Abstract
A rapid lateral flow immunochromatographic strip (ICS) using fluorescein isothiocyanate (FITC) labeled antigen and antibody was developed for the detection of Acidovorax citrulli (Ac) in melons and vegetable samples. In the ICS, signal amplification was realized based on antigen Ac and anti-Ac monoclonal antibody (McAb) 4F conjugated with FITC, respectively, which were forming two probes. The control line and the test line were obtained by immobilizing the goat anti-mouse IgG antibody and anti-Ac McAb 6D on both sides of the nitrocellulose membrane. The visual detection limit of the strip was 105 CFU/mL, which was 10-fold sensitive compared to the strip of FITC only labeling antigen or antibody. Signal amplification ICS was successfully applied to the detection of Ac in melon and vegetable samples with less detection time and operation procedures compared to the traditional enzyme-linked immunosorbent assay (ELISA) and PCR methods. This is the first report of using FITC labeled antigen and McAb as dual fluorescent probes to develop a direct-type immunofluorescence strip for the rapid and sensitive detection of Ac, which demonstrates a powerful tool for rapidly screening Ac in plant materials and other samples.

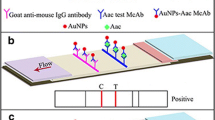
The schematic presentation of the test strip (a) and the positive result (b) or negative result (c) of the test strip
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial fruit blotch (BFB) caused by gram-negative bacterium Acidovorax citrulli (Ac) is a devastating bacterial disease to many cucurbitaceous plant species, e.g., watermelon, melon, cucumber, pumpkin, and other cucurbits [1]. Ac, as a seed-borne pathogen, can infect seeds, seedlings, and fruits, and the infested seeds are usually the most important source for BFB outbreaks [2]. Since the first report in commercial products in 1965 [3], BFB has caused millions of dollars of loss around the world, such as United States, Australia, Brazil, Turkey, Japan, Thailand, Israel, Iran, Hungary, Greece, and China. So far, there are still no commercial species that could completely resist BFB [4]. Hence, relatively effective BFB management is early detection, diagnosis, and clearance of Ac from all commercial seeds and seedlings.
As we all know, membrane-based lateral flow immunochromatographic strip (ICS) has been a powerful tool for simple, rapid, and low-cost detection. The assay procedure could be significantly simplified to avoid the requirements for a long incubation time and complex operation steps, which is especially suitable for personal detection or in places where complicated equipment is not available. Lately, ICS tests based on colloidal gold as a tracer have been widely used for the detection of foodborne pathogens [5], mycotoxins [6], pesticide residues [7], heavy metal ions [8], prohibited additives [9], antibiotics residues [10] etc. However, ICS based on colloidal gold shows a serious limit when high sensitivity is needed. Many strategies, such as selecting new labels, e.g., quantum dot [11] and upconverting nanophosphors [12], combining with other reagent or other techniques [13], e.g. magnetic capture [14] and PCR-based methods [15], designing new patterns [16], and developing signal amplification system [17], were applied to enhance the sensitivity of lateral flow immunoassay.
Fluorescein isothiocyanate (FITC), a fluorochrome dye, which could emit a visible yellow-green light after absorbing blue or ultraviolet light. As a fluorescent probe, FITC has been widely used to show the intracellular localization of pathogens [18], visualize live cells [19], trace drugs in vivo delivery [20, 21], estimate in vivo counts during passage of probiotics [22], and other fields. FITC was also regarded as a promising tracer for immunochromatographic assay duo to the unique properties, such as strong luminescence, photo-stability, satisfactory labeling effect, and low cost. These properties make it possible to be a new label material for developing highly sensitive ICS assays.
Recently, monoclonal antibody (McAb) 4F and 6D against Ac have been prepared in our lab, which possessed sufficient sensitivity and specificity for the analysis of Ac. In this study, a novel signal amplification fluorescent immunoassay method based on a common fluorochrome dye, FITC, was developed. ICS was successfully applied with the target bacteria as a novel fluorescent probe after incubation with FITC and the FITC labeled McAb 4F as the second fluorescent probe. The results indicated that the dual labeled method could enhance the sensitivity for the detection of Ac, and the assay would be a useful tool for the early diagnosis, prevention, and control of BFB in the future.
Materials and methods
Bacterial strains and materials
The McAbs (6D and 4F) against Ac were produced in our lab as previously reported [23]. Strains used for testing the cross-reaction of the strip are listed in Table 1. All the strains were obtained from the Chinese Academy of Inspection and Quarantine (Beijing, China). Brain heart infusion (BHI) medium was purchased from Land Bridge (Beijing, China). The nitrocellulose (NC) membrane, sample pad, conjugate pad, and absorbent pad were purchased from Millipore (Bedford, MA, USA). FITC ≥ 90% (HPLC) and dimethylsulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA). All solvents and other chemicals were of analytical reagent grade.
Conjugate of antibodies to FITC
Bicarbonate/carbonate buffer at pH 9.0 was used to dilute the purified monoclonal antibody (McAb) 4F from 10 mg/mL to 4 mg/mL. Freshly prepared FITC solution dissolved in DMSO was added slowly dropwise to the antibody solution until a final concentration of about 150 μg FITC per mg McAb. The mixture was kept in the dark and reacted at room temperature for 2 h. Then the reaction mixture was dialyzed to separate the conjugated antibody from the free FITC. The labeled antibody was stored at 4 °C before use.
Bacteria culture and sample pretreatment
Freshly prepared FITC solution dissolved in DMSO was added to BHI medium, which was used for bacteria culture. A single colony was added to the modified BHI (45 mL) containing plant juice sample (5 mL) and incubated at 37 °C overnight. Bacterial concentration was diluted by sterile saline and determined by plate count or by microplate reader (SpectraMax M2; Molecular Devices, Sunnyvale, CA, USA). The fluorescence intensity of bacteria was determined by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
The optimum concentration of FITC
The concentration of FITC solution for sample incubation could influence the background and final fluorescence intensity of the test line. Freshly prepared FITC solution was diluted to different concentrations of 10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, 300 μg/mL, 400 μg/mL to culture Ac. After incubating at 37 °C for 12 h, fluorescence intensity was detected by flow cytometry.
Fabrication of the immunoassay strip and results judgment
The immunofluorescence strip is usually composed of the following five parts: a sample pad, a conjugate pad, a NC membrane, an absorbent pad, and a plastic backing board. A schematic presentation of the test strip is shown in Fig. 1a. The sample pad (2.5 × 30 cm) and conjugate pad (0.5 × 30 cm) were made of the same type of glass fiber, and the adsorbent pad (3 × 30 cm) was made of cellulose fiber. The anti-Ac McAb 6D and goat anti-mouse IgG were transferred onto both sides of the NC membrane (2.5 × 30 cm) using an XYZ arrayer (Bio-Dot, Irvine, CA, USA) with 1 μL/cm volume at 4 cm/s dispensed rate to form test line and control line, respectively. FITC labeled anti-Ac McAb 4F was dispensed onto the conjugate pad followed by drying at room temperature. The spotted NC membrane, absorbent pad, conjugate pad, and sample pad were assembled sequentially on the backing board (8 × 30 cm) with approximately 2 mm overlap. The assembly was cut into 3 mm wide strips using a guillotine cutter (Bio-Dot). All strips were sealed with desiccant and stored at room temperature.
The preparation of the strip is based on the classic double antibodies sandwich theory. The complex FITC Ac from sample was first combined with FITC-4F coated on conjugate pad, and then the complex FITC Ac-4F FITC flowed through the NC membrane via capillary action. Subsequently, the FITC Ac-4F FITC bound to McAb 6D was immobilized on test line, which formed a tripolymer FITC 4F-FITC Ac–6D in test line area. Accumulative FITC displayed yellow-green fluorescence under ultraviolet light as shown in Fig. 1b. Conversely, if there was no Ac in the samples, the FITC did not accumulate on the test line (Fig. 1c).
Sensitivity and specificity of the test strip
To evaluate the sensitivity, the SD01 strain diluted to 104–108 CFU/mL concentrations were analyzed with the strip. The Ac detection was carried out by applying 75 μL sample solution to the sample pad. Modified BHI medium containing FITC was used as negative control. After 10 min, the results of test line and control line were visualized in a black box equipped with an ultraviolet light source and a fluorescent filter of 525 nm (Semrock, Rochester, NY, USA), and then recorded with a digital camera.
The strip was stored at room temperature for 1, 2, and 3 mo to assess the stability by evaluating the sensitivity in 105 CFU/mL. The same test was conducted as described above.
Eight strains of Ac (SD01, ATCC 29625, 99-5, xj112, PLSB1, 00-1, plsb91, tw31) and six strains of Ac genetic nearly plant pathogens (ATCC 33996, NCPPB 961, ATCC 19307, NCPPB 2597, NCPPB 540, NCPPB 2975) were cultured in modified BHI medium and tested to evaluate the specificity of the strip. All bacterial suspensions were adjusted to the same concentrations using a microplate reader.
Application to real samples and comparison with PCR methods
The Ac free plant samples collected from the field, including watermelon, muskmelon, cantaloupe, luffa, wax gourd, pumpkin, cucumber, and summer squash, were verified by PCR methods. The modified BHI (45 mL) containing juice (5 mL) from plant samples was used to cultivate SD01 strain. Then, these inoculums were diluted to 105 CFU/mL for strip detection. The purpose was to determine whether the impurities in the juice from the plants could impact the smallest detectable concentration.
Results
Optimization of parameters
To develop the strip, many factors should be optimized to increase the sensitivity. After incubation at different FITC concentrations of 10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, 300 μg/mL, 400 μg/mL, the Ac was with different fluorescence intensity. As shown in Fig. 2, the Ac without fluorescence was distributed in M1 region (Control), and the fluorescent bacteria were distributed in M2, M3, or M4 areas according to the fluorescence intensity from 10 to 104. The percentage of fluorescent bacteria (in M2+M3+M4 area) and the distribution in M2, M3, or M4 areas were different. Since there were almost no bacteria in the M4 region, the proportion of bacteria distributed in M3 region was the basis for determining the optimum FITC concentration.
Comparison with the proportion of the M3 area, 25 μg/mL was considered to be the optimal FITC concentration for detection. In addition, when the FITC concentration was at 25 μg/mL, the proportion of fluorescent bacteria (M2+M3+M4) was up to 98.9%, which was higher than others. Figure 3 was the morphological picture of Ac cultured with FITC viewed under fluorescent microscope with blue light. After incubating with FITC, the bacteria became fluorescent probe.
The concentration of McAb 6D is also important for the performance of the strip since the color of the test line deepened with the increase of 6D concentration. However, the blank control had a false-positive result when the 6D concentration was higher than 2 mg/mL. Thus, the optimal concentration of 6D was determined to be 2 mg/mL. The concentration of FITC-4F measured by NanoDrop (Thermo, Waltham, MA, USA) was 2 mg/mL.
Sensitivity and stability of the ICS
To identify the sensitivity, a diluted gradient of SD01 (108–104 CFU/mL) was added to the sample pad of the dual FITC labeled ICS. As shown in Fig. 4c, the yellow-green light of the test line was evident for concentrations ranging from 108 to 105 CFU/mL. When the concentration was below 104 CFU/mL, there were no lines observed, which indicated that the detection limit was 105 CFU/mL. However the immunoassay strip based on FITC just labeling antigen (Fig. 4a) or antibody (Fig. 4b) displayed the sensitivity of 106 CFU/mL. All the results indicated that the method of FITC labeling antigen and antibody successfully achieved the effect of signal amplification, which realized a 10-fold increase in sensitivity compared with FITC just labeling antigen or antibody.
The stability of the strip was assessed through the sensitivity test. After storing at room temperature for 1, 2, and 3 the sensitivity test was performed and no exceptional results appeared. Therefore, we concluded that the strip could be stored for at least 3 mo without losing stability.
Specificity of the ICS
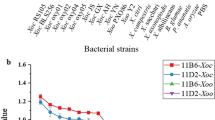
Since the common antigen of most bacteria, including Ac, is still unknown, the detection of different serotypes to evaluate the specificity of the strip are of critical importance. The specificity of the ICS was evaluated using eight strains of Ac and six genetically similar species to Ac. As shown in Fig. 5, eight strains of Ac (1 to 8) demonstrated positive results, and the other strains similar to Ac (9 to 14) produced negative results, which indicated that the strip possessed high specificity for the detection of Ac.
The fluorescence intensity of the control line for the positive results (1 to 8) was visibly higher than the fluorescence intensity of the negative results (9 to 14). The fluorescence of control line for positive results was from fluorescent bacteria and 4F-FITC, and the fluorescence was just from 4F-FITC after 4F-FITC combining with goat anti-mouse IgG antibody for negative results.
Sample analysis
To ensure the applicability and accuracy of the strip in real samples, eight species of cucurbitaceous plant, including watermelon, cantaloupe, muskmelon, pumpkin, cucumber, luffa, wax gourd, and summer squash were used to simulate Ac infection. As shown in Fig. 6, all Ac samples presented positive results, indicating the impurities in plant juice would not impact the accuracy of detection results, and the smallest detectable concentration in real samples was 105 CFU/mL, which was consistent with the PCR results (Fig. 7).
To further confirm the specificity of the strip and the detection performance for real samples, 14 strains of cucurbitaceous common pathogen, including eight strains of Ac and six stains of plant pathogens, were mixed with watermelon juice for strip test. As shown in Table 2, the detection results were not affected by the juice, indicating the strip possessed high specificity and was suitable for the detection of Ac in real samples.
Discussion
In this study, a new antigen and antibody dual labeled method using FITC was developed. FITC was used to make the bacteria fluoresce during bacterial enrichment process; the fluorescence stability of the labeling method has been proven before. The assay of FITC-labeled probiotic during gastrointestinal transit in broilers [22] and FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon Caco-2 cell uptake [24] verified that the bacterial fluorescence was very stable and was not quenched after entering the cell. In summary, the labeling method in vivo could make the bacteria emit stable and bright fluorescence.
Many methods for Ac detection were reported in recent years. The sensitivity of ELISA [25] and microsphere-based assay [26] were 104 CFU/mL with long time incubation and complex steps. PCR-based methods [27, 28] with the sensitivity of 32 and 10 CFU/mL, required trained technicians and precise instruments. Recently, DNA strip [29] has gained much attention owing to the high sensitivity of 4 nM. However, the process of pretreating sample to obtain DNA before detection was complex and time-consuming. The colloidal gold-based strip for Ac detection has been reported with the detection limit of 105 CFU/mL, while the chemical and colloidal instability in biological environment may lead to inaccurate result [30].
Compared with the methods mentioned above, antigen and antibody dual labeled immunofluorescence strip developed in this study is simple, fast, convenient, and easy to operate. The detection limit was successfully realized to 105 CFU/mL, which was 10-fold more sensitive compared with the strip of FITC only labeling antigen or antibody. In addition, there was a positive correlation between the Ac concentration and the fluorescence intensity of test line. The developed strip could detect the majority of Ac and had no cross-reactions with other plant pathogens. This antigen and antibody dual labeled, one-step Ac detection method have many advantages, such as reducing the cost and possessing high stability, which will be a more popular and useful tool for the early diagnosis, prevention, and control of BFB in the future.
References
Feng JJ, Li JQ, Walcott RR, Zhang GM, Luo LX, Kang L, et al. Advances in detection of Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbits. Seed Sci Technol. 2013;41(1):1–15.
Rane KK, Latin RX. Bacterial fruit blotch of watermelon: association of the pathogen with seed. Plant Dis. 1992;76(5):761–5.
Webb RE, Goth RW. A seedborne bacterium isolated from watermelon. Plant Dis Rep. 1965;49:818–21.
Bahar O, Kritzman G, Burdman S. Bacterial fruit blotch of melon: screens for disease tolerance and role of seed transmission in pathogenicity. Eur J Plant Pathol. 2009;123(1):71–83.
Singh J, Sharma S, Nara S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015;170:470–83.
Ren W, Huang Z, Xu Y, Li Y, Ji Y, Su B. Urchin-like gold nanoparticle-based immunochromatographic strip test for rapid detection of fumonisin B1 in grains. Anal Bioanal Chem. 2015;407(24):7341–8.
Liu Y, Wu A, Hu J, Lin M, Wen M, Zhang X, et al. Detection of 3-phenoxybenzoic acid in river water with a colloidal gold based lateral flow immunoassay. Anal Biochem. 2015;483(1):7–11.
Zhou Y, Li YS, Meng XY, Zhang YY, Yang L, Li ZH, et al. Production of a monoclonal antibody and development of an immunoassay for detection of Cr(III) in water samples. Chemosphere. 2013;93(10):2467–72.
Zhang MZ, Wang MZ, Chen ZL, Fang JH, Fang MM, Liu J, et al. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal Bioanal Chem. 2009;395(8):2591–9.
Byzova NA, Smirnova NI, Zherdev AV, Eremin SA, Shanin IA, Lei HT, et al. Rapid immunochromatographic assay for ofloxacin in animal original foodstuffs using native antisera labeled by colloidal gold. Talanta. 2014;119:125–1232.
Di Nardo F, Anfossi L, Giovannoli C, Passini C, Goftman VV, Goryacheva IY, et al. A fluorescent immunochromatographic strip test using quantum dots for fumonisins detection. Talanta. 2016;150:463–8.
Sirkka N, Lyytikäinen A, Savukoski T, Soukka T. Upconverting nanophosphors as reporters in a highly sensitive heterogeneous immunoassay for cardiac troponin I. Anal Chim Acta. 2016;925:82–7.
Anfossi L, Di Nardo F, Giovannoli C, Passini C, Baggiani C. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal Bioanal Chem. 2013;405(30):9859–67.
Liu D, Huang Y, Wang S, Liu K, Chen M, Xiong Y, et al. A modified lateral flow immunoassay for the detection of trace aflatoxin M1 based on immunomagnetic nanobeads with different antibody concentrations. Food Control. 2015;51:218–24.
Nagatani N, Yamanaka K, Ushijima H, Koketsu R, Sasaki T, Ikuta K, et al. Detection of influenza virus using a lateral flow immunoassay for amplified DNA by a microfluidic RT-PCR chip. Analyst. 2012;137(137):3422–6.
Liu Q, Liu XP, Wei YP, Mao CJ, Niu HL, Song JM, et al. Electrochemiluminescence immunoassay for the carcinoembryonic antigen using CdSe:Eu nanocrystals. Microchim Acta. 2017;184(5):1353–60.
Wang C, Wu J, Zong C, Ju H, Yan F. Highly sensitive rapid chemiluminescent immunoassay using the DNAzyme label for signal amplification. Analyst. 2011;136(20):4295–300.
Saniee P, Siavoshi F, Nikbakht BG, Khormali M, Sarrafnejad A, Malekzadeh R. Localization of H.pylori within the vacuole of Candida yeast by direct immunofluorescence technique. Arch Iran Med. 2013;16(12):705–10.
Yeo KB, Kim HB, Choi YS, Pack SP. Highly effective detection of inflamed cells using a modified bradykinin ligand labeled with FITC fluorescence. Enzyme Microb Technol. 2016;82:191–6.
Kim BS, Oh JM, Kim KS, Seo KS, Cho JS, Khang G, et al. BSA-FITC-loaded microcapsules for in vivo delivery. Biomaterials. 2009;30(5):902–9.
Murányi J, Gyulavári P, Varga A, Bökönyi G, Tanai H, Vántus T, et al. Synthesis, characterization, and systematic comparison of FITC-labelled GnRH-I, -II and -III analogues on various tumour cells. J Peptide Sci. 2016;22(8):552–60.
Blajman JE, Astesana DM, Zimmermann JA, Rossler E, Scharpen AR, Berisvil AP, et al. Quantification of FITC-labelled probiotic Lactobacillus salivarius DSPV 001P during gastrointestinal transit in broilers. Beneficial Microbes. 2017;8(1):55–64.
Zeng H, Guo W, Liang B, Li J, Zhai X, Song C, et al. Self-paired monoclonal antibody lateral flow immunoassay strip for rapid detection of Acidovorax avenae subsp. citrulli. Anal Bioanal Chem. 2016;408(22):6071–8.
Hu C, Chiang C, Hong P, Yeh M. Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers. Int J Nanomed. 2012;7(8):4861–72.
Himananto O, Luxananil P, Kumpoosiri P. Novel and highly specific monoclonal antibody to Acidovorax citrulli and development of ELISA-based detection in cucurbit leaves and seed. Plant Dis. 2011;95(95):1172–8.
Charlermroj R, Himananto O, Seepiban C, Kumpoosiri M, Warin N, Oplatowska M, et al. Multiplex detection of plant pathogens using a microsphere immunoassay technology. Plos One. 2013;8(4):e62344.
Bahar O, Efrat M, Hadar E, Dutta B, Walcott RR, Burdman S. New subspecies-specific polymerase chain reaction-based assay for the detection of Acidovorax avenae subsp. citrulli. Plant Pathol. 2008;57(4):754–63.
Ha Y, Fessehaie A, Ling KS, Wechter WP, Keinath AP, Walcott RR. Simultaneous detection of Acidovorax avenae subsp. citrulli and Didymella bryoniae in cucurbit seedlots using magnetic capture hybridization and real-time polymerase chain reaction. Phytopathology. 2009;99(6):666–78.
Zhao W, Lu J, Ma W, Xu C, Kuang H, Zhu S. Rapid on-site detection of Acidovorax avenae subsp. citrulli by gold-labeled DNA strip sensor. Biosens Bioelectron. 2011;26(10):4241–4.
Qu H, Zhang Y, Qu B, Kong H, Qin G, Liu S, et al. Rapid lateral-flow immunoassay for the quantum dot-based detection of puerarin. Biosens Bioelectron. 2016;81:358–62.
Acknowledgements
This research is supported by the Natural Science Foundation of China (No. 31371776), Science and Technology Innovation Plan of Shanghai: Yangtze River Delta joint research (No. 15395810900), Minimally Motivated Innovation Fund (YS30809102). and Graduate Education Innovation Program of Shanghai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors confirm that no conflict of interest exists for this manuscript and all of the authors agree to the submission of this paper.
Rights and permissions
About this article
Cite this article
Zeng, H., Zhang, D., Zhai, X. et al. Enhancing the immunofluorescent sensitivity for detection of Acidovorax citrulli using fluorescein isothiocyanate labeled antigen and antibody. Anal Bioanal Chem 410, 71–77 (2018). https://doi.org/10.1007/s00216-017-0690-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0690-2