Abstract
A multianalyte lateral-flow immunochromatographic technique using colloidal gold-labeled polyclonal antibodies was developed for the rapid simultaneous detection of clenbuterol and ractopamine. The assay procedure could be accomplished within 5 min, and the results of this qualitative one-step assay were evaluated visually according to whether test lines appeared or not. When applied to the swine urines, the detection limit and the half maximal inhibitory concentration (IC50) of the test strip under an optical density scanner were calculated to be 0.1 ± 0.01 ng mL−1 and 0.1 ± 0.01 ng mL−1, 0.56 ± 0.08 ng mL−1, and 0.71 ± 0.06 ng mL−1, respectively, the cut-off levels with the naked eye of 1 ng mL−1 and 1 ng mL−1 for clenbuterol and ractopamine were observed. Parallel analysis of swine urine samples with clenbuterol and ractopamine showed comparable results obtained from the multianalyte lateral-flow test strip and GC-MS. Therefore, the described multianalyte lateral-flow test strip can be used as a reliable, rapid, and cost-effective on-site screening technique for the simultaneous determination of clenbuterol and ractopamine residues in swine urine.

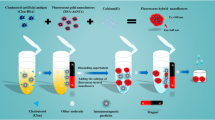
The colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clenbuterol (CLE) and ractopamine (RAC) are synthesized β-adrenergic agonists which enhance animal growth and increase feeding efficiency by inhibiting fat synthesis, stimulating lipolysis, increasing protein synthesis and carcass leanness [1, 2]. There is an increasing concern of the hazards posed to human health by the presence of β-adrenergic agonists residues in animal tissues [3, 4]. Most of the β-adrenergic agonists except RAC, which has been approved as a feed additive for swine and cattle in the United States and some other countries, are now banned as feed additives for growth promotion in food animals in China, the United States and most European countries.
Because of the potential risk of β-adrenergic agonist residues for human health and for monitoring the illegal use of them, there is an urgent need for a sensitive method for β-adrenergic agonist analyses. Several assays for detection of CLE, RAC, and other β-adrenergic agonist residues have been reported, including confirmatory and screening methods. The confirmatory methods, such as liquid chromatography (LC) [5–7], gas chromatography–mass spectrometry (GC-MS) [8–12] and liquid chromatography–mass spectrometry (LC-MS) [13–15], require extensive sample clean-up and personnel with professional training to operate the sophisticated instruments. The screening methods are often immunoassays [10, 15–31], which provide the advantages of sensitivity, specificity and user-friendly analysis.
Moreover, the number of substances with β-agonistic activity, illegally introduced in meat production or in sports doping as anabolic or beta-blocking agents, is increasing. Analytical methods suited for their multianalyte detection are thus necessary. The simultaneous determination of several β-adrenergic agonists in food and feed samples with one single test is the most attractive approach for practical purpose. It can reduce the time and cost per analysis, allow for simpler assay protocols, and decrease the sample volume required. The development of multianalyte immunoassays, such as enzyme-linked immunosorbent assays (ELISA), immunoaffinity chromatographic technique, and immunomagnetic separation-ELISA, allowing the simultaneous determination of co-occurring β-adrenergic agonists, have been described [32–37]. To our knowledge, the lateral-flow colloidal gold-based technique for the simultaneous detection of β-adrenergic agonists has not been reported.
The use of membrane-based lateral-flow immunoassay tests for on-site screening provides a simple, low-cost, sensitivity, specificity, and user-friendly alternative to expensive, laborious, and time-consuming instrumental methods and more sophisticated immunoassay formats [38]. The primary aim of this paper was to develop a lateral-flow colloidal gold-based technique for the simultaneous detection of β-adrenergic agonist residues (CLE and RAC) in swine urine.
Materials and methods
Reagents
Clenbuterol hydrochloride, salbutamol hemisulfate, terbutaline, cimbuterol, cimetrol, salmeterol 1-hydroxy-2-naphthoate, bamethane sulfate, fenoterol hydrochloride, isoproterenol hydrochloride, isoxsuprine hydrochloride, ritodrine hydrochloride, zilpaterol hydrochloride, bovine serum albumin (BSA), Tween 20, gold chloride, polyvinyl alcohol, and sucrose (no. S9378) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ractopamine hydrochloride was obtained from Eli Lilly & Co. (Indianapolis, IN, USA). CLE-BSA, RAC-BSA conjugates, polyclonal antibody against CLE and RAC were provided by International Diagnostic Systems Corp (St. Joseph, MO, USA). The polyclonal antibody against CLE was obtained from New Zealand white rabbit immunized with a conjugate prepared by the direct coupling of diazotized clenbuterol to keyhole limpet hemocyanin (KLH). CLE-BSA was also synthesized by the diazotization reaction as the T-line coating antigen. RAC hapten was synthesized by coupling RAC with glutarate anhydride and then was coupled to KLH as immunogen and coupled to BSA as the T-line coating antigen using the mixed anhydride method according to Shelver and Smith [17]. The polyclonal antibody against RAC was also obtained from New Zealand white rabbit immunized with RAC-KLH. Goat anti-rabbit IgG was prepared in our laboratory. Unless otherwise noted, all other reagents were of analytical grade or higher. The water used in all experiments was purified with a Milli-Q system (Millipore, Bedford, MA, USA). Phosphate-buffer saline (PBS, 0.05 M, pH 7.4) and sodium borate buffer (0.02 M, pH 9.2) were used in the experiments.
Hi-Flow Plus 135 membrane from Millipore (Bedford, MA, USA), conjugate pad grade 8964 and absorbent pad type 133 from Pall (Saint Germain-en-Laye, France), glass fiber grade F075-17 from Whatman (Maidstone, Kent, England) were used.
Swine urine samples and preparation of standard samples
Swine urine samples were collected manually in glass vials and stored at −20 °C from several locally small farms where swine with mix of genders such as Duroc, Landrace, and Yorkshire were bread and fed in Zhejiang Province, China.
Standard solutions of CLE, RAC, and other β-adrenergic agonists were prepared by diluting stock solutions of these compounds (1 mg mL−1, in methanol, store at -20 °C). CLE and RAC stock solutions were diluted in normal swine urine, which was determined to be the negative content of β-adrenergic agonists by GC-MS, at 0, 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, and 10.0 ng mL−1 and other β-adrenergic agonists of bamethane, cimbuterol, cimetrol, fenoterol, isoproterenol, isoxsuprine, ritodrine, salbutamol, salmeterol, terbutaline and, zilpaterol at 10, 25, 50, 100, 250, 500, 1,000, 2,000, 4,000, 8,000 ng ml−1.
Colloidal gold-based lateral-flow immunoassay
Preparation of colloidal gold-labeled polyclonal antibodies
According to the procedure described by Hayat [39], colloidal gold with an average diameter of 40 nm were prepared by controlled reduction of gold chloride with 1% sodium citrate [39]. Briefly, 100 mL of 0.2% gold chloride trihydrate solution in super purified water was heated to boil and 1.5 mL of 1% sodium citrate solution was added while stirring. After the color changed from light yellow to brilliant red, the solution was boiled for another 5 min, and then cooled to and stored at room temperature with 0.05% sodium azide added.
The colloidal gold-labeled polyclonal antibody against CLE (anti-CLE pAb) and polyclonal antibody against RAC (anti-RAC pAb) were prepared as described by Yokota [40] with some modification [40]. Briefly, 1 mL of anti-CLE pAb and anti-RAC pAb at the optimum concentration of 40 and 50 μg mL−1 were incubated with 10 mL of colloidal gold solution (pH 9.2) for 30 min at room temperature, respectively. Blocking with 1 mL 10% BSA solution in 0.02 M sodium borate buffer (pH 9.2) at room temperature for another 10 min, the mixtures were centrifuged at 4 °C, 20,000×g for 30 min and then the labeled pAb washed by repeated centrifugation (20,000×g) with 1% BSA in 0.02 M sodium borate buffer (pH 9.2) at 4 °C for 30 min. The precipitates were resuspended with 1 mL PBS (0.05 M, pH 7.4) containing 1% BSA and 0.05% sodium azide and stored at 4 °C for use.
Preparation of the membrane
Test and control lines were spotted on the Hi-Flow Plus 135 membrane (300 × 25 mm) using a Quanti 3000 Biojets attached to a XYZ Bioatrip Dispenser (Bio-Dot, CA, USA). The test lines (for CLE and RAC, respectively) were separately coated with CLE-BSA and RAC-BSA conjugates at the bottom of the membrane. Goat anti-rabbit IgG was dispensed on the top of the membrane as the control line. The distance between the lines was 50 mm. These BSA conjugates and goat anti-rabbit IgG were separately diluted in PBS containing 7% methanol (v/v) to the concentration of 0.5, 0.7, and 1.0 mg mL−1, respectively, and applied in the form of dots at 50 dots/μL/cm to form the test and control lines. After drying at 37 °C for 60 min, the membrane was blocked with PBS (0.05 M, pH 7.4) containing 1% (w/v) casein at room temperature for another 60 min. Then, the membrane was dried at 37 °C for 60 min, vacuum-packaged in plastic bag containing silica as moisture absorbent and stored under dry condition at room temperature for use.
Preparation of the conjugate pad
The conjugate pad (300 × 8 mm) was dispensed with 300 μL of the optimum mixture of colloidal gold-labeled anti-CLE (200 μL) and anti-RAC (100 μL) pAbs diluted with 700 μL PBS containing 5.0% (w/v) sucrose, 5.0% (w/v) BSA, 0.8% (w/v) NaCl, 0.1% (w/v) EDTA , 0.3% (v/v) Tween 20, and 0.05% (w/v) sodium azide by using a Quanti 3000 Biojets attached to a XYZ Bioatrip Dispenser. The volume dispensed was 3 μL per 1 mm pad. After dispensing, the pad was dried at 37 °C for 60 min and then stored in a desiccator at room temperature.
Preparation of sample pad and absorbent pad
Glass fiber grade F075-17 from Whatman (Maidstone, Kent, England) was used as the sample pad. The sample pad (300 × 20 mm) was saturated with sodium borate buffer (0.02 M, pH 9.2) containing 2.0% (w/v) sucrose, 1.0% (w/v) BSA, 0.8% (w/v) NaCl, 0.2%(w/v) polyvinyl alcohol, and 0.05% (w/v) sodium azide at room temperature for 30 min. Then the sample pad was dried at 37 °C for 60 min and stored as described above. The absorbent pad was cut to 300 × 30 mm for use.
Assembly of the test strip
On a plastic baking plate (300 × 80 mm), the conjugate pad was attached to the bottom of the membrane with 1–2 mm overlapping on the membrane, and then the sample pad was attached to the bottom of the conjugate pad in a similar manner. The absorbent pad was attached to the top of the membrane with 1–2 mm overlapping on the membrane also. The prepared master card was cut to 3.8 mm width strips using a CM 4000 Cutter (Bio-Dot, CA, USA). The strips were then enclosed in the plastic box and sealed in the aluminum foil bag containing desiccant gel, then stored under dry conditions at room temperature until use.
Assay procedure and principle
The principle of test strips was illustrated as in Fig. 1. The test strips were oriented flatwise. Eighty to 100 μL of swine urine samples were dripped into the sample holder of the test strip cell at the sample pad side and allowed the liquid to migrate for 5 min. The specific colloidal gold-labeled anti-CLE and anti-RAC pAb, which were redissolved from the conjugate pad, reacted with CLE and RAC (if they were present in the urine samples). On the mean while, excess of colloidal gold-labeled anti-CLE and anti-RAC pAbs were trapped by the BSA-CLE and BSA-RAC immobilized on the membrane forming red test lines and further trapped by the goat anti-rabbit IgG antibodies forming the control line while the whole complex were migrating along the membrane. After 3–10 min, the test results were evaluated visually or test lines were scanned with a Bio-Dot TSR3000 Membrane Strip Reader (Bio-Dot, CA, USA) as described by Zhang et al. [29]. G/Peak and G/D × Area of the relative optical (ROD) decreased as the CLE or RAC concentration in the standard samples increased. The concentration of CLE or RAC and the ROD (%) produced a sigmoidal dose–response curve that fits to a four-parameter logistic curve pattern indicating the classical competition. The negative test (for both CLE and RAC) results in three red lines (test and control lines). The more CLE and or RAC present in the sample, the weaker the test lines appear. The positive samples (for both CLE and RAC) gave only one red line (the control line). If two lines appeared (control line and one test line), the sample was positive for either CLE or RAC. If no control line was present, the test was considered to be invalid.
GC-MS analysis of CLE and RAC in swine urine
In parallel with the strip tests, GC-MS analysis of CLE and RAC was performed with an Agilent model 6890 gas chromatograph and an Agilent model 5973 mass-selective detector (Palo Alto, CA, USA). Compounds were separated on a 30 m × 0.25 mm i.d. fused silica capillary column coated with a 0.25 μm film of the bonded phase HP5 Trace (Agilent). Splitless injection (0.75 min) of 1 μL solution was performed at a helium flow rate of 1.0 mL min−1, and an injection port temperature of 250 °C. The column temperature was maintained at 80 °C for 2 min, then ramped at 18 min−1 from 80 °C to 250 °C, and finally at 25 min−1 to 300 °C, which was held for 3 min. The transfer line temperature was set at 280 °C. The mass spectrometer was operated in electron-impact (EI) ionization mode with an electron energy of 70 eV, a source temperature of 230 °C, and a detector temperature of 280 °C. Acquisition in selected-monitoring (SIM) mode was performed with a dwell time of 100 ms. Data acquisition and integration were achieved by use of MSD ChemiStation software.
Results and discussion
Simultaneous detection of two β-adrenergic agonists with the test strips
The colloidal gold-based competitive and multianalyte immunoassay was developed as a rapid visual qualitative test which gave a simple yes/no response to the levels of two and up two target analytes. Therefore, first, the optimal conditions for the negative test which gave the most intensely red-colored test lines and the smallest amount of two β-adrenergic agonists that resulted in no red color development at the test lines should be studied; in addition, the difference between positive and negative samples should be easily distinguished with the naked eye. Second, the multianalyte test allowed the visual evaluation of both β-adrenergic agonists test lines simultaneously should be determined. For these purposes, the optimal condition experiments for the lateral-flow assay for each single β-adrenergic agonist were tested similar to the “checkerboard titration” in competitive ELISA. Several dilutions of CLE-BSA and RAC-BSA conjugates coated on the membrane against different amounts of colloidal gold-labeled anti-CLE pAb and anti-RAC pAb were investigated using PBS and PBS containing 3 ng mL−1 CLE and RAC. Combinations satisfying the abovementioned criteria (data not shown) were selected for the experiments of multi-β- agonist assay.
For the simultaneous detection of CLE and RAC by the lateral-flow test, the mixture of different ratio of colloidal gold-labeled anti-CLE pAb and anti-RAC pAb were investigated using a blank swine urine sample and samples containing 1 ng mL−1 CLE and RAC. The optimal conditions were selected for the further experiments under the following conditions: CLE-BSA and RAC-BSA concentrations of 0.5 and 0.7 mg mL−1, respectively, forming the test lines; 300 μL of the mixture of colloidal gold-labeled anti-CLE (200 μL) and anti-RAC (100 μL) pAbs diluted with 700 μL PBS containing 5.0% (w/v) sucrose, 5.0% (w/v) BSA, 0.8% (w/v) NaCl, 0.1% (w/v) EDTA , 0.3% (v/v) Tween 20 and 0.05% (w/v) sodium azide dispensing on conjugate pad. In accordance with the optimal conditions, red color development was observed for both test lines (CLE and RAC), only the color of the corresponding test line appeared (CLE or RAC, respectively) and no color developed at the test line corresponding to the other β-adrenergic agonist (RAC or CLE, respectively; Fig. 2). Thus, it was possible to detect multiple β-adrenergic agonists using the colloidal gold-labeled pAb mixture. Moreover, the binding between one β-adrenergic agonist and the corresponding specific colloidal gold-labeled pAb was not affected by the presence of the other β-adrenergic agonist, demonstrated by the comparison of the visual results of single- and multi β-adrenergic agonist assay formats (Fig. 2).
Lateral-flow immunoassay of swine urine samples (at 25 °C). a CLE/RAC concentrations (from left to right): 0/0; 0.1/0; 0.5/0 and 1.0/0 ng mL−1. b CLE/RAC concentrations (from left to right): 0/0; 0/0.1; 0/0.5 and 0/1.0 ng mL−1. c CLE / RAC concentrations (from left to right): 0/0; 0.1/0.1; 0.5/0.5 and 1.0/1.0 ng mL−1. Upper line is the control line; middle and bottom lines are the CLE and RAC test lines, respectively
Determination of immunochromatographic time with the test strips
The colloidal gold immunoassay was developed as a rapid visual qualitative test. Therefore, the appearance of a clear red color on the test lines for negative samples or samples with low concentrations of CLE or/and RAC was optimized within a reasonably short immunochromatographic time to satisfy the purpose. Moreover, the multianalyte test which will be intended to allow the visual evaluation or investigation with strip reader of both test lines simultaneously should be studied. The performance of test lines were investigated with a Bio-Dot TSR3000 Membrane Strip Reader, using a blank swine urine sample (0 ng mL−1) and samples containing 1 ng mL−1 CLE and RAC. The relative optical density (ROD) of CLE and RAC increased simultaneously for 5 min, and not increased obviously after 5 min (Fig. 3). The results showed that after 5 min, almost all the of colloidal gold-labeled antibodies will bind to the CLE-BSA and RAC-BSA coated on the nitrocellulose membrane (the test lines), if the CLE and RAC levels in the urine samples are negative or below the particular level. At the immunochromatographic time of 5 min, the difference between positive and negative samples could be also easily distinguished with the naked eye. So the immunochromatographic time of 5 min was selected for further experiments.
Sensitivity of the test strips
The multianalyte test of CLE and RAC was based on the competitive principle, the inverse relationship between concentrations of two β-adrenergic agonists in sample and development of red color on the test lines. Therefore, the sensitivity of the test strips should be determined by testing the CLE and RAC standard samples. The relative optical densities (ROD) decreased as the CLE and RAC concentrations in the standard samples increased. The relationship between the concentrations of CLE, RAC, and the B/B 0 (%) showed the sigmoidal dose–response curves which fit to a four-parameter logistic curve pattern indicating the classical competition (Fig. 4). Similar to the ELISA assay, the lower detection limit (LDL) with the strip reader was quantitatively defined here as the amount of CLE or RAC in the standard samples that caused 10% decrease of the ROD than that produced by the 0 ng mL−1 sample. The half maximal inhibitory concentration (IC50) was also set at the amount of CLE or RAC in the standard samples causing 50% decrease of the ROD than that produced by the 0 ng mL−1 sample. In the present study, the LDL of CLE and RAC were calculated to be 0.1 ± 0.01 ng mL−1 and 0.1 ± 0.01 ng mL−1, and the IC50 of CLE and RAC were 0.56 ± 0.08 ng mL−1 and 0.71 ± 0.06 ng mL−1, respectively.
The colloidal gold immunoassay was studied as rapid visual qualitative test which gave a simple yes or no response to the levels of the target analytes. The cut-off value with the naked eye was defined here as the amount of CLE or RAC in the standard samples that resulted in no red color development at the test lines. In accordance with visual evaluation, the cut-off values of CLE and RAC were 1.0 and 1.0 ng mL−1, respectively (Fig. 2).
Specificity of the test strips
Bamethane, cimbuterol, cimetrol, fenoterol, isoproterenol, isoxsuprine, ritodrine, salbutamol, salmeterol, terbutaline and zilpaterol are analogs of clenbuterol and ractopamine. The cross-reactivity of CLE and RAC simultaneous test strip with these β-adrenergic agonist compounds were examined at 25 °C. The cross-reactivity with the scanner was quantitatively defined here as the percentage of IC50 concentrations of the β-adrenergic agonist compounds divided by that of CLE or RAC. The IC50 and the cross-reactivities of the test strip to CLE, RAC, and other β-adrenergic agonists were analyzed with the four-parameter logistic equation and shown in Table 1. No cross-reactivity of anti-CLE pAb to other tested β-adrenergic agonists except salbutamol and terbutaline for concentrations up to 1,000 ng mL−1 was observed. Anti-CLE pAb had cross-reactivity with salbutamol with IC50 of 25 ± 1.79 ng mL−1 (2.24%; n = 10) and terbutaline with IC50 of 100 ± 8.12 ng mL−1 (0.56%; n = 10) because of their structures resembling portions of CLE’s structure. Except for isoxsuprine and ritodrine which structurally resembling RAC, no β-adrenergic agonists at concentrations up to 1,000 ng mL−1 showed binding with the anti-RAC pAb. Anti-RAC pAb cross-reacted with isoxsuprine with IC50 of 250 ± 9.47 ng mL−1 (0.284 %; n = 10) and ritodrine with IC50 of 50 ± 2.19 ng mL−1 (1.420%; n = 10). Of particular importance was the fact that anti-CLE pAb did not cross-react with RAC and anti-RAC pAb did not cross-react with CLE. The specificity will reduce the possibility of false positive result.
Limit of detection and limit of quantification
The limit of detection (LOD) was calculated as the mean of the measured content of blank different samples (n = 20) plus three standard deviations (mean + 3SD). The limit of quantification (LOQ) was calculated as the measured content of blank different samples (n = 20) plus six standard deviations (mean + 6SD; Commission Decision 87/410/EEC) [35]. The 20 blank swine urine samples, obtained by 20 different animals, were analyzed in triplicate for both CLE and RAC by using the CLE and RAC simultaneous test strips with the scanner. In this work, the LOD for CLE in swine urine samples was 0.34 ng mL−1 and the LOQ was 0.55 ng mL−1. The LOD for RAC was 0.39 ng mL−1 and the LOQ was 0.66 ng mL−1 (Table 2). The LOD and LOQ of the present study could meet the requirement of simultaneous screening detection for CLE and RAC residues in swine urine samples.
Determination of recovery and variation
CLE and RAC standards were simultaneously spiked into the blank swine urine samples with concentrations of 1, 3, 6, 8, and 10 ng mL−1. The average recovery and variation were measured for 20 replicates of each concentration of spiked urine samples. Table 3 showed the average recoveries ranged from 74 to 104% for CLE and 79–106% for RAC. The coefficients of variation (CV) ranged from 4.8 to 8.1% for CLE and 4.2 to 13.3% for RAC. The results indicated that recoveries within 25% of theoretical values and coefficients of variation below 15% were acceptable for screening detection of CLE and RAC residues in swine urine.
Temperature effects determination
The test strips will be usually performance outside the laboratory. The effect of temperature was examined by running the spiked swine urine samples with CLE and RAC standards of 0, 0.1, 0.5, and 1.0 ng mL−1 at 4, 15, 25, and 35 °C, respectively. The effects of various temperatures on the ROD and B/B 0 (%) values were evaluated. Comparison with the ROD at 25 °C, the ROD of corresponding standards at 35 °C were slightly changed with no significant difference (Fig. 5). There was a systematic decrease in the ROD values at both 15 °C and 4 °C with significant difference at P < 0.05 and P < 0.01, respectively. There were no obviously changes of B/B 0 values at various examined temperature comparing with at 25 °C. The results indicated that the determination of test strips with the naked eye may be affected especially at the lower temperature; there may be no effect on performance for test strips with the scanner.
Comparative studies between test strips and GC-MS
GC-MS analysis, which was considered as one of confirmatory methods for identification and quantification of β-adrenergic agonists, was performed to quantify the amount of CLE and RAC residues in 30 unknown swine urine samples in parallel with the strip tests with the scanner. The values showed in the figures are the average of three repeated tests. Comparisons were made using linear regression analysis with the lines modeled having a zero intercept. The resulting correlation coefficients served as measures of assay variability between test strips and GC-MS methods, whereas slopes of the correlations served as indicators of differences in assay responsiveness.
The correlation coefficient (r 2) for the test strips analysis and the GC-MS analysis of CLE, RAC in swine urine samples was 0.94 and 0.93, respectively, indicating an acceptable agreement between the two methods for the detection of CLE and RAC. The slope of the correlation was 1.06 and 1.09, indicating that both the detected results of CLE and RAC by the test strips method were little greater than the quantitative results by GC-MS (Fig. 6). The results suggested that the test strips method based colloidal gold-based lateral-flow immunoassay is reliable for the CLE and RAC detection in swine urine samples, meanwhile, the method offers advantages of ease of sample preparation and high throughput.
Conclusions
In the present study, a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection at the parts-per-billion level in swine urine of two β-adrenergic agonists residues, clenbuterol and ractopamine (the most often found illicitly used in China), was developed. The assay could be accomplished within 5 min without the need for any sample preparation. By scanning the relative optical density (ROD) of the test lines, this test strip format assay could be quantitatively analyzed in accordance with the mathematical model of RPNA (Qian and Bau [41]). Similar to the competitive ELISA, the LDL, IC50, sensitivity and specificity of CLE and RAC simultaneous test strip were easy calculated and analyzed. Results from visual evaluation of the lateral-flow tests of spiked swine urine samples were shown that the cut-off values of CLE and RAC were 1.0 and 1.0 ng mL−1, respectively. Results with test strips and GC-MS analysis for detection of CLE and RAC residues in factual swine urine samples proved the reliability of the immunoassay. In conclusion, the described multianalyte technique and immunoassay format could be used for rapid and cost-effective simultaneous screening CLE and RAC residues in swine urine samples.
References
Rick CA, Baker PK, Dalrymple RH (1984) Recipr Meat Conf Proc 37:5
Xiao RJ, Xu ZR, Chen HL (1999) Anim Feed Sci Technol 79:119–127
Martinez-Navarro JF (1990) Lancet 336:1311
Mitchell GA, Dunnavan G (1998) J Anim Sci 76:208–211
Hooijerink H, Schilt R, Haasnoot W, Courtheijn D (1991) J Pharm Biomed Anal 485-492
Botterblom MH, Feenstra MG, Erdtsieck-Ernste EB (1993) J Chromatogr 613:121–126
Turberg MP, Macy TD, Lewis JJ, Coleman MR (1994) J AOAC Int 78:1394–1402
Abukhalaf IK, von Deutsch DA, Parks BA, Wineski L, Paulsen D, Aboul-Enein HY, Potter DE (2000) Biomed Chromatogr 14:99–105
Harkins JD, Woods WE, Lehner AF, Fisher M, Tobin T (2001) J Vet Pharmacol Ther 24:7–14
Haasnoot W, Stouten P, Lommen A, Cazemier G, Hooijerink D, Schilt R (1994) Analyst 119:2675–80
Bocca B, Fiori M, Cartoni C, Brambilla G (2003) J AOAC Int 86:8–14
Wang JP, Li XW, Zhang W, Shen JZ (2006) Chromatographia 64:613–617
Lehner AF, Harkins JD, Karpiesiuk W, Woods WE, Robinson NE, Dirikolu L, Fisher M, Tobin T (2001) J Anal Toxicol 25:280–287
Antignac JP, Marchand P, Le Bizec B, Andre F (2002) J Chromatogr B 774:59–66
Shishani E, Chai SC, Jamokha S (2003) Anal Chim Acta 483:137–145
Elliott CT, Thompson CS, Arts CJ, Crooks SR, van Baak MJ, Verheij ER, Baxter GA (1998) Analyst 123:1103–1107
Shelver WL, Smith DJ (2000) J Immunoassay 21:1–23
Shelver WL, Smith DJ (2002) J Agric Food Chem 50:2742–2747
Shelver WL, Smith DJ (2003) J Agric Food Chem 51:3715–3721
Shelver WL, Smith DJ (2004) J Agric Food Chem 52:2159–2166
Shelver WL, Kim HJ, Li QX (2005) J Agric Food Chem 53:3273–3280
Wang JP, Zhang SX, Shen JZ (2006) J Anim Sci 84:1248–1251
Degand G, Bernes-Duyckaerts A, Maghuin-Rogister G (1992) J Agric Food Chem 40:70–75
Mcconnell RI, Mccormick A, Lamont JV, Fitzgerald SP (1994) Food Agric Immunol 6:147–153
Gleixner A, Meyer HHD (1995) Food Agric Immunol 7:221–225
Petruzzelli E, Ius A, Berta S, Dovis M, Albertini A (1996) Food Agric Immunol 8:3–10
Rodgers ES, Elliott CT, Wan Po AL, Mackie DP, Scott EM, Kreuter J (1997) Food Agric Immunol 9:159–166
Johansson MA, Hellens KE (2003) Food Agric Immunol 15:197–205
Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM, Zhao D, Chai SJ, Guo JQ (2006) J Immunol Methods 312:27–33
Lai WH, Xu Y, Fung DY, Xiong Y (2007) Asia Pac J Clin Nutr 16:106–110
Lai WH, Fung DY, Xu Y, Xiong YH (2008) J Food Prot 71:865–869
Kolosova AY, De Saeger S, Sibanda L, Verheijen R, Van Peteghem C (2007) Anal Bioanal Chem 389:2103–2107
Courtheyn D, Bakeroot V, De Volder F, Vercammen J (1994) Food Agric Immunol 6:131–139
Drsch I, Malucelli A, Meyer HHD (1994) Food Agric Immunol 6:141–145
Cerni L, Biancotto G, Tondolo A, Bogoni P (1998) Food Agric Immunol 10:307–315
Cooper AD, Shepherd MJ (1996) Food Agric Immunol 8:205–213
Wang JP, Shen JZ (2007) Food Agric Immunol 18:107–115
Vanoosthuyze K, Van Peteghem C, Courtheyn D, Vercammen J (1994) Food Agric Immunol 6:241–249
Hayat MA (1989) Colloidal Gold: Principles, Methods and Applications. Academic Press, New York, p 421
Yokota S, Fujimori O (1992) Methods of Immunogold Staining. Soft Science Publications, Japan
Qian S, Bau HH (2004) Anal Biochem 326:211–224
Acknowledgment
This work was supported by the Important Science and Technology Specific Program of Zhejiang Province (Grant No. 2006C12102) and the Innovation Fund for Technology-based Firms from the Ministry of Science and Technology of China (Grant No. 08C26213300756).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, MZ., Wang, MZ., Chen, ZL. et al. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Anal Bioanal Chem 395, 2591–2599 (2009). https://doi.org/10.1007/s00216-009-3181-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3181-2