to Elez-Martínez et al. [14] and Yeom et al. [36], who found that PME activity of treated orange juice was not recovered for 66 and 112 days, respectively, HIPEF processing can cause irreversible inactivation of PME. From an industrial point of view, a juice can be considered enzymatically stabilized when the residual enzymatic activity remains below 10%. This guarantees the shelf life and the quality of the product demanded by the consumer [37].
PME residual activity of thermal treatments was below the values achieved by HIPEF after 21 days of storage, remaining, afterwards, constant until 77 days of storage. PME activity of untreated tomato juice decreased dramatically until it reached 46% of the initial activity at day 14. PME catalyses the hydrolysis of the methylester groups from pectin. Tomato juice does not contain large amounts of pectin and the pectin content diminishes in the process of ripening [38]. This fact could contribute to decrease the PME activity during the tomato juice storage.
Significantly differences (P < 0.05) between treatments were observed on the reduction of tomato juice PG (Fig. 4). HIPEF processing inactivated only 12% of the PG found in the untreated juice, whereas a thermal treatment at 90 °C for 30 s achieved 22% inactivation. The highest inactivation (44%) was obtained when a thermal treatment at 90 °C for 1 min was applied. It is known that PG is present in tomato fruit in two isoforms: PG1 as heat stable form and PG2 as the heat labile form. Some studies [39] described that thermal inactivation phase for PG1 starts at around 55 °C and for PG2 around 80 °C in purified crude tomato extract. Both enzyme fractions were almost completely inactivated after 5 min at 90 °C. The low PG inactivation obtained under all treatment conditions could be attributed to the resistance to inactivation exhibited by the heat stable form PG1.Studies about the effects of HIPEF on PG are scarce, and further research is needed. Nevertheless, Giner et al. [40] studied the PG reduction in an aqueous solution of a commercial enzyme. They attained a 98% inactivation with HIPEF treatment consisting of 200 bipolar pulses of 160 μs at 10.28 kV/cm. On the other hand, a decrease in the residual PG activity of the treated tomato juices was observed, irrespective of the treatment applied (P > 0.05) (Fig. 4). In general, the changes in PG residual activity during all storage followed an exponential decay trend in both treated and untreated tomato juice. HIPEF and thermal treatment at 90 °C for 1 min showed residual PG activity of 14 and 11%, respectively, at the last day of storage.
In this work, PME was inactivated by HIPEF treatment while PG activity was not affected. Similar results were seen by Nguyen and Mittal [41]. They observed how the combination of different hurdles such as moderately high temperatures (<50 °C), antimicrobial compounds (100 U/mL nisin) and HIPEF (20 pulses of 80 kV/cm) had an effect on PME and PG inactivation in tomato juice. They observed the same PME and PG activity reduction (up to 55%) between these combined treatments and heating the juice at 50 °C. PME de-esterifies the pectin yielding methanol and pectin acid with lower degree of esterification, whereas PG catalyses the hydrolytic cleavage of the glycosidic α-d-(1–4) bonds in the pectin acid. PME prepares an ideal substrate for PG action. Therefore, PME inactivation may lead to a decrease in the PG action [41].
Correlation between the physical parameters and the enzymes
In order to estimate correlations between color, viscosity and the enzymatic residual activities of POD, PME and PG of HIPEF and heat-treated tomato juice storage for 77 days at 4 °C, principal component analysis (PCA) was performed. The correlation circle obtained (Fig. 5) shows that the first two principal components (PC1 and PC2) represented 80.89% of the total variance of the data. The main component (PC1) explained 56.55% of total variance, which was mainly described by the variables viscosity, PG, PME and POD. Figure 5 confirms a positive correlation between PG and viscosity. Therefore, it is suggested that changes in viscosity during the storage of tomato juice may be affected by the pectolytic enzymes such as PG inactivation. Moreover, a close relationship between POD and PME was observed. These enzymes are considered among the most HIPEF-labile enzymes, and the treatment applied to the product could inactivate them. Elez-Martínez et al. [14] observed the same trend for both enzymes during the storage of heated and HIPEF-treated orange juice. On the other hand, no correlation exists between POD, L* and h°.
Conclusions
The HIPEF-treated tomato juice presented better color preservation than the heat-processed, evidencing higher lightness than heat treatments during the storage. Furthermore, the HIPEF treatment was more effective than heat treatments in obtaining a tomato juice with higher viscosity than an unprocessed tomato juice.
On the other hand, HIPEF processing at 35 kV/cm for 1,500 μs using 4-μs bipolar pulses at 100 Hz achieved higher POD inactivation than thermal treatments and showed a stable behaviour for 56 days of storage. The PME inactivation by HIPEF was similar to that reached by a thermal treatment at 90 °C for 1 min and remained constant throughout storage, showing that HIPEF processing can cause irreversible PME inactivation. The principal component analysis related the viscosity values to the PG inactivation and showed a close relationship between POD and PME due to the HIPEF-labile characteristics of these enzymes. Nevertheless, further research is needed to know the effect of different HIPEF treatments on the shelf life of juices.
References
Braddock RJ (1999) Handbook of citrus by-products and processing technology. Wiley, New York
El-Beghiti K, Rabhi Z, Vorobiev E (2005) J Sci Food Agric 85:213–218
Schilling S, Alber T, Toepfl S, Neidhart S, Knorr D, Schieber A, Carle R (2007) Innov Food Sci Emerg Technol 8:127–134
Espachs-Barroso A, Barbosa-Cánovas G.V, Martín-Belloso O (2003) Food Rev Int 19:253–273
Sobrino-López A, Martín-Belloso O (2006) J Food Prot 69:345–353
Cortés C, Esteve MJ, Frígola A, Torregrosa F (2005) Food Chem 91:319–325
Gould WA (1992) Tomato production, processing and technology. CTI, Baltimore
Marsellés-Fontanet AR, Martín-Belloso O (2007) J Food Eng 83:452–462
Bruemmer JH, Roc B, Bowen ER (1976) J Food Sci 41:186–189
BeMiller JN (1986) An introduction to pectins: structure and properties. In: Fishman ML, Jen JJ (eds) Chemistry and function of pectins. American Chemical Society, Washington, pp 1–12
Chou TD, Kokini JL (1987) J Food Sci 52:1658–1664
Kimball DA (1991). Quality control and technology. Van Nostrand Reinhold, New York
Seacheol M, Jin ZT, Zhang QH (2003) J Agric Food Chem 51:3338–3344
Elez-Martínez P, Soliva-Fortuny R, Martín-Belloso O (2006) Eur Food Res Technol 222:321–329
Hunter RS (1975) The measurement of appearance. Wiley, New York
Marcus RT (1999) Colorymetry. In: Webster JG (ed) The measurement, instrumentation and sensors handbook. CRC Press, IEEE Press, chap 58
Elez-Martínez P, Aguiló-Aguayo I, Martín-Belloso O (2006) J Sci Food Agric 86:71–81
Kimball DA (1991) Citrus processing. In: Kimbal DA (ed) Quality control and technology. Van Nostrand Reinhold, New York, pp 117–125
Gross KC (1982) Hortic Sci 17:933–934
Yeom HW, Streaker CB, Zhang QH, Min DB (2000) J Agric Food Chem 48:4597–4605
Klim M, Nagy S (1988) J Agric Food Chem 36:1271–1274
Goodman CL, Fawcett S, Barringer SA (2002) J Food Sci 67:404–408
Cserhalmi Z, Sass-Kiss Á, Tóth-Markus M, Lechner N (2006) Innov Food Sci Emerg Technol 7:49–54
Tiziani S, Vodovotz Y (2005) J Agric Food Chem 53: 7267–7273
Irwe S, Olsson I (1994) Reduction of pectinesterase activity in orange juice by high-pressure treatment. In: Singh RP, Oliveira FAR (eds) Minimal processing of foods and process optimization. CRC, Ann Arbor, pp 35–42
Van Loey A, Verachtert B, Hendrickx M (2002) Trends Food Sci Technol 12:94–102
Martín-Belloso O, Elez-Martínez P (2005) Enzymatic inactivation by pulsed electric fields. In: Sun D-W (ed) Emerging technologies for food processing. Elsevier, London, pp 155–181
Nguyen P, Mittal GS (2007) Chem Eng Process 46:360–365
Antohon GE, Sekine Y, Watanabe N, Barrett FM (2002) J Agric Food Chem 50:6153–6159
Castro AJ, Swanson BG, Bárbosa-Cánovas GV, Zhang QH (2001) Pulsed electric fields denaturation of bovine alkaline phosphatase. In: Barbosa-Cánovas GV, Zhang QH (eds) Pulsed electric fields in food processing. Technomic Publishing, Lancaster, pp 65–82
Zhong K, Hu X, Zhao G, Chen F, Liao X (2005) Food Chem 92:473–479
Porretta S (1996) Fruit Process 2:58–65
Fachin D, Van Loey AM, Nguyen BL, Verlent I, Indrawati, Hendrickx M (2002) Biotechnol Prog 18:739–744
Espachs-Barroso A, Van Loey AM, Hendrickx M, Martín-Belloso O (2006) Innov Food Sci Emerg Technol 7:40–48
Giner J, Gimeno V, Espachs A, Elez P, Barbosa-Cánovas GV, Martín O (2000) Innov Food Sci Emerg Technol 1:57–67
Yeom HW, Sreaker CB, Zhang QH, Min DB (2002) J Food Sci 65:1359–1363
Sentandreu E, Carbonell L, Rodrigo D, Carbonell JV (2006) J Food Prot 69:2016–2018
Vovk I, Simonovska B (2007) J Chromatogr 1144:90–96
Rodrigo D, Cortés C, Clynen E, Schoofs L, Van Loey AM, Hendrickx M (2006) Food Res Int 39:440–448
Giner J, Gimeno V, Palomes M, Barbosa-Cánovas GV, Martín O (2003) Eur Food Res Technol 217:43–48
Fachin D, Van Loey AM , Nguyen BL, Verlent I, Indrawati, Hendrickx M (2003) Innov Food Sci Emerg Technol 4:135–142
Acknowledgments
This work was supported by the Ministerio de Ciencia y Tecnología (Spain) through the Project ALI 2005-05768. This study has also been carried out with financial support from the Commission of the European Communities, Framework 6, Priority 5 ‘Food Quality and Safety’, Integrated Project NovelQ FP6-CT-2006-015710 that also financed a pre-doctoral grant for author Aguiló-Aguayo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiló-Aguayo, I., Soliva-Fortuny, R. & Martín-Belloso, O. Comparative study on color, viscosity and related enzymes of tomato juice treated by high-intensity pulsed electric fields or heat. Eur Food Res Technol 227, 599–606 (2008). https://doi.org/10.1007/s00217-007-0761-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0761-2