Abstract
Sioma® is a variety of red palm oil produced in Ecuador; it is mainly unsaturated, has no flavor, odor, nor cholesterol, and it is GMO-free and free of trans fatty acids. The main objectives of this study were: (a) to study changes in fatty acids, color coordinates, total carotenoids and carotenoid composition during deep-frying simulations; (b) to develop a mathematical model that allows quantification of total carotenoids (antioxidant compounds) using routine color measurements. Two different deep-frying temperatures were assayed 180 and 240 °C. The main fatty acids and carotenoids found in this oil were: (a) oleic, palmitic, and linoleic acids, and (b) α-, β-, and δ-carotenes. During these deep-frying simulations, Sioma® oil became lighter and more yellowish (L* and b* values increased) and more greenish (a* values decreased); these changes were more evident for higher temperatures. At 180 °C, total carotenoids decreased linearly, from almost 500 mg L−1, at a rate of 5 mg /kg1 min−1; however, at 240 °C the degradation of carotenoids was almost complete after 40 min. Finally, the mathematical models developed using multiple linear regressions allowed an easy and fast quantification of total carotenoids using color measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep-fat frying is a widely used food process, which basically consists of immersion of food pieces in hot vegetable oil [1]. The correct choice of oil for frying is important for two reasons. Firstly, the oil is used as the heat transfer medium during frying and must not only be able to withstand frying temperature but also have a high enough stability to allow it to be used repeatedly. Secondly, products being fried will take up some of the oil, and therefore the oil also needs to maintain a high oxidative stability during the life of the product, and be both palatable and nutritious [2].
During frying, a range of reactions occurs within the oil (for instance, oxidation polymerization, darkening and hydrolysis); these physico-chemical reactions together with the fatty acid composition and the presence of antioxidant compounds in the oil will affect the frying-life of the oil. In addition, components in the food being fried can also have an effect on the end quality of the oil.
In general, the optimum frying oil should be non-hydrogenated (for nutritional reasons), have a high oxidative stability (for frying-life reasons) and contain limited amount of linoleic acid. Palm oil and particularly Sioma® oil fulfill all of these requirements [2]. Besides, the oil used in this study, Sioma® oil, is free of cholesterol, trans fatty acids, and GMO, and has no odor or flavor, which can disturb the typical odor/flavor of food being fried.
The oils preferred for frying have changed considerably over the years and reflect flavor preferences, nutritional requirements, oil stability and economics. For example, animal fats have now been widely replaced by vegetable oils. This change has been mainly on the grounds of nutritional requirements; animal fats being rich in saturated fat, despite the good flavor they impart to the food, and vegetable oils having a lowering effect on cholesterol [3]. Partially hydrogenated vegetal oils (PHVO) were once widely used in industrial frying because of their high oxidative stability. Over the past few years, however, the use of such oils has decreased significantly, because of the effects of trans fatty acids on cardiovascular health [2]. PHVO were initially replaced by vegetable oils such as rapeseed oil and soya bean oil, but their low oxidative stability, especially when used repeatedly at frying temperatures, has lead to other choices, such as palm oil [2].
In contrast to animal fats which are predominantly saturated and hence do not react readily with other chemicals especially oxygen, unsaturated vegetable oils are more reactive. Exposure to air [4]), heat [5], light [6], trace metals [7] and moisture enhances their chemical reactivity [3].
Antioxidants naturally present in unsaturated oils can help to extend their frying-life and the shelf-life of fried foods. Natural antioxidants such as carotenoids, tocopherols, and tocotrienols are especially useful in prolonging the frying-life of oils. Oils containing naturally high levels of these components are maize oil, cotton seed oil, soya bean oil and especially red palm oil, including Sioma ® oil [2, 8].
Palm oil is the second largest vegetable oil in terms of world production, and today, about 50% of traded oils in the world is palm oil [9]. Particularly high levels of antioxidant compounds, mainly carotenoids, are found in Sioma® oil because of its careful deodorization process [10]. In this way, the high levels of natural antioxidants have become palm oil, and therefore Sioma® oil, as a perfect ingredient for functional foods [11, 12]. For instance, palm oil has been used for the improvement of the vitamin A status in children at risk of vitamin A deficiency in India [13]. However, most of these natural antioxidants are thermo-sensitive and their concentration will decrease during their use as deep-frying medium. This is an important issue that needs research.
The first objective of this study was to study the degradation of the naturally present carotenoids in Sioma® oil and describe the changes in the fatty acid composition and color. The second and final objective of this study was to develop a mathematical model (multiple linear regression) that allows food industrials to know exactly and easily the content of total carotenoids (natural antioxidants) present in their Sioma® oil during deep-frying operations based on the measurements of routine color coordinates. In this way, people of the quality control departments of the frying-companies will easily determine when their oil should be changed and if required to know the exact content of carotenoids present in the frying oil for a particular batch of samples.
Materials and methods
Materials
Sioma® oil elaborated in Ecuador was provided by Industrial Danec S.A (Quito, Ecuador). Standards of α-carotene and β-carotene were obtained from Sigma Chemical Co. (St Louis, MO, USA). Lutein and β-cryptoxanthin standards were obtained from Extrasynthese (Genay, France).
The experiment was carried out at two different temperatures to show the temperature dependence of degradation of carotenoids and fatty acids, and changes in color. The first one, 180 °C, is the optimal temperature of deep-frying processes. The smoke points of the studied oils were 205, 230, and 235 °C for extra virgin olive, Sioma® and sunflower oils, respectively. Therefore, a temperature above these smoke points (physical degradation of the oils) was needed; the selected temperature was 240 °C.
Color measurement
Color determinations were made, at 25 ± 1 °C, using a Minolta CR-300 colorimeter (Minolta, Osaka, Japan). This spectrophotometer uses an illuminant D65 and a 10° observer as references and an aperture diameter of 11 mm.
Color data are provided as CIELab coordinates [14, 15], which define the color in a three-dimensional space. L* indicates lightness and a* and b* are the chromaticity coordinates, green-red and blue-yellow coordinates, respectively. L* is an approximate measurement of luminosity, which is the property according to which each color can be considered as equivalent to a member of the gray scale, between black and white, taking values within the range 0–100; a* takes positive values for reddish colors and negative values for the greenish ones, whereas b* takes positive values for yellowish colors and negative values for the bluish ones.
C* is chroma \( {\left[ {C^{*}\; = \;{\sqrt {{\left( {a^{*2} } \right)} + {\left( {b^{*2} } \right)}} }} \right]}, \) and is 0 at the center of a color sphere and increases according to the distance from the center. Finally, h ab is the hue angle \( {\left[ {h_{{{\text{ab}}}} \; = \;arc\;tg\;{\left( {\frac{{b^{*}}} {{a^{*}}}} \right)}} \right]}, \) which is defined as starting at the +a* axis and is expressed in degrees; 0° would be +a* (red), 90° would be +b* (yellow), 180° would be −a* (green), and 270° would be −b* (blue) [14, 15].
All color analyses were run five times in three different oil samples (total of 15 measurements per value) and results were expressed as “mean ± standard error”.
Total carotenoids
An aliquot of Sioma® oil (15 mL) was homogenized in a Kika Labortechnik, T25 basic homogenizer (Jankel & Kunkel Gmbh & Co, Staufen, Germany) with 50 mL of extracting solvent (hexane:acetone:ethanol, 50:25:25, v/v) and centrifuged (Heraeus BioFuge, Heraeus Instruments, Hanau, Germany) for 5 min at 6,500 rpm at 5 °C. The top layer of hexane containing the color was recovered and transferred to a 25-mL volumetric flask. The volume of recovered hexane was then adjusted to 25 mL with hexane [16].
Total carotenoid determination was carried out on an aliquot of the hexane extract by measuring the absorbance at 450 nm in a UV-Vis Uvikon XS spectrophotometer (Bio-Tek Instruments, Saint Quentin Yvelines, France). Total carotenoids were calculated using calibration curves of β-carotene (Sigma) [16, 17].
Carotenoid profiles
Samples were first saponified by adding 5 mL methanol to 400 mg of the oil sample in a 25 mL test tube. This was followed by 0.3 mL of 60 % aqueous potassium hydroxide and 1 mL of 0.5 % BHT as an antioxidant. The mixture was sealed under nitrogen and heated at 100 °C for 30 min with occasional shaking. After cooling, 10 mL of water and 10 mL diethyl ether were added and the mixture was shaken for 1 min. The ether layer was then transferred to a 125 mL separatory funnel containing 10 mL water. The soap solution was extracted two more times with 10 mL ether. The separatory funnel was gently swirled to prevent the formation of an emulsion [18].
After drawing off the water layer, the ether layer was washed three more times with 10 mL water each. Then 10 mL aqueous 0.5 N KOH solution was added followed by 10 mL water for washing. This KOH addition and water washing was repeated two more times followed by five more times of water washing or until the water wash was neutral. The ether layer was dried by adding anhydrous Na2SO4 and then filtered through 0.2 mm filter. The solvent was evaporated to dryness under nitrogen and the extract was kept at −20 °C until analysis. Upon analysis, the extract was dissolved in the HPLC mobile phase and 20 μL of sample was injected [18].
The HPLC system consisted of an HP-1100 Series unit with a photodiode array detector equipped with HP ChemStation software (Hewlett Packard, Palo Alto, CA, USA). The column used was a 250 mm × 4.6 mm i.d., YMC C30, S-5 μm (YMC, Schermbeck, Germany). The mobile phase for this column was 81:15:4 methanol:methyl tertiary butyl ether (MTBE):H2O (solvent A) and 91:9 MTBE: methanol (solvent B). The gradient elution was 100% A to 50% A and 50% B in 45 min followed by 100% B in the next 10 min and 100% A in the next 5 min at a flow rate of 0.8 mL min−1 [18]. Carotenoids were monitored at 450 nm.
Fatty acids analysis
Fatty acids were analyzed by GC-MS after derivatization to fatty acid methyl esters (FAMEs) with KOH 2 N in methanol, according to the IUPAC standard method [19].
A Shimadzu GC-17A coupled with a Shimadzu mass spectrometer detector GC-MS QP-5050A and a Tracsil Meta .X5 (Teknokroma, Barcelona, Spain) column (30 m × 0.25 mm × 0.25 μm film thickness) were used. Samples (0.2 μL) were injected in split mode (split/column flow ratio 40:1) and helium was used as carrier gas at a flow rate of 0.8 mL min−1. The injection temperature was 250 °C and the oven temperature program was 180 °C (4 min), and 4 °C min−1 up to 230 °C (15 min) [20, 21].
The output from the GC column entered the ionization chamber of the mass spectrometer via an interface tube maintained at 250 °C. Mass spectrometry (EI, 70 eV, ion source temperature 200 °C) was performed in full scan between 35 and 450 amu. The GC-MS apparatus was linked to a PC running software (LabSolutions, GCMSsolution version 1.01; Shimadzu Corporation, Kyoto, Japan) and spectrometric data were compared with those of the Wiley 229 library mass-spectra. Under the GC-MS conditions used, FAMEs eluted in order of increasing molecular weight and, for a given molecular weight, in order of decreasing saturation [20, 21].
Statistical analyses
A theoretical modeling for predicting the value of the total carotenoids in Sioma® oil was established with a multiple linear regression [22] using experimental values of the CIELab color coordinates:
Statistical analyses were carried out using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Changes on physico-chemical parameters during deep-frying simulation
Color
As the deep-frying simulation moved forward, the Sioma® oil become lighter as described by increases in the values of L*, lightness (Fig. 1a). Lightness values increased from 26.54 at 0 min up to 29.15 at 90 min (mean values for both studied temperatures) (Table 1); however, the increase of L* was more evident for the highest temperature assayed, 240 °C. The blue-yellow coordinate, b*, showed a similar behavior to that previously described for L* during the deep-frying simulations (Fig. 1c). Mean values of b* (both temperatures) increased from 1.82 at 0 min to 5.96 after 90 min of oil heating (Table 1). Again significantly higher values of b* were found for 240 °C compared to 180 °C, especially within the time range between 10 and 70 min (Fig. 1c). On the other hand, the values of the green-red coordinate, a*, decreased as the deep-frying process moved forward. Values decreased from 4.63 at 0 min down to 1.51 at 90 min (Table 1); always the oil heated at 240 °C showing lower values of a* than the oil heated at 180 °C (Fig. 1b). Finally, the values of both C* and h ab increased during the deep-frying process at both assayed temperatures (Fig. 2). In summary, the intense red color of the initial Sioma® oil became more greenish and yellowish oil. This loss of the red color is without a doubt a reflection of the carotenoids degradation due to the heat treatment of the oil during the deep-frying simulation.
Fatty acids, FA
In general, the fatty acids profile of Sioma® oil was dominated by oleic acid (62.3%), followed by palmitic acid (25.5%), linoleic and stearic acids (7.6 and 3.2%, respectively) (Table 2). This profile clearly justifies why Sioma® oil is considered an unsaturated oil; its initial composition presented 29.6% saturated FA, 62.8% of mono-unsaturated FA, and 7.6% poly- unsaturated FA (total of 70.4% of unsaturated FA). Contrarily to what some people may think, Sioma® oil have contents of saturated FA much lower than other fats and oils such as oil of African palm (48%), butter (58%), cacao butter (60%), and coconut oil (85%).
Table 2 presents the FA composition of the two oils more frequently used in Spain for deep-frying operations, sunflower and extra virgin olive oil. Extra virgin olive oil can be considered as a mono-unsaturated oil (17.4, 79.2 %, and 3.5 saturated, mono-unsaturated, and poly-unsaturated FA) while sunflower is clearly poly-unsaturated oil (12.1, 28.5 %, and 59.5 saturated, mono-unsaturated, and poly-unsaturated FA).
The FA composition of Sioma® oil did not change significantly after 90 min of deep-frying simulation at neither of the two temperatures assayed, 180 nor 240 °C. Perhaps the time used in these simulations was not long enough for causing significant effects on the FA profiles of the Sioma® oil due to the high amount of antioxidant compounds (carotenoids and tocopherols) present in this oil.
Total carotenoids
The total content of carotenoids found in Sioma® oil was 481 ± 20 mg L−1. This value is extremely high when compared to other oils, especially those widely used for frying purposes in Spain: (a) sunflower (8.5 ± 0.7 mg L−1) and (b) extra virgin olive oil (11.0 ± 0.3 mg L−1). These experimental values represent that Sioma® oil has approximately 57 times more carotenoids (antioxidant compounds) than the studied sunflower oil and approximately 44 times more carotenoids than the studied extra virgin olive oil.
Darnoko et al. [18] reported a total content of 381.2 mg carotenoids L−1 in red palm oil (Nutrolein, produced in Malaysia). On the other hand, the values reported in this study for the extra virgin olive oil are slightly lower than those reported by Giuffrida et al. [23] for Sicilian monovarietal virgin olive oils. These authors found 27.4, 32.0 and 19.7 mg carotenoids L−1 in virgin olive oils from the olive varieties Cerasuola, Nocellara and Biancolilla, respectively.
The degradation of carotenoids followed an almost linear trend when the Sioma® oil was heated at 180 °C, optimum temperature of deep-frying processes (Fig. 3). However, if the deep-frying temperature is increased up to 240 °C (temperature above its smoke point, 230 °C), the carotenoid degradation followed an exponential decay pattern. In this way, almost all carotenoids were lost after just 40 min; only remained 13.6 ± 7.7 mg carotenoids L−1 (at 240 °C) while at 180 °C remained 230 ± 3 mg carotenoids L−1. Figure 3 clearly showed how important is to have a right control over the temperature during deep-frying processes; if temperatures higher than the smoke point are used, not only the degradation of the oil will be important and higher emission rates of aldehydes will occur [20, 21] but also the degradation of the antioxidants present in the initial oil will be more significant.
Carotenoid profile
As many as nine different carotenoids were identified in Sioma® oil (Table 3 and Fig. 4). Identification was based on the order of elution, retention time, and spectra of absorbance maxima of a particular peak. These data were compared with information in the literature especially the study by Darnoko et al. [18]; these authors developed a HPLC analysis for the simultaneous quantification of carotenoids and tocopherols. Some of the peaks can only be tentatively identified due to limited information for comparison.
In comparison with classical C-18 stationary phase, the use of the much more hydrophobic C-30 phase has shown a better resolving power for compounds with a clear hydrophobic character, such as carotenoids [23].
Darnoko et al. [18] identified 13 different carotenoids in red palm oil from Malaysia. In the present study only 9 of these 13 carotenoids were found; the three compounds missing compounds in Sioma® oil were β-cryptoxanthin, 9,15-dicis-β-carotene, and 9-cis-β-carotene. These differences in the carotenoids compositions could be due to genetic factors (palm variety), environmental conditions, stage of fruits ripening, extraction process and storage conditions.
Lutein has been previously identified in red palm oil by Leitz and Henry [24] and Darnoko et al. [18] and was the first carotenoid resolved in this study (26.59 min). However, other researchers did not observe lutein in palm oil, perhaps due to differences in the extraction and saponification methods; lutein is a polar carotenoid which has limited solubility in non-polar solvents.
It is not clear whether a-carotene-5,6-epoxide occurs naturally in Sioma® oil or if it is a product of carotenoid oxidation taking place during sample preparation or storage.
γ-Carotene and ε-carotene were tentatively identified based on their spectral maxima and these carotenoids have also been reported by other researchers in palm oils [25, 26].
The most important carotenoids in Sioma® oil were hydrocarbon carotenoids (α-, β-, and δ-carotenes) with their geometric isomers. The major single carotenoid found in Sioma® oil was α-carotene and was resolved at 32.65 min; it represented approximately 47% of the total carotenoids (mean of experimental values for both assayed temperatures and times 0, 10, and 90 min). The β- and δ-carotenes were resolved at 33.95 and 36.85 min, and were the second and third most abundant carotenoids, representing about 17 and 14% of total carotenoids. The geometric isomers of this compound are 15-cis-β-carotene and 9-cis-β-carotene. Finally, two geometric isomers of β-carotene were also resolved at 30.81 and 36.25 min, 15-cis-β-carotene and 9-cis-β-carotene, respectively.
The changes of the individual carotenoids during the deep-frying simulations are depicted in Figs. 4a (180 °C), b (240 °C). The deep-frying temperature did not have a differential effect on individual carotenoids; all of them were degraded in a similar way. In this way, α-, β-, and δ-carotenes were always the predominant carotenoids. No carotenoids could be identified after 90 min of oil heating at 240 °C, due to the complete degradation of these antioxidant compounds.
The presence of a specific carotenoid profile in palm oils, including Sioma® oil, could be used to guarantee the genuineness and typicality of the product, since the quality control of foods requires a precise knowledge of the antioxidant composition of the original products.
Mathematical modeling
Total carotenoid can be determined after extracting the oil using a mixture of organic solvents and measurement of absorbance in a spectrophotometer Lee and Castle [16]. This method is more complicated than the routine measurement of CIELab color coordinates.
Predicting the value of one variable based on the values of one or more other variables has become commonplace [27]. Regression analysis is used to relate the value of a continuous dependent variable to the values of one or more independent variables. In the present study, the experimental values of total carotenoids (mg L−1) have been mathematically described using experimental values of the CIELab color coordinates (L*, a*, and b*) (Table 1). The inclusion of C* and h ab did not improve the model.
The regression findings were presented in standardized regression coefficient form and the variables shown to be significant beyond the 0.05 level were identified with an asterisk:
where Y = total carotenoid (mg L−1); X 1 = time (min); X 2 = L*; X 3 = a*; X 4 = b*; X 5 = C*; X 6 = h ab*.
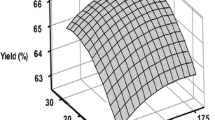
In this particular case, the response (Y) was significantly influenced by six different independent variables. The coefficients of determination, R 2, of these multiple linear regressions were 0.979 and 0.958, which means that 97.9 and 95.8% of the variance of the dependent variable were explained by these two temperature-dependent models (180 and 240 °C, respectively). The adjusted R 2 were 0.977 and 0.947, respectively; in this way the two statistics (R 2 and adjusted R 2) for each model agreed qualitatively, implying that the models were good. Figure 5 shows the relationship among the experimental and the modeled total carotenoids in samples of Sioma® oil at 180 and 240 °C and shows how good the models were.
Three independent variables with a higher weight (identified with an asterisk) in the model were time, green-red coordinate (a*), and blue-yellow coordinate (b*). According to visual observation of the color changes during the deep-frying simulations, the initial oil has an intense red color and becomes more yellow and green with time; thus, it was expected that the red and yellow coordinates changed with frying time as it happened.
The mathematical models developed using multiple linear regressions in this study (especially that at 180 °C, optimum temperature of deep-frying operations) will be of great help to the people responsible for Quality Control in the agro-food industry. They will be able to quantify how much of the initial content of carotenoids is still in their Sioma® oil just be running a routine color analysis, which will only take few seconds.
Conclusions
The fatty acids and carotenoids profiles of Sioma® oil were dominated by: (a) oleic, palmitic, and linoleic acids, and (b) α-, β-, and δ-carotenes. During deep-frying simulations, Sioma® oil (initially of intense reddish color) became lighter and more yellowish (L* and b* values increased) and greenish (a* values decreased); these changes were more evident as the frying temperature increased from 180 to 240 °C. At 180 °C total carotenoids decreased linearly, at a rate of 5 mg kg1 min−1, from 481 mg L−1 (0 min) down to 45 mg L−1 (90 min); however, at 240 °C the degradation of carotenoids was almost complete after 40 min. Finally, the mathematical models developed using multiple linear regressions (especially that at the optimal temperature of deep-frying operations, 180 °C) will allow an easy and fast quantification of the total carotenoids remaining in the oil only using routine color information.
References
Moyano PC, Pedreschi F (2006) LWT 39:285–291
Talbot G, Zand I (2006) Food Eng Ingr 8:34–35
Naz S, Siddiqi R, Sheikh H, Sayeed SA (2005) Food Res Int 38:127–134
Paz I, Molero M (2001) Afinidad 58:190–196
Fukumoto Y, Iibuchi S (2001) Kaseikei-Hen 40:25–35
Chenh XC, Ahn DU. (1998) J Amer Oil Chem Soc 75:1717–1721
Anderson K, Lingnert H (1998) J Amer Oil Chem Soc 75:1041–1046
Farombi EO, Britton G (1999) Food Chem 64:315–321
Schroeder MT, Miquel-Becker E, Skibsted LH (2006) J Agric Food Chem 54:3445–3453
Andreu-Sevilla A, Sayas E, Hartmann A, Carbonell-Barrachina AA (2007) Alimentación, Equipos y Tecnología 223(5):64–68
Reddy V (2000) Food Nutr Bull 21:247
Al Hooti SN, Sidhu JS, Al Saqer JM, Al Amiri HA, Al Othman A, Mansour IB, Johari M (2004) Int J Food Prop 7:15–25
van Stuijvenberg ME, Faber M, Dhansay MA, Lombard CJ, Vorster N, Benadé AJS (2000) Int J Food Sci Nutr 51:S43-S50
Minolta (1994) Precise color communication. Color control from feeling to instrumentation. Minolta Co Ltd, Osaka, Japan
Pérez-López A, Beltrán F, Serrano-Megias M, Saura D, Carbonell-Barrachina AA (2006) Eur Food Res Technol 222:516–520
Lee HS, Castle WS (2001) J Agric Food Chem 49:877–882
Ritter ED, Purcell AE (1981) Carotenoid analytical methods. In: Bauernfeind JC (ed) Carotenoids as colorants and vitamin A precursors. Academic, New York, pp 815–923
Darnoko D, Cheryan M, Moros E, Jerrel J, Perkins EG (2000) J Liq Chrom Rel Technol 23:1873–1885
IUPAC (International Union of Pure, Applied Chemistry) (1992) Standard methods for the analysis of oils, fats and derivatives, 7th edn. Pergamon Press, Oxford, UK
Fullana A, Carbonell-Barrachina AA, Sidhu S (2004) J Agric Food Chem 52:5207–5214
Fullana A, Carbonell-Barrachina AA, Sidhu S (2004) J Sci Food Agric 84:2015–2021
Verdú A, Vázquez-Araújo L, Carbonell-Barrachina AA (2006) Eur Food Res Technol (accepted for publication)
Giuffrida D, Salvo F, Salvo A, La Pera L, Dugo G (2007) Food Chem 101:833–837
Leitz G, Henry CJK (1997) Food Chem 60:107–117
Ng JH, Tan B (1988) J Chrom Sci 26:463–469
Tan B, Grady CM, Gawienowski AM (1986) J Amer Oil Chem Soc 63:1175–1179
Meilgaard M, Civille GV, Carr BT (1999) Sensory evaluation techniques, 3rd edn. CRC Press, Boca Raton
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreu-Sevilla, A., Hartmann, A., Sayas, E. et al. Mathematical quantification of total carotenoids in Sioma® oil using color coordinates and multiple linear regression during deep-frying simulations. Eur Food Res Technol 226, 1283–1291 (2008). https://doi.org/10.1007/s00217-007-0656-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0656-2