Abstract
The effect of static and multi-pulsed high pressure processing (HPP) on the activity of hydrolyzing enzymes and the rheological properties of cloudy carrot juice during refrigerated storage (4 °C for 12 weeks) were investigated. The changes in the carotenoid profile, total polyphenolic content (TPC), antioxidant potential, and color were also studied. After HPP treatment, the activity of polygalacturonase (PG) and pectinmethylesterase (PME) decreased significantly. During the storage, the activity of PG increased by a maximum of 40% for 450 MPa, whereas PME activity decreased by a maximum of 76% for 300 MPa × 3 and 600 MPa. The apparent dynamic viscosity and turbidity changed after HPP treatment and storage. Seven carotenoids were identified in the untreated juice and their concentration increased significantly as increased pressure during the treatment. The carotenoids underwent significant degradation during the storage, which also correlated with changes in the a* and b* color parameters. The storage time also had a significant impact on the TPC and antioxidant capacity determined with DPPH and ABTS tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carrot juice is one of the most commonly consumed vegetable juices in Poland. It is rich in β-carotene, which is a major source of pro-vitamin A in the human diet. Pasteurized carrot juice differs significantly from home-made freshly prepared juice. However, unprocessed raw carrot juice has limited market potential due to its short shelf life and should usually be consumed within 1 to 2 days (Gosavi et al. 2019; Miȩkus et al. 2019; Zhang et al. 2016).
The color of the juice is one of the most important sensory attributes perceived by consumers. Nowadays, consumers are paying more attention to health-promoting nutritional compounds with high antioxidant activity such as carotenoids (Patras et al. 2009; Barba et al. 2012; Zhang et al. 2016). Fresh unpasteurized juices arouse the interest of consumers. Considering that fresh juice has a very limited shelf life and might even be unsafe for consumers, food technologists are looking for techniques that allow a high sensorial and nutritional value to be maintained along with microbial safety. High pressure processing (HPP) is one of the non-thermal techniques commonly used for food preservation, sufficiently extending shelf life due decreasing the concentration of microorganisms in juices (Abid et al. 2014; Zhao et al. 2014; Marszałek et al. 2015; Huang et al. 2018; Buerman et al. 2020). By applying pressure of up to 600 MPa, HPP can extend the shelf life of fruit juices by even up to 3 months in refrigerated conditions (Donsì et al. 2010; Marszałek et al. 2016). Unfortunately, the high activity of native enzymes decreases the shelf life due to the fast degradation of nutritional compounds and consistency during the storage. On the other hand, using pressure as high as 600 MPa or even higher decreases durability of HPP devices, therefore multi-pulsed HPP with the application of relatively lower pressure might be a good alternative for extension of equipment lifetime. Although HPP devices are still expensive, the HPP treatment is more environmentally friendly compared to pasteurization (Cacace et al. 2020).
There are very few studies to verify the HPP stability of enzymes from the hydrolases group, especially polygalacturonase (PG) and pectinmethylesterase (PME), which are responsible for the changes in the rheological properties of juices, such as turbidity and viscosity, due to pectin degradation (Eisenmenger and Reyes-De-Corcuera 2009; Houben et al. 2014; Hurtado et al. 2015; Picouet et al. 2016). However, there are more and more studies describing rheological changes such as viscosity and turbidity after HPP treatment (Keenan et al. 2012; Picouet et al. 2016). Furthermore, HPP often results in the better extractivity of nutritional compounds, which can lead to the better bioaccessibility of these compounds in pressure treated juices (Briones-Labarca et al. 2011). Although there are several studies about the effect of HPP on the bioactive compounds in fruit and vegetable juices (Cao et al. 2012; Gao et al. 2015; Liu et al. 2016; Marszałek et al. 2016; Patras et al. 2009), the number of publications on the impact of multi-pulsed HPP on quality of juices is very limited (Buzrul 2014; Donsì et al. 2010).
Our previous study confirmed the microbiological safety of HPP-treated (using the same process parameters) carrot juices. The total aerobic mesophilic and lactic acid bacteria, yeast and mold concentration allowed for 12 weeks of storage in refrigerated conditions with good microbial quality for juice treated at 450 MPa and 600 MPa, whereas juice treated at 300 MPa × 3 had a shorter the shelf life (Stinco et al. 2019). In another study by our group, the polyphenolic profile and activity of oxidoreductive enzymes in HPP-treated carrot juice were investigated (Szczepańska et al. 2020). Therefore, in the current work, we have focused on the effect of static and multi-pulsed HPP on the activity of hydrolytic enzymes, the rheological properties, such as apparent dynamic viscosity and turbidity, antioxidant capacity, color parameters, and, finally, the carotenoid profile changes during the refrigerated storage, in order to obtain more general conclusions about the quality of HPP-treated carrot juice during storage. The above manuscript complements the previous works (Stinco et al. 2019; Szczepańska et al. 2020) and constitutes a comprehensive evaluation of the static and multi-pulsed HPP on the quality of carrot juice.
Materials and Methods
Preparation of Carrot Juice
Carrots of unspecified variety were purchased from a local market in Poland (Warsaw). They were purchased at commercial maturity stage and kept at 4 °C in the laboratory until further processing. Carrot juice was obtained by squeezing (J 80 Ultra, Robot Coupe, France) and bottled in high density polyethylene bottles (50 mL) and immediately treated using HPP. Experiments were performed in duplicate and each sample was analyzed 2 times (n = 4).
High Pressure Processing Preservation
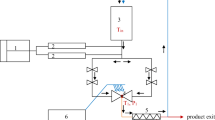
HPP treatment of carrot juices was performed in a 100-l capacity prototype chamber (model 6-CAL70, Exdin Solutions, Poland). The maximum value of working pressure is up to 600 MPa, whereas the maximum value of working temperature is up to 40 °C. Tap water was used as the pressurization fluid. The process was carried out using various pressures: static pressure of 450 MPa and 600 MPa for 5 min and three pulses of 300 MPa for 5 min (total 15 min) at ambient temperature (≈ 22–24 ± 1 °C). The initial temperatures of the pressure transmitting fluid were 13 °C, 11 °C, and 4 °C, respectively for pressures of 300 MPa, 450 MPa and 600 MPa. The initial temperatures of the tap water were lower, because temperature is increasing about 3 °C per every 100 MPa as a consequence of the adiabatic heat of compression. The initial temperature of the juices was 4 °C. Pressure was generated in 20–60 s, whereas the release time was 8–10 s, depending on the pressure applied. Pressure time generation and release were not included in the total pressurization time. After processing, the samples were immediately cooled and analyzed. Color changes were assessed immediately after the HPP process, whereas samples for performing the remaining analyses were stored at − 25 °C. The control sample was fresh untreated juice.
Storage Conditions
Samples were stored in refrigerated conditions (≈ 4 °C), protected from light, for up to 12 weeks and subjected to testing in 2-weeks intervals.
Physico-Chemical Analysis
The pH was measured using an HI 991001pH meter (Hanna Instruments, USA), while the density was measured using a DME 4500 density meter (Anton Paar, Austria). The total soluble solids (TSS) content was determined using a digital refractometer (PAL-3, Atago, USA) and expressed as Brix.
HPLC Analysis of Sugars
Glucose, fructose, and sucrose were determined according to the EN 12630:1999 standard. For the analysis, a Sugar-Pak I, 10 μm, 6.5 mm × 300 mm analytical column with a Sugar-Pak and Guard-Pak insert, 10 μm (both Waters, USA), was used. The separation of the 2 μL samples was performed within 20 min at a flow rate of 0.5 mL/min and a column temperature of 90 °C, and a detector temperature of 35 °C. The compounds were quantified using a refractive index detector (Waters 2414, USA). Samples were eluted isocratically using 0.1 mM calcium disodium EDTA.
Determination of Hydrolytic Enzyme Activity
Determination of Polygalacturonase (PG) Activity
The activity of polygalacturonase (PG) was determined using the spectrophotometrical method proposed by Pires and Finardi-Filho (2005) with some modifications. The carrot juices and 1 M NaCl (5 mL:5 mL) were adjusted to pH = 4.0 by adding 1.0 M CH3COOH. The mixture was centrifuged (Rotina 380R, Hettich Instruments, Germany) at 13,000×g for 30 min at 4 °C. The supernatant was used to determine PG activity.
For the PG assay, 100 μL of the supernatant was added to 90 μL of 37.5 mM Na-acetate (pH = 4.4) and 300 μL of the same Na-acetate buffer, with 0.2% of polygalacturonic acid, were incubated at 30 °C for 2 h. To quantify the released reducing groups, the reaction was stopped by adding 2.0 mL of cold 100 mM borate buffer (pH 9.0), followed by 0.4 mL of 1% 2-cyanoacetamide. The samples were mixed and immersed in a boiling water bath for 10 min. After equilibration at 25 °C, the absorbance was measured at λ = 276 nm using a UV–visible spectrophotometer (6705 UV–vis Spectrophotometer, Jenway, UK). The blank sample was prepared with the same components, but with the supernatant previously boiled for 5 min. The residual activity of PG was calculated as a quotient of the HPP-treated juice and fresh juice activities.
Determination of Pectinmethylesterase (PME) Activity
The activity of pectinmethylesterase (PME) was determined spectrophotometrically according to Hagerman and Austin (1986) with some modifications. The carrot juices were adjusted to pH = 7.5 by adding 0.1 M NaOH. The mixture was centrifuged (Rotina 380R, Hettich Instruments, Germany) at 10,000×g for 10 min at 4 °C. The supernatant was used to determine PME activity.
For the PME assay, 0.3 mL of the supernatant was added to a reaction mixture composed of 2.0 mL of 0.5% (w/v) citrus pectin (pH = 7.5), 0.15 mL of 0.01% (w/v) bromothymol blue in 0.003 M sodium phosphate buffer (pH = 7.5), and 0.75 mL distilled water. The absorbance was measured at λ = 620 nm using a UV–visible spectrophotometer (6705 UV–vis Spectrophotometer, Jenway, UK). The residual activity of PME was calculated as a quotient of the HPP-treated juice and fresh juice activities.
Apparent Dynamic Viscosity and Turbidity
Apparent dynamic viscosity was determined by using a viscometer (Brookfield DV-II+, USA) coupled to Brookfield Wingather software. The base of this operation is to drive a spindle, which is immersed in the test fluid through a calibrated spring. The viscous drag of the fluid against the spindle is measured by the spring deflection and spring deflection is measured with a rotary transducer.
The apparent dynamic viscosity (Pa x s) and shear stress were determined at varying shear rates, ranging from 7.5 to 263 s−1, while possible range of shear rate was 1500 s−1. CP-40 spindle with cone 0.8° was used for analysis. Shear stress values were recorded during 80 s. The measurements of a 1 mL sample were carried out at 20 °C.
The turbidity of the samples was determined using a nephelometer (Hach-2100 N turbidymeter, USA). The samples were diluted 5 times with water before analyzing turbidity.
Total Polyphenolic Content (TPC) and Antioxidant Capacity (DPPH•, ABTS•+)
Five milliliters of methanol 80% (v/v) containing 0.1% (v/v) of HCl were added to 5 mL of carrot juices. The samples were treated with ultrasound for 5 min (45 kHz, 200 W, 25 °C, MKD Ultrasonic, Poland) and centrifuged (Rotina 380R, Hettich Instruments, Germany) for 5 min at 4 °C at RCF 3670×g. The supernatant was transferred to a 25 mL volumetric flask. The extraction was repeated four times. After filtration (pore size 0.45 μm, Macherey-Nagel, Germany), the supernatant was used for analyzing TPC and the antioxidant capacity (DPPH and ABTS tests).
The supernatant used to determine TPC. The phenolic compounds were determined spectrophotometrically (6705 UV–vis Spectrophotometer, Jenway, UK) using a method proposed by Gao et al. (2000). The results were expressed as mg of gallic acid equivalents (GAE) per 1 L of juice.
The antioxidant activity was measured using the DPPH• (2,2-diphenyl-1-picrylhydrazyl) method as described by Yen and Chen (1995) with some modifications. The stable free radical DPPH• is reduced to the corresponding hydrazine by reaction with hydrogen donors. One hundred twenty microliter of supernatant, prepared according to above, was mixed with 2.0 mL of a 0.1 mM 80% methanolic solution of DPPH. The absorbance was measured after 20 min at λ = 520 nm and 25 °C (6705 UV–vis Spectrophotometer, Jenway, UK). The results were calculated using a calibration curve prepared at different concentrations of DPPH in 80% methanol and expressed as μM Trolox equivalents (μM Tx).
The antioxidant activity was measured using the ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) method as described by Re et al. (1999). The ABTS•+ solution was made with ABTS stock solution (7 mM) and potassium persulfate solution (2.45 mM). Before the assay, the ABTS•+ solution was diluted with 80% methanol to an absorbance value of 0.740–0.750 at λ = 734 nm. Forty microliter of supernatant, prepared according to above, was mixed with 2 mL of an ABTS•+ solution. The absorbance, after 6 h of incubation at 30 °C was measured at λ = 734 nm and 25 °C (6705 UV–vis Spectrophotometer, Jenway, UK). The results were calculated using a calibration curve prepared at different concentrations of ABTS•+ solution and expressed as μM Trolox equivalents (μM Tx).
Carotenoid Profile
For carotenoid analyses, samples were extracted in duplicate according to the method proposed by Mapelli-Brahm et al. (2017) with some modifications. One milliliter of BHT (500 mg of BHT in 1 L of hexane), 3.5 mL of hexane, and 4.5 mL of acetone were added to 4 mL of carrot juice. The samples were shaken using an orbital shaker for 1 min at 400 RPM (Sk-0330-Pro, DLab, China), treated with ultrasound for 5 min (45 kHz, 200 W, 25 °C, MKD Ultrasonic, Poland), and centrifuged (Rotina 380R, Hettich Instruments, Germany) for 5 min at 4 °C at RCF 2500×g. The organic phase was transferred to a 50-mL tube. The aqueous phase was extracted four times with 4.5 mL of hexane and 2 mL of saturated aqueous sodium chloride solution and three more times with 4.5 mL hexane. The organic phases were mixed. Two milliliter of the organic phase was evaporated at 30 °C and 160 mbar (Rotavapor R-300, Buchi, Switzerland). Two milliliter of acetone was added to the dried organic phase and the HPLC analysis was performed according to Melendez-Martinez et al. (2013). A Waters 2695 HPLC system (Waters, Milford, MA) equipped with an YMC Carotenoid column (3 μm, 4.6 mm × 150 mm). The mobile phase solvents were phase A, methanol with 0.1% ammonium acetate, and phase B, methyl-tert-butyl ether, as follows: from 0 to 44 min, 100% (A); then 45–54 min, 85% (A) and 15% (B); 55–59 min, 40% (A) and 60% (B); 60–69 min, 30% (A) and 70% (B); and finally 70–75 min, 100% (A). The separation of the 25 μL samples was performed within 75 min at a flow rate of 1.0 mL/min and a column temperature of 25 °C. The compounds were quantified using a photodiode array detector (Waters 2996, USA) at λ = 450 nm. The results were calculated using a calibration curve prepared at different concentrations of β-carotene and expressed as mg of β-carotene equivalents per 1 L of carrot juice.
Color Parameter
Color analysis was carried out using a Color Quest XE colorimeter (HunterLab, 166 USA) equipped with a xenon flash lamp in glass cuvettes with an optical path of 1 cm. The results were presented in accordance with the CIE L*a*b* system, using illuminant D65 and 10° observer. The data was expressed as Hunter scale parameters: L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) values. The total color difference (ΔE) was calculated using Eq. 1, where L*o, a*o and b*o were the values of the control sample.
Depending on the value of ΔE, the color difference between the treated and untreated samples could be estimated such as unnoticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), visible (3.0–6.0), and excellent (6.0–12.0) (Cserhalmi et al. 2006). Therefore, a larger ΔE denotes greater color change from the reference juice. The chroma value (C) (Eq. 2) and hue angle (h) (Eq. 3) were calculated based on the Hunter L, a, and b values (Maskan 2001).
Statistical Analysis
Experiments were performed in duplicate and each sample was analyzed 2 times (n = 4). The results were expressed as a mean value ± standard error of the mean (SEM). A statistical analysis was conducted using STATISTICA 7.1 software (StatSoft, Tulsa, OK, USA) with a repeated measures ANOVA for a stored samples and one-way analysis of the variance (ANOVA) to verify if the fresh sample differed from the HPP-treated samples. A statistical analysis was conducted using Tukey’s test at a 95% confidence level.
Results and Discussion
Physico-chemical Characteristic of Carrot Juice
The fresh carrot juice, with a pH of 6.29, density 1.034 g/cm3, contained 8.31°Brix of total soluble solids (TSS). The total sugar content was 65.15 g/L (33.37 g/L sucrose, 17.29 g/L glucose, and 14.49 g/L fructose). All parameters were in accordance with the requirements of the Code of Practice of the Association of the Industry of Juices and Nectars (Anonymous 2015) and there were no significant differences under static and multi-pulsed HPP treatment.
Polygalacturonase and Pectinmethylesterase Activity
Table 1 shows the residual activity of polygalacturonase (PG) of HPP-treated carrot juices. Application of the 300 MPa × 3, 450 MPa and 600 MPa resulted in a decrease in PG activity after juice preservation by 5.4%, 17.9% and 31.9%, respectively. It is clear that the higher the pressure applied the lower the RA of PG noted. The RA was measured also during storage time in cold conditions (4 °C) and it was observed that the activity of PG increased during the storage. However, changes observed in the first 2 weeks of storage were insignificant, but a longer storage time caused a significant increase in PG residual activity. The highest activity (RA 129.8%) of PG during refrigerated storage was noted for samples treated at 450 MPa, whereas the lowest (RA 96.2%) at 600 MPa after 12 weeks of storage. It transpired that the application of HPP at lower pressure in pulses (300 MPa × 3) resulted in a lower RA of PG than 450 MPa in one pulse, which is very important considering processing costs. Our results confirmed the findings published by other authors that the inactivation of PG was correlated with the level of pressure applied (Jolie et al. 2012). In this regard, Fachin et al. (2003) noticed that increasing the pressure from 200 MPa up to 550 MPa at 25 °C resulted in faster inactivation of tomato PG. In addition, Hsu (2008) also reported that PG activity in tomato juice was reduced by 90% in pressures exceeding 400 MPa for 10 min at 4 °C and 25 °C. Pressure level of around 500 MPa was sufficient for irreversible enzyme inactivation in tomato pieces and juices, even without additional heating (Jolie et al. 2012). However, Sun et al. (2019) found insignificant changes of PG activity in carrot sticks at 400 MPa and 600 MPa after 2 min but a pressure higher than 800 MPa resulted in the complete inactivation of PG.
An inverse relationship was observed in the RA of pectinmethylesterase (PME) (Table 1). Although the decrease in the activity of this enzyme after processing was lower compared to PG, the RA decreased significantly during the storage time, which indicated that PME was unable to regenerate. This may be connected with the changes in the native structure of enzymes under HPP treatment. The application of HPP may induce the partial or complete irreversible denaturation of enzymes by unfolding their native structure, which leads to the inability of enzymes to regenerate (Chakraborty et al. 2014). In the present study, the residual activity of PME in carrot juice after pressurization was 94.2%, 92.5%, and 91.6%, for 300 MPa × 3, 450 MPa, and 600 MPa, respectively. In the 2nd week of refrigerated storage, the residual activity of this enzyme decreased and the highest degradation (RA 51.3%) was observed in samples treated with 300 MPa × 3 pulses, whereas the RA of samples treated at a higher pressure was about 80%. Longer storage time caused further degradation of PME up to 25% RA after 12 weeks of the storage for samples treated with 300 MPa × 3 and 600 MPa. Our finding agrees with data reported by Jabbar et al. (2014) who found that carrot juice PME gradually decreased together with increased pressure in the range of 250 MPa to 450 MPa at 25 °C and 10 min. In addition, Balogh et al. (2004) observed that a combination of very high pressure (800 MPa) and a long period of time (36 min) at 10 °C was required to reduce the PME activity in carrot juice by 90%.On the contrary, Sun et al. (2019) observed that carrot PME was stable in a pressure range of 400–1000 MPa for 2 min. This phenomenon might be connected with more efficient enzyme extraction due to greater tissue disruption caused by intensified pressures. These authors obtained significant inactivation at 1200 MPa, with a reduction of PME activity to 22.5%.
Apparent Dynamic Viscosity and Turbidity
Figure 1 shows the dependence between apparent dynamic viscosity and the shear rate (at a range from 7.5 to 263.0 s−1) for fresh carrot juice and after HPP treatment. A significant increase in apparent dynamic viscosity after HPP was observed, compared to fresh juice. Higher apparent viscosity may be caused by important changes in the particle size of the tissue due to particle agglomeration. The apparent dynamic viscosity of carrot juice subjected to varying HPP treatments decreased in line with the increased shear rate. Shear-thinning behavior is very common in fruit and vegetable products as was observed by Steffe et al. (1983) and Opazo-Navarrete et al. (2012). The above authors explained the phenomenon as the molecules becoming less dependent on each other and consequently presenting less resistance to flow under an increase in the shear rate. There were no statistical changes on apparent dynamic viscosity during the refrigerated storage (data not presented). This outcome agrees with data reported by Gao et al. (2016) who observed no significant differences with the apparent dynamic viscosity in strawberry syrup treated with 400 MPa during 5 min and storage at 4 °C and 25 °C. On the other hand, other authors noticed significant changes on apparent dynamic viscosity during the storage time of HPP-treated fruit beverages. For example, Cao et al. (2012) noticed decreased viscosity of 67% during 6 months of storage at 4 °C in cloudy strawberry juice treated with 600 MPa during 4 min. Liu et al. (2014) reported that the viscosity of mango nectars decreased gradually throughout the storage time, except for pressurized nectars stored at 4 °C. These authors explained the loss of viscosity due to pectin degradation, because pectin may be degraded through enzymatic and non-enzymatic degradation mechanisms. The enzymatic degradation of pectin may be caused by incompletely inactivated PME (Sila et al. 2009). In the present study, HPP did not completely inactivate PME and the activity of this enzyme did not correlate with the viscosity. Figure 1 also shows the relationship between shear stress and shear rate (range from 7.5 to 263.0 s−1) for fresh and HPP-treated carrot juices. Changes on shear stress were observed and depended on the pressure level applied. Generally, bigger particles are more resistant to shear and show greater friction during the flow, which is probably due to an increase of shear stress (Steffe et al. 1983).
The turbidity of fresh carrot juice was 8061.7 ± 23.1 NTU. The results obtained are in the range (from 6000 to 12,000 NTU) determined by Reiter et al. (2003a) for different commercial carrot juices. It was observed that the turbidity increased by a maximum of 25.4% after pressurization for carrot juice treated at 600 MPa (Table 2). It is evident that HPP treatment significantly affected the turbidity of carrot juices. The long-time cold storage decreased the turbidity of HPP-treated juices, which can be justified by particle agglomeration. Larger particles sediment faster compared to smaller particles. Zhang et al. (2016) observed differing results in their study on carrot juice preserved using HPP and HTST techniques. These authors summarized that the increase in viscosity and turbidity during the storage time might be connected with the diminishing of juice particles and finally with the dispersion of carrot cells and tissue. These results are inconsistent with our study, most probably because the carrots used in the present study could contained more protein, which promoted agglomeration and/or protein polymerization. Reiter et al. (2003b) reported that different kinds of pretreatment may promote protein coagulation and in this way influence the stability of carrot juice. Furthermore, the same authors explained that the proteins had a greater effect on the stability of carrot juice than pectin concentration. When proteins precipitate, particles tend to aggregate. In the present study, the polygalacturonase activity might also show a partial effect on turbidity. In this regard, the correlation coefficient for PG with turbidity was weak, r = − 0.5472 but significant at p ≤ 0.01.
Total Phenolic Content (TPC)
The TPC in fresh carrot juice was 163.80 ± 2.8 mg GAE/L. After pressurization, the measured TPC increased slightly, up to 13% at 600 MPa (Table 2). This result can be justified by plant cell disruption caused by HPP and in consequence the better extraction of bonded phenols from tissue and also by the probable formation of new polyphenols. The formation of new polyphenols under HPP was proved in our previous study where new polyphenols such as oleuropein, 4-vinylsyringol, isocoumarin, and 4-hydroxybenzaldehyde were detected after HPP treatment at static (450 MPa, 600 MPa at 5 min) and multi-pulsed HPP (300 MPa × 3 pulses at 5 min) of carrot juice (Szczepańska et al. 2020).
During the storage at 4 °C, the polyphenol content of carrot juice began to increase, but the upward trend is not clear. After 12 weeks of storage, TPC increased by 26.4% for juices treated with 300 MPa × 3 pulses (Table 2) and it was the highest result noted in all samples. This phenomenon can be attributed to differences in chemical structure responsible for the different affinity of polyphenols with Folin–Ciocalteu reagent. On the other hand, Folin–Ciocalteu reagent reacts not only with phenols but also with other compounds reducing sugars, carotenoids, amino acids and vitamin C (Vinson et al. 2001). Other authors also confirmed that the Folin–Ciocalteu reagent is not selective and may react with other antioxidants (Klimczak et al. 2007). An increase in TPC was also reported in pressurized cape gooseberry pulp (300 MPa at 3 and 5 min) after 60 days of storage (Torres-Ossandón et al. 2018). Different results were obtained by Zhang et al. (2016). They reported that 20 days of refrigerated storage of carrot juice treated with HPP at 550 MPa revealed TPC degradation of 35.8%. The above authors suggested that this fact was caused by the oxidation, degradation and polymerization of polyphenols with proteins.
Antioxidant Capacity (DPPH•, ABTS•+)
The DPPH assay is based on the reduction in the purple DPPH to 1,1-diphenyl-2-picryl hydrazine, whereas the ABTS assay is based on the generation of a blue/green ABTS+ that can be reduced by antioxidants. The DPPH assay takes place in an alcoholic medium, while the ABTS radical is soluble in water and organic solvents (Apak et al. 2016; Floegel et al. 2011). Table 2 shows the antioxidant capacity of fresh and HPP-treated carrot juices. The antioxidant activity of fresh carrot juice measured with DPPH• and ABTS•+ tests were 340.2 ± 4.7 and 164.7 ± 2.9 μM of Trolox equivalent, respectively. The antioxidant capacity after HPP treatment increased, especially at 600 MPa, by 48.8% and 10.9% as measured by the DPPH• and ABTS•+ tests, respectively. In this regard, Varela-Santos et al. (2012) also reported an increase in the antioxidant activity expressed as IC50 after HPP treatment at 450 MPa for 30–150 s in pomegranate juice. The above authors concluded that the increase in the antioxidant potential may be related to the increased extractability of some hydrolysable tannins, which were present in the fruits. In the present study, the antioxidant potential increased during the refrigerated storage, especially measured with the DPPH• radicals (Table 2). In addition, Varela-Santos et al. (2012) obtained similar results after 30 days of storage at 4 °C in pomegranate juice treated with 350 MPa and 550 MPa at 30 s. These authors explained that a possible reason for the increase in antioxidant capacity could be connected with anthocyanin polymerization during the storage and the creation of higher polyphenols, for example ellagitannins from ellagic acid and other phenolic compounds. After HPP treatment and 30 days of storage at 4 °C and 25 °C, an increase in the antioxidant capacity (about 40%) of strawberry syrup treated at 400 MPa during 5 min was also observed Gao et al. (2016).
In the present study, a significant correlation was noted between the antioxidant capacity measured with ABTS•+ test and TCP (r = 0.7443, significant at p ≤ 0.01), while the correlation between the DPPH• test and TPC was insignificant (p ≥ 0.05). Furthermore, inverse, but a significant correlation between the DPPH• test and carotenoid content (r = − 0.7001, significant at p ≤ 0.01) was also observed, whereas the correlation between the ABTS•+ test and carotenoids was insignificant (p ≥ 0.05). The discrepancies noted may be due to the types of reactions occurring in both methods. Measuring of antioxidant capacity depends on the conditions used in the protocols and the affinity of different radicals to specific substrates. The inverse relationship between the DPPH• test and carotenoids content may be due to the interaction of carotenoids, especially β-carotene and free radicals forming a conjugated polyene, carbon centered radicals (Liu et al. 2008). In this regard, Wootton-Beard et al. (2011) observed higher values of antioxidant activity for FRAP assay followed by DPPH• and ABTS•+ methods in vegetables juices such as carrot and tomato juices and they explained these differences by the fact that ABTS•+ radical is soluble in both water and lipophilic solvents whereas DPPH• radical has better solubility in organic solvents.
Carotenoid Content
The carotenoid concentration in fresh carrot juice and their changes after HPP treatment and during the refrigerated storage are presented in Table 3. In fresh carrot juice, the sum of individual carotenoids was 161.60 ± 0.33 mg/L. The results obtained are in accordance with the AIJN code of practice (Anonymous 2015), where the total carotenoid content in carrot juices may range from 30 to 300 mg/L. In the present study, the major carotenoids detected in fresh juice were β-carotene (86.29 ± 0.16 mg/L) and α-carotene (64.83 ± 0.31 mg/L), which accounted for 93.5% of all carotenoids. Other carotenoids identified in fresh juice were: lutein, β-cryptoxanthin, ε-carotene, 9-Z-β-carotene. In addition, one unidentified carotenoid was determined. After pressurization, the measured concentration of carotenoids increased significantly by 4.8–6.8% with the increase pressure. This phenomenon can be justified by better extraction of these bioactive compounds from tissue treated by high pressure, which was also confirmed by other authors (Jabbar et al. 2014; Patras et al. 2009), who proved that HPP makes carotenoids and other bioactive compounds more accessible for extraction. Disruption of cell membranes and protein–carotenoids complex under HPP treatment was also observed by Patras et al. (2009) in carrot purée (600 MPa for 15 min at ambient temperature), which resulted in a 58.5% increase in total carotenoids. According to Pokhrel et al. (2019), pressure treatment of carrot juice at 500 MPa for 2 min at 20 °C resulted in a 18.5% increase in total carotenoids.
During the refrigerated storage, a decrease trend in carotenoids was observed (Table 3). The total content of carotenoids in HPP-treated samples decreased by 37.3%, 57.9% and 39.7%, for 300 MPa × 3, 450 MPa and 600 MPa, respectively after 12 weeks of storage. This phenomenon can be explained by the degradation of carotenoids and other phenolic compounds due to the high residual activity of oxidoreductive enzymes and their synergistic activity (Stinco et al. 2019; Szczepańska et al. 2020). Our previous studies confirmed that even slight enzymes activity might be responsible for the faster degradation of bioactive compounds during the storage time. In this regard, Picouet et al. (2015) reported a decrease in β-carotene of 18% in acidified and non-acidified carrot juice treated with 600 MPa for 5 min at an initial temperature of 10 °C and a further decline of 12% during 29 days of refrigerated storage. However, the α-carotene concentration remained stable. In the present study, both α- and β-carotene degraded at a similar level during the storage time, whereas lutein was particularly degraded, especially at 450 MPa. Similar results were found by Zhang et al. (2016) who noticed than lutein exhibited a higher degradation than β-carotene in carrot juice. They also observed degradation of lutein by 66.7% and β-carotene by 11.1% during 20 days of storage.
In the current study, the carotenoid content correlated positively (significant at p ≤ 0.01) with Hunter a* and b* values, as evidenced by the correlation coefficient r = 0.8282 and r = 0.8335 respectively. Correlation between the concentration of carotenoids and a* value was also noticed by Patras et al. (2009) in HPP-treated carrot and tomato purées at 400, 500 and 600 MPa for 15 min at ambient temperature.
Color Parameters
The colorimetric parameters of the samples studied were summarized in Table 4. There were significant differences in all the color parameters between the control and HPP-treated samples. In carrot juice, the L*, a*, and b* values decreased after HPP and during the storage time, which are in accordance with the previous studies (Jabbar et al. 2014). These results are also in accordance with the research obtained by Trejo Araya et al. (2009), where the values of the color parameters of carrot treated at 600 MPa for 2 min decreased. During the storage at 4 °C, the lightness of the carrot decreased, especially in the first week. Furthermore, the above authors observed higher color retention of HPP-treated carrots than of raw carrots during the storage time. Research conducted by Pokhrel et al. (2019) showed a reduction in the L* value in carrot juice treated with HPP under different conditions (200–500 MPa, 20–50 °C, 2 min). They observed that HPP-treated samples became gradually lighter during the storage for 4 weeks at 4 °C, which also confirms our results.
The total color difference (ΔE) is used to characterize the variation of color in foods during processing. Immediately after the HPP treatment, the ΔE parameter was noticeable, but visible only by an experienced observer. In the 12 weeks after pressurization, the ΔE parameter increased, making the changes in color more visible, also for the inexperienced observer. In a study conducted by Cao et al. (2012) during 6 months of storage in cloudy strawberry juice at 4 °C the parameter (ΔE = 7.8) was at a comparable level to the present study, which indicates the degradation of colored compounds. Chen et al. (2015) reported that ΔE values of HPP-treated papaya beverage under different conditions (350–650 MPa for 5, 10 min at 20 °C) increased with the time extension. The above authors suggested oxidation, degradation and polymerization of pigments and non-enzymatic reactions as the main reasons for changes on color during the refrigerated storage.
Parameter C (chroma) indicates the degree of color saturation, while the h angle shows an angular position. Pressurized carrot juice had lower chroma values compared to the control samples and there was a decrease during the storage time, whereas the differences in parameter h were not so large, but also statistically significant (Table 4). Similar results were obtained by Trejo Araya et al. (2009) who observed decreasing C values during the storage time and no significant changes in the h angle values in carrot juice treated with 600 MPa for 2 min. Also, in research carried out by Pokhrel et al. (2019), C and h parameters in carrot juice decreased after pressurization (200–500 MPa, 20–50 °C, 2 min) and during 4 weeks of refrigerated storage.
Conclusions
HPP had an impact on the activity of hydrolyzing enzymes in carrot juice, PG was more sensitive to inactivation under HPP treatment than PME, but the residual activity of PG increased during the storage time. This phenomenon may be connected with enzyme proteins folding under high pressure and unfolding during the storage time. On the contrary, PME activity was more resistant to inactivation, whereas the storage time did not favor increasing its residual activity. A significant increase was observed in apparent dynamic viscosity and turbidity after HPP treatment. TPC increased under HPP treatment and during the refrigerated storage and correlated significantly with the antioxidant capacity measured with ABTS•+ assays. Increase in individual carotenoid content after pressurization may be a result of the better extraction of these compounds from juice tissue, whereas decrease of carotenoids content during storage may be caused by oxidative degradation. The color of HPP-treated juices was stable and high pressure did not affect the total color difference, whereas the refrigerated storage resulted in a noticeable change on the ΔE coefficient.
Multi-pulsed HPP treatment at 300 MPa had a similar effect that treatment at 600 MPa in all the measured quality attributes. Taking this phenomenon into account, the use of lower pressure (two times lower compared to that used commercially) in pulses could be a good alternative for reducing the processing costs of carrot juice preservation using HPP.
References
Abid, M., Jabbar, S., Hu, B., Hashim, M. M., Wu, T., Wu, Z., Khan, M. A., & Zeng, X. (2014). Synergistic impact of sonication and high hydrostatic pressure on microbial and enzymatic inactivation of apple juice. LWT - Food Science and Technology, 59(1), 70–76. https://doi.org/10.1016/j.lwt.2014.04.039.
Anonymous. (2015). Code of practice for evaluation of fruit and vegetable juices. In: AIJN (Ed.), Association of juices and nectars from fruits and vegetables of European Union. Brussels.
Apak, R., Özyürek, M., Güçlü, K., & Çapanoʇlu, E. (2016). Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agricultural and Food Chemistry, 64(5), 997–1027. https://doi.org/10.1021/acs.jafc.5b04739.
Balogh, T., Smout, C., Nguyen, B. L., Van Loey, A. M., & Hendrickx, M. E. (2004). Thermal and high-pressure inactivation kinetics of carrot pectinmethylesterase: From model system to real foods. Innovative Food Science and Emerging Technologies, 5(4), 429–436. https://doi.org/10.1016/j.ifset.2004.06.002.
Barba, F. J., Cortés, C., Esteve, M. J., & Frigola, A. (2012). Study of antioxidant capacity and quality parameters in an orange juice–milk beverage after high-pressure processing treatment. Food and Bioprocess Technology, 5(6), 2222–2232. https://doi.org/10.1007/s11947-011-0570-2.
Briones-Labarca, V., Venegas-Cubillos, G., Ortiz-Portilla, S., Chacana-Ojeda, M., & Maureira, H. (2011). Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chemistry, 128(2), 520–529. https://doi.org/10.1016/j.foodchem.2011.03.074.
Buerman, E. C., Worobo, R. W., & Padilla-Zakour, O. I. (2020). High pressure processing of spoilage fungi as affected by water activity in a diluted apple juice concentrate. Food Control, 107, 106779. https://doi.org/10.1016/j.foodcont.2019.106779.
Buzrul, S. (2014). Multi-pulsed high hydrostatic pressure inactivation of microorganisms: A review. Innovative Food Science and Emerging Technologies, 26, 1–11. https://doi.org/10.1016/j.ifset.2014.07.004.
Cacace, F., Bottani, E., Rizzi, A., & Vignali, G. (2020). Evaluation of the economic and environmental sustainability of high pressure processing of foods. Innovative Food Science and Emerging Technologies, 60, 102281. https://doi.org/10.1016/j.ifset.2019.102281.
Cao, X., Bi, X., Huang, W., Wu, J., Hu, X., & Liao, X. (2012). Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innovative Food Science and Emerging Technologies, 16, 181–190. https://doi.org/10.1016/j.ifset.2012.05.008.
Chakraborty, S., Kaushik, N., Rao, P. S., & Mishra, H. N. (2014). High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Comprehensive Reviews in Food Science and Food Safety, 13(4), 578–596. https://doi.org/10.1111/1541-4337.12071.
Chen, D., Pang, X., Zhao, J., Gao, L., Liao, X., Wu, J., & Li, Q. (2015). Comparing the effects of high hydrostatic pressure and high temperature short time on papaya beverage. Innovative Food Science and Emerging Technologies, 32, 16–28. https://doi.org/10.1016/j.ifset.2015.09.018.
Cserhalmi, Z., Sass-Kiss, A., Tóth-Markus, M., & Lechner, N. (2006). Study of pulsed electric field treated citrus juices. Innovative Food Science and Emerging Technologies, 7(1–2), 49–54. https://doi.org/10.1016/j.ifset.2005.07.001.
Donsì, G., Ferrari, G., & Maresca, P. (2010). Pasteurization of fruit juices by means of a pulsed high pressure process. Journal of Food Science, 75(3), E169–E177. https://doi.org/10.1111/j.1750-3841.2010.01535.x.
Eisenmenger, M. J., & Reyes-De-Corcuera, J. I. (2009). High pressure enhancement of enzymes: A review. Enzyme and Microbial Technology, 45(5), 331–347. https://doi.org/10.1016/j.enzmictec.2009.08.001.
EN 12630. (1999). Fruit and vegetable juices. Determination of glucose, fructose, sorbitol and sucrose contents. Method using high-performance liquid chromatography.
Fachin, D., Van Loey, A. M., Ly Nguyen, B., Verlent, I., Indrawati, A., & Hendrickx, M. E. (2003). Inactivation kinetics of polygalacturonase in tomato juice. Innovative Food Science and Emerging Technologies, 4(2), 135–142. https://doi.org/10.1016/S1466-8564(02)00090-5.
Floegel, A., Kim, D.-O., Chung, S.-J., Koo, S. I., & Chun, O. K. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, 24(7), 1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008.
Gao, X., Ohlander, M., Jeppsson, N., Björk, L., & Trajkovski, V. (2000). Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. Journal of Agricultural and Food Chemistry, 48(5), 1485–1490. https://doi.org/10.1021/jf991072g.
Gao, G., Zhao, L., Ma, Y., Wang, Y., Sun, Z., & Liao, X. (2015). Microorganisms and some quality of red grapefruit juice affected by high pressure processing and high temperature short time. Food and Bioprocess Technology, 8(10), 2096–2108. https://doi.org/10.1007/s11947-015-1556-2.
Gao, G., Ren, P., Cao, X., Yan, B., Liao, X., Sun, Z., & Wang, Y. (2016). Comparing quality changes of cupped strawberry treated by high hydrostatic pressure and thermal processing during storage. Food and Bioproducts Processing, 100, 221–229. https://doi.org/10.1016/j.fbp.2016.06.017.
Gosavi, N. S., Salvi, D., & Karwe, M. V. (2019). High pressure-assisted infusion of calcium into baby carrots part II: Influence of process variables on β-carotene extraction and color of the baby carrots. Food and Bioprocess Technology, 12(4), 613–624. https://doi.org/10.1007/s11947-019-2236-4.
Hagerman, A. E., & Austin, P. J. (1986). Continuous spectrophotometric assay for plant pectin methyl esterase. Journal of Agricultural and Food Chemistry, 34(3), 440–444. https://doi.org/10.1021/jf00069a015.
Houben, K., Kermani, Z. J., Van Buggenhout, S., Van Loey, A. M., & Hendrickx, M. E. (2014). Thermal and high-pressure stability of pectin-converting enzymes in broccoli and carrot purée: Towards the creation of specific endogenous enzyme populations through processing. Food and Bioprocess Technology, 7(6), 1713–1724. https://doi.org/10.1007/s11947-013-1166-9.
Hsu, K.-C. (2008). Evaluation of processing qualities of tomato juice induced by thermal and pressure processing. LWT - Food Science and Technology, 41(3), 450–459. https://doi.org/10.1016/j.lwt.2007.03.022.
Huang, H.-W., Chen, B.-Y., & Wang, C.-Y. (2018). Comparison of high pressure and high temperature short time processing on quality of carambola juice during cold storage. Journal of Food Science and Technology, 55(5), 1716–1725. https://doi.org/10.1007/s13197-018-3084-3.
Hurtado, A., Picouet, P., Jofré, A., Guàrdia, M. D., Ros, J. M., & Bañón, S. (2015). Application of high pressure processing for obtaining “fresh-like” fruit smoothies. Food and Bioprocess Technology, 8(12), 2470–2482. https://doi.org/10.1007/s11947-015-1598-5.
Jabbar, S., Abid, M., Hu, B., Muhammad Hashim, M., Saeeduddin, M., Lei, S., Wu, T., & Zeng, X. (2014). Influence of sonication and high hydrostatic pressure on the quality of carrot juice. International Journal of Food Science and Technology, 49(11), 2449–2457. https://doi.org/10.1111/ijfs.12567.
Jolie, R. P., Christiaens, S., De Roeck, A., Fraeye, I., Houben, K., Van Buggenhout, S., et al. (2012). Pectin conversions under high pressure: Implications for the structure-related quality characteristics of plant-based foods. Trends in Food Science and Technology, 24(2), 103–118. https://doi.org/10.1016/j.tifs.2011.11.003.
Keenan, D. F., Brunton, N. P., Mitchell, M., Gormley, R., & Butler, F. (2012). Flavour profiling of fresh and processed fruit smoothies by instrumental and sensory analysis. Food Research International, 45(1), 17–25. https://doi.org/10.1016/j.foodres.2011.10.002.
Klimczak, I., Małecka, M., Szlachta, M., & Gliszczyńska-Świgło, A. (2007). Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. Journal of Food Composition and Analysis, 20(3–4), 313–322. https://doi.org/10.1016/j.jfca.2006.02.012.
Liu, D., Shi, J., Colina Ibarra, A., Kakuda, Y., & Jun Xue, S. (2008). The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT - Food Science and Technology, 41(7), 1344–1349. https://doi.org/10.1016/j.lwt.2007.08.001.
Liu, F., Li, R., Wang, Y., Bi, X., & Liao, X. (2014). Effects of high hydrostatic pressure and high-temperature short-time on mango nectars: Changes in microorganisms, acid invertase, 5- hydroxymethylfurfural, sugars, viscosity, and cloud. Innovative Food Science and Emerging Technologies, 22, 22–30. https://doi.org/10.1016/j.ifset.2013.11.014.
Liu, F., Liao, X., & Wang, Y. (2016). Effects of high-pressure processing with or without blanching on the antioxidant and physicochemical properties of mango pulp. Food and Bioprocess Technology, 9(8), 1306–1316. https://doi.org/10.1007/s11947-016-1718-x.
Mapelli-Brahm, P., Corte-Real, J., Meléndez-Martínez, A. J., & Bohn, T. (2017). Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chemistry, 229, 304–311. https://doi.org/10.1016/j.foodchem.2017.02.074.
Marszałek, K., Mitek, M., & Skąpska, S. (2015). The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innovative Food Science and Emerging Technologies, 27, 48–56. https://doi.org/10.1016/j.ifset.2014.10.009.
Marszałek, K., Woźniak, Ł., & Skąpska, S. (2016). The application of high pressure–mild temperature processing for prolonging the shelf-life of strawberry purée. High Pressure Research, 36(2), 220–234. https://doi.org/10.1080/08957959.2016.1172072.
Maskan, M. (2001). Kinetics of color change of kiwifruits during hot air and microwave drying. Journal of Food Engineering, 48(2), 169–175. https://doi.org/10.1016/S0260-8774(00)00154-0.
Melendez-Martinez, A. J., Stinco, C. M., Liu, C., & Wang, X.-D. (2013). A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chemistry, 138(2–3), 1341–1350. https://doi.org/10.1016/j.foodchem.2012.10.067.
Miȩkus, N., Iqbal, A., Marszałek, K., Puchalski, C., & Świergiel, A. (2019). Green chemistry extractions of carotenoids from Daucus carota L.–Supercritical carbon dioxide and enzyme-assisted methods. Molecules, 24(23). https://doi.org/10.3390/molecules24234339.
Opazo-Navarrete, M., Tabilo-Munizaga, G., Vega-Gálvez, A., Miranda, M., & Pérez-Won, M. (2012). Effects of high hydrostatic pressure (HHP) on the rheological properties of Aloe vera suspensions (Aloe barbadensis Miller). Innovative Food Science and Emerging Technologies, 16, 243–250. https://doi.org/10.1016/j.ifset.2012.06.006.
Patras, A., Brunton, N., Da Pieve, S., Butler, F., & Downey, G. (2009). Effect of thermal and high pressure processing on antioxidant activity and instrumental color of tomato and carrot purées. Innovative Food Science and Emerging Technologies, 10(1), 16–22. https://doi.org/10.1016/j.ifset.2008.09.008.
Picouet, P. A., Sárraga, C., Cofán, S., Belletti, N., & Dolors Guàrdia, M. (2015). Effects of thermal and high-pressure treatments on the carotene content, microbiological safety and sensory properties of acidified and of non-acidified carrot juice. LWT - Food Science and Technology, 62(1), 920–926. https://doi.org/10.1016/j.lwt.2014.07.027.
Picouet, P. A., Hurtado, A., Jofré, A., Bañon, S., Ros, J.-M., & Guàrdia, M. D. (2016). Effects of thermal and high-pressure treatments on the microbiological, nutritional and sensory quality of a multi-fruit smoothie. Food and Bioprocess Technology, 9(7), 1219–1232. https://doi.org/10.1007/s11947-016-1705-2.
Pires, T. D. C. R., & Finardi-Filho, F. (2005). Extraction and assay of pectic enzymes from Peruvian carrot (Arracacia xanthorriza Bancroft.). Food Chemistry, 89(1), 85–92. https://doi.org/10.1016/j.foodchem.2004.02.023.
Pokhrel, P. R., Toniazzo, T., Boulet, C., Oner, M. E., Sablani, S. S., Tang, J., & Barbosa-Cánovas, G. V. (2019). Inactivation of Listeria innocua and Escherichia coli in carrot juice by combining high pressure processing, nisin, and mild thermal treatments. Innovative Food Science and Emerging Technologies, 54, 93–102. https://doi.org/10.1016/j.ifset.2019.03.007.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3.
Reiter, M., Neidhart, S., & Carle, R. (2003a). Sedimentation behaviour and turbidity of carrot juices in relation to the characteristics of their cloud particles. Journal of the Science of Food and Agriculture, 83(8), 745–751. https://doi.org/10.1002/jsfa.1367.
Reiter, M., Stuparić, M., Neidhart, S., & Carle, R. (2003b). The role of process technology in carrot juice cloud stability. LWT - Food Science and Technology, 36(2), 165–172. https://doi.org/10.1016/S0023-6438(02)00221-9.
Sila, D. N., Van Buggenhout, S., Duvetter, T., Fraeye, I., De Roeck, A., Van Loey, A., & Hendrickx, M. (2009). Pectins in processed fruits and vegetables: Part II-Structure-function relationships. Comprehensive Reviews in Food Science and Food Safety, 8(2), 86–104. https://doi.org/10.1111/j.1541-4337.2009.00071.x.
Steffe, J. F., Mohamed, I. O., & Ford, E. W. (1983). Rheological properties of fluid foods. In Paper - American Society of Agricultural Engineers. Retrieved from https://www.scopus.com/inward/record.uri?eid=2-s2.0-0020936174&partnerID=40&md5=692037c845a85fdb0375f590c9c3e5a8.
Stinco, C. M., Szczepańska, J., Marszałek, K., Pinto, C. A., Inácio, R. S., Mapelli-Brahm, P., & Meléndez-Martínez, A. J. (2019). Effect of high-pressure processing on carotenoids profile, color, microbial and enzymatic stability of cloudy carrot juice. Food Chemistry, 299, 125112. https://doi.org/10.1016/j.foodchem.2019.125112.
Sun, Y., Kang, X., Chen, F., Liao, X., & Hu, X. (2019). Mechanisms of carrot texture alteration induced by pure effect of high pressure processing. Innovative Food Science and Emerging Technologies, 54, 260–269. https://doi.org/10.1016/j.ifset.2018.08.012.
Szczepańska, J., Barba, F. J., Skąpska, S., & Marszałek, K. (2020). High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and color. Food Chemistry, 307. https://doi.org/10.1016/j.foodchem.2019.125549.
Torres-Ossandón, M. J., Vega-Gálvez, A., López, J., Stucken, K., Romero, J., & Di Scala, K. (2018). Effects of high hydrostatic pressure processing and supercritical fluid extraction on bioactive compounds and antioxidant capacity of Cape gooseberry pulp (Physalis peruviana L.). Journal of Supercritical Fluids, 138, 215–220. https://doi.org/10.1016/j.supflu.2018.05.005.
Trejo Araya, X. I., Smale, N., Zabaras, D., Winley, E., Forde, C., Stewart, C. M., & Mawson, A. J. (2009). Sensory perception and quality attributes of high pressure processed carrots in comparison to raw, sous-vide and cooked carrots. Innovative Food Science and Emerging Technologies, 10(4), 420–433. https://doi.org/10.1016/j.ifset.2009.04.002.
Varela-Santos, E., Ochoa-Martinez, A., Tabilo-Munizaga, G., Reyes, J. E., Pérez-Won, M., Briones-Labarca, V., & Morales-Castro, J. (2012). Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innovative Food Science and Emerging Technologies, 13(JANUARY), 13–22. https://doi.org/10.1016/j.ifset.2011.10.009.
Vinson, J. A., Su, X., Zubik, L., & Bose, P. (2001). Phenol antioxidant quantity and quality in foods: Fruits. Journal of Agricultural and Food Chemistry, 49(11), 5315–5321. https://doi.org/10.1021/jf0009293.
Wootton-Beard, P. C., Moran, A., & Ryan, L. (2011). Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Research International, 44(1), 217–224. https://doi.org/10.1016/j.foodres.2010.10.033.
Yen, G.-C., & Chen, H.-Y. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry, 43(1), 27–32. https://doi.org/10.1021/jf00049a007.
Zhang, Y., Liu, X., Wang, Y., Zhao, F., Sun, Z., & Liao, X. (2016). Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innovative Food Science and Emerging Technologies, 33, 135–144. https://doi.org/10.1016/j.ifset.2015.10.012.
Zhao, L., Wang, Y., Qiu, D., & Liao, X. (2014). Effect of ultrafiltration combined with high-pressure processing on safety and quality features of fresh apple juice. Food and Bioprocess Technology, 7(11), 3246–3258. https://doi.org/10.1007/s11947-014-1307-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szczepańska, J., Skąpska, S., Lorenzo, J.M. et al. The Influence of Static and Multi-Pulsed Pressure Processing on the Enzymatic and Physico-Chemical Quality, and Antioxidant Potential of Carrot Juice During Refrigerated Storage. Food Bioprocess Technol 14, 52–64 (2021). https://doi.org/10.1007/s11947-020-02577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02577-9