Abstract
A method based on gas chromatography–tandem mass spectrometry after derivatization with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide was developed for the analysis of monohydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) in hair. The method focused on 52 target compounds corresponding to two- to six-ring monohydroxylated metabolites of polycyclic aromatic hydrocarbons (PAHs). The limits of quantification ranged from 0.2 to 50 pg mg−1. The method was then applied to the analysis of hair samples collected from rats exposed to 12 PAHs at 0.01, 0.1, and 1 mg kg−1, by intraperitoneal injection, for 28 days. The results of this study confirm that these metabolites can be incorporated in hair after intraperitoneal administration of the corresponding parent compound. Only 20 of the 52 metabolites were actually detected in hair samples and corresponded to nine parent PAHs. The mean concentrations of OH-PAHs in rat hair samples exposed to PAHs at 1 mg kg−1 ranged from 0.6 ± 0.2 pg mg−1 for 8-hydroxybenzo[b]fluoranthene to 6.7 ± 1.0 pg mg−1 for 1-hydroxypyrene. The results also demonstrated that hair pigmentation has no influence on the concentration of most OH-PAHs. This animal experiment confirmed the incorporation of PAH metabolites in hair and demonstrated that the method was sufficiently sensitive to detect low levels of exposure to PAHs. These results confirmed the usefulness of hair analysis in the biomonitoring of human exposure to PAHs.

Analysis of 52 monohydroxylated polyccyclic aromatic hydrocarbons in a supplemented hair sample by GC-EI-MS/MS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since exposure to polycyclic aromatic hydrocarbons (PAHs) is suspected to be responsible for numerous health issues, significant efforts have been made to develop efficient strategies for the assessment of human exposure to these ubiquitous compounds. Over the last few decades, PAH metabolites have been analyzed in urine [1–4], with a special focus on 1-hydroxypyrene and 3-hydroxybenzo[a]pyrene, which were considered the most representative biomarkers of global exposure to PAHs [5–14]. Measurements of these biomarkers in urine provided useful evaluation of recent exposure to pyrene and benzo[a]pyrene in occupationally exposed workers [9, 10, 15–19]. Since individuals are generally exposed to mixtures of PAHs, more extensive methods covering a number of PAH metabolites have been developed for a more comprehensive exposure assessment [3, 12, 20–22]. The urinary metabolites of PAHs have been analyzed by different methods, such as high-performance liquid chromatography with fluorescence detection [1, 2], gas chromatography (GC)–mass spectrometry (MS) after derivatization with an alkylating agent or a silylating agent [23–25] or with a fluorinating agent with use of negative chemical ionization (NCI) mode [4, 26], or liquid chromatography–tandem MS (MS/MS), directly [27–29] or after derivatization [30, 31].
Along with urine, which is the matrix classically used for the assessment of recent exposure to PAHs, the possibility to detect PAHs and their metabolites in hair has also been demonstrated [32, 33]. Hair has been recognized to be a relevant matrix for analytical toxicology studies. Many studies performed on humans have demonstrated that this matrix is suitable for the analysis of drugs of abuse, medical drugs, metals, and organic pollutants [34–38]. The use of hair for the biomonitoring of long-term exposure to environmental pollutants offers several advantages: (1) this matrix allows the assessment of the average level of exposure (integrating all exposure routes) over several months depending on the hair sample length; (2) in contrast to biological fluids, the accumulation of pollutants in hair in the course of time and their presence even after exposure has stopped increases the likelihood of positive detection; (3) it relies on an easy sampling and simple storage conditions (no refrigeration). Some precautions have, however, to be taken with regard to hair analysis in order to avoid misinterpretation of the results. A main limitation concerns the possible effect of hair pigmentation, which was demonstrated to influence the incorporation of basic drugs such as codeine and cocaine [37]. Nevertheless, as the influence of pigmentation seems to be compound-dependent, with no or a limited effect for some chemicals [39, 40], this parameter has to be evaluated for each method development focused on the analysis in hair of new compounds.
Although hair analysis was first limited to the detection of illicit and medical drugs, technical advances in analytical methods resulting in increased sensitivity have allowed the analysis of pollutants such as dioxins [41–44] and pesticides [45–48]. PAHs were first detected in hair by Toriba et al. [33], who described the use of this matrix for the biomonitoring of human exposure to PAHs. More recently, human exposure to 3,6-dinitrobenzo[e]pyrene, a strong mutagen essentially produced by diesel exhaust, was also investigated in hair [49]. The presence of the latter compounds in air and dust can lead to contamination of hair surface. This external contamination corresponds to chemicals recently deposited on hair and is thus not representative of people’s internal dose. In contrast, the chemicals incorporated from blood during hair growth following an individual’s exposure (e.g., via ingestion or inhalation) are located inside the hair shaft [50]. For the proper determination of the level of exposure, external contamination has, therefore, to be removed prior to analysis. For this purpose, different solvents can be used. However, the efficiency of washing remains uncertain in that contamination can be only partially removed and also on the grounds that washing with organic solvents can alter hair structure and reach chemicals located inside the hair [50]. The origin of the removed chemicals is therefore unknown, and the interpretation of the results obtained from the analysis of washed hair or washing solvents remains contentious [32]. A possible way to avoid interferences due to contamination lies in the determination of metabolites which are produced during biological processes and are unlikely to be deposited via the air [32]. For this purpose, monohydroxylated metabolites of PAHs appear to be suitable candidates since their atmospheric concentration is generally below the detection levels, except in areas where coal combustion is still an important source of energy production in facilities unable to efficiently remove this waste from released fumes [51–54]. For this purpose, recent studies performed in our laboratory have allowed the detection of hydroxylated metabolites of PAHs in hair [32, 55]. This method was based on NCI after derivatization of monohydroxylated PAHs (OH-PAHs) with (2S,4R)-N-heptafluorobutyryl-4-heptafluorobutoyloxyprolyl chloride [(S,R)-HFBOPCl] [32, 56]. Unlike with electron ionization (EI), whereby molecules are directly ionized through interaction with high-energy electrons, NCI generates interactions between analytes and a lower-energy reagent gas, such as methane. OH-PAH derivatives with (S,R)-HFBOPCl form negative ions through electron capture. NCI is particularly useful for providing molecular mass information as little or no fragmentation occurs, unlike with EI. For instance, the characteristic ions observed for (S,R)-HFBOPCl OH-PAH derivatives are [M]•- and [M - 20]•−. Moreover, this derivatization produced higher molecular mass compounds, which allowed higher specificity (from m/z 629 for naphthols to m/z 799 for 3-hydroxydibenzo[a,h]anthracene). However, recently obtained results demonstrated that although this approach is suitable for two- to four-ring OH-PAHs, it is unsuitable for compounds with higher molecular mass [57]. In fact, (S,R)-HFBOPCl derivatives generated peak broadening, which did not allow appropriate resolution and separation of OH-PAH isomers, particularly those with more than four aromatic rings. A clearer separation of isomer analytes as well as a higher analytical response have been obtained by means of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) derivatization and EI–triple quadrupole detection [57].
The objective of this study consisted in the development of an analytical method based on GC–MS/MS after derivatization with MTBSTFA for the analysis of OH-PAHs in hair. The method focused on 52 target compounds corresponding to two- to six-ring monohydroxylated metabolites of PAHs. The method was then applied to the analysis of hairs of rats submitted to controlled exposure to PAHs in order to assess the incorporation of PAH metabolites in this matrix and to confirm that the method was sufficiently sensitive to highlight low levels of exposure.
Materials and methods
Reagents and standards
1-Hydroxyphenanthrene and 4-hydroxyphenanthrene standard solutions were supplied at 10 mg L−1 by Dr. Ehrenstorfer (Augsburg, Germany). Stable-isotope-labeled analogues 1-hydroxypyrene-d 9 and naphthol-d 7 were obtained from Chiron (Trondheim, Norway) and Medical Isotopes (Pelham, NH, USA), respectively. 3-Hydroxyfluoranthene-13 C 6, 1-hydroxybenz[a]anthracene-13 C 6, and the 52 OH-PAHs investigated in this study were purchased in powder form from MRIGlobal (Kansas City, MO, USA). The purity of all the standards investigated was above 98 %, except for 2-hydroxychrysene, 9-hydroxybenzo[a]anthracene, 11-hydroxybenzo[a]anthracene, and 8-hydroxyindeno[1,2,3-c,d]pyrene, which had purity ranging from 91 to 96 %. The level of purity was taken into account for the preparation of the standard solutions. ENVI-Chrom P solid-phase extraction (SPE) columns and the derivatization reagent MTBSTFA (purity 97 % or greater) containing 1 % tert-butyldimethylchlorosilane were purchased from Chromoptic (Courtaboeuf, France) and Sigma-Aldrich (Bornem, Belgium), respectively. A stock solution of each compound at 1 g L−1 and mixed-standard solutions of OH-PAHs and internal standards were prepared in volumetric flasks at 10 mg L−1 in acetonitrile. Working solutions were prepared by successive tenfold dilutions at concentrations ranging from 10 to 1,000 μg L−1 and were stored at −20 °C. Ultrapure water was produced by means of an AFS-8 system from Millipore (Brussels, Belgium).
Instrumentation
Analyses were conducted with an Agilent 7890A gas chromatograph equipped with an HP-5MS capillary column (30 m, 0.25-mm inner diameter, 0.25-μm film thickness), coupled to an Agilent 7000A triple-quadrupole mass spectrometer operating in EI mode and an Agilent CTC PAL autosampler.
Gas chromatography
The inlet was at a temperature of 260 °C. Two microliters of extract was injected in pulsed splitless mode with a pressure of 47 psi for 1.5 min. Chromatographic separation was performed using the column described earlier. Helium was used as a carrier gas at 1.8 mL min−1. The oven temperature was kept at 100 °C for 2 min. It was later increased to 235 °C at a rate of 40 °C min−1 and was then raised to 280 °C at 10 °C min−1, and was maintained for 3 min. Finally, after it had been increased at a rate of 10 °C min−1, the temperature was maintained at 300 °C for 14 min. After each run, the temperature was set to 300 °C for 4 min in backflush mode in order to remove high-boiling compounds through the split vent.
Mass spectrometry
The source temperature was set to 230 °C. The spectrometer was operated in EI mode with multiple reaction monitoring. Detailed parameters are presented in Table S1. The collision-induced-dissociation gas and the quench gas in the collision cell were nitrogen (1.5 mL min−1) and helium (2.25 mL min−1), respectively, and were used following the supplier’s recommendations for flow and quality.
Administration of PAHs and sampling
Twenty healthy bicolor (white and black hair) Lister hooded rats (males of 180–200 g) obtained from Harlan (Horst, Netherlands) were kept in plastic cages and acclimatized to the conditions in the animal facility for 1 week under a timed 12 h/12 h light/dark cycle (light on at 7 pm) at 22 ± 2 °C and 40 ± 5 % relative humidity. Food and water were available ad libitum. The water, food, and oil were tested according to NF ISO 15302 to confirm that all these matrices were PAH-free down to a limit of detection (LOD) of 1 ng g−1 fat and 10 ng L−1 water. To avoid external contamination of OH-PAHs in hair by urine excretion, special bedding with a high absorption capacity (4.8 L kg−1, Limogen® 8-15), was used and replaced every 2 days. All procedures complied with the rules set out in European Union Council Directive 2010/63/EU. The PAH stock solution (made of naphthalene, fluorene, phenanthrene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-c,d]pyrene, and dibenzo[a,h]anthracene) was prepared in oil weekly. Five rats were randomly assigned to each of the experimental groups receiving the PAH mixture at a dose of 0.01, 0.1, or 1 mg kg−1, the levels corresponding to the levels of exposure detected in smokers, heavy consumers of smoked or grilled meat and fish, or individuals with heavy occupational exposure [58, 59]. Each rat received intraperitoneal injections of the PAH mixture solubilized in vegetable oil (1 mL kg−1 body weight, ISIO4, Lesieur, Neuilly-sur-Seine, France) three times per week over a 28-day period. Control rats received blank vegetable oil only. The back of the rats was shaved with an electric shaver before and after the PAH exposure period to ensure that the hairs analyzed represented the period of exposure. White and black hairs were collected separately and stored in aluminum foil at room temperature until analysis.
Extraction and purification
Hair specimens were decontaminated by washing a 50-mg sample with 10 mL water (for 2 min) and were dried at room temperature. Hydrolysis was performed with 1 mL 1 N NaOH and samples were incubated overnight at 40 °C. Neutralization was achieved with 500 μL 2 N HCL and 1 mL ammonium acetate buffer (pH 1, 0.2 M). A first liquid–liquid extraction (LLE) was conducted twice with 2 mL dichloromethane (300 agitations per minute for 10 min and centrifugation for 3 min at 1,800 g at room temperature). The organic layer containing OH-PAHs was collected and dried under a nitrogen flow at 37 °C. Then, the residue was dissolved in 2 mL cyclohexane and 2 mL methanol–water (80:20; v/v), shaken at 300 agitations per minute for 10 min, and centrifuged at 1,800 g for 3 min. The upper layer was collected with a glass Pasteur pipette and dried under a nitrogen flow at 37 °C. The residue was dissolved in 1 mL cyclohexane and applied onto an Envi-Chrom P SPE column that had previously been conditioned with water, methanol, and cyclohexane successively. After they had been cleaned with 1 mL cyclohexane, OH-PAHs were eluted with 2 mL cyclohexane–ethyl acetate (50:50; v/v). The extract that contained hydroxylated metabolites was evaporated to dryness (at 37 °C using nitrogen), and 50 μL MTBSTFA was added. Derivatization of target analytes was completed after 30 min at 60 °C, and 2 μL of sample was injected into the GC–MS/MS system.
Data analysis
Peaks were identified by absolute retention time and quantifier and qualifier ion peak area ratios. The transition yielding the highest signal-to-noise ratio for each analyte was used for quantification; the second and third transitions were used as qualifiers (Table S1). Data were processed as peak area ratios of target analytes and their internal standards.
Statistical analysis
The concentrations of OH-PAHs were analyzed using one-way analysis of variance with different doses of the PAH mixture as an independent factor. Given that the data did not follow a Gaussian distribution, nonparametric statistical procedures were used. The data from controls were compared with those from PAH-exposed rats using a Kruskal–Wallis test followed by a modified Dunn’s procedure for post hoc comparisons. The statistical analysis was performed using SigmaPlot (Systat Software, Erkrath, Germany). Differences were considered to be significant at the level of p < 0.05.
Safety considerations
General guidelines for working with organic solvents, acids, and alkalis were respected. All standard compounds have been designated as “chemical carcinogens.” This does not necessarily imply that the sample is a known carcinogen, but merely implies that it is intended for use in research involving chemical carcinogens and that it should be treated as a carcinogen.
Results and discussion
Extraction of OH-PAHs from hair
The efficiency of the extraction method was evaluated using blank rat hairs supplemented with OH-PAHs as well as hairs from animals after controlled exposure to PAHs. The hair samples were washed to remove external contamination such as residues of sebum and the dust typically present on hair and that generates an increase in the analytical noise. The hair washing with water was conducted as previously described by Schummer et al. [32]. This washing step allows impurities from hair to be removed without OH-PAHs being removed. In accordance with previous studies, hydrolysis with NaOH (1 N) was performed in order to increase the recovery of OH-PAHs from hair, and LLE with dichloromethane was used for the extractions of OH-PAHs [32]. Incubation of hair in NaOH, which leads to the complete hydrolysis of the matrix, is particularly suitable for chemically stable compounds such as drugs (Δ9-tetrahydrocannabinol and methamphetamine) or organic micropollutants (PCBs and PAHs) [32, 33, 42, 60, 61].
Purification
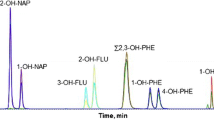
The LLE using dichloromethane did not allow clear separation of the four- to six- ring OH-PAHs. Therefore, an additional SPE combining ethyl acetate–cyclohexane (50:50, v/v) as a mobile phase with Envi-Chrom P as a stationary phase was included in the method. Except for naphthols, the peak area increased by a factor ranging from 1.2 for 3-hydroxydibenzo[a,h]anthracene to 11.7 for 2-hydroxyfluorene. Although this SPE permitted clear separation and a higher response (peak area) of OH-PAHs, a greasy deposit remained in the extract, resulting in rapid clogging of the system and decreased performances. An additional purification step consisting in use of cyclohexane–methanol–water (50:40:10; v/v/v) LLE was thus added, as described in previous studies, with a view to removing impurities (e.g., lipids and proteins) [57, 62] (data not shown). The benefit of this additional LLE for the extraction of OH-PAHs from hair was tested through two different protocols: (1) SPE followed by LLE with cyclohexane–methanol–water (50:40:10; v/v/v); (2) SPE after LLE with cyclohexane–methanol–water (50:40:10; v/v/v). For most of the compounds tested here, the highest response (peak area and signal-to-noise ratio) was obtained when LLE was performed before SPE. For example, the signal-to-noise ratio increased by a factor ranging from 1.1 for 1-hydroxypyrene to 4.2 for 3-hydroxyfluoranthene. The purified extract was then dried before derivatization with MTBSTFA. Derivatization was completed after 30 min at 60 °C, and 2 μL of sample were injected into the GC–MS/MS system. Figure 1 displays the analysis of OH-PAHs in hair samples supplemented at 10 pg mg−1.
Analysis of monohydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) in hair samples supplemented at 10 pg mg−1. OH-PAH standards were derivatized with N-tert-butyldimethylsilyl->N-methyltrifluoroacetamide. [1] hydroxynaphthalenes, [2] hydroxyfluorenes, [3] hydroxyphenanthrenes, [4] hydroxyfluoranthenes/hydroxypyrene, [5] hydroxybenzo[c]phenanthrene/hydroxybenzo[a]anthracene/hydroxychrysenes, [6] hydroxybenzo[a]pyrene/hydroxybenzo[b]fluoranthene/hydroxybenzo[k]fluoranthene, [7] hydroxyindeno[1,2,3-c,d]pyrene, [8] hydroxydibenzo[a,h]anthracene
Chromatographic aspects
Although most of the OH-PAHs were clearly separated, some of them which exhibited the same molecular mass and the same specific transitions ([M]•− → [M-16] •−, [M]• −→ [M-57]−, [M-57]− → [M-57-16]−, and [M-57] −→ [M-57-16-15]•−) remained coeluted. Despite many attempts to separate these isomers (alteration of stationary phase, column characteristics, and temperature program) ten OH-PAH pairs were still coeluted (Table S1). Therefore, for these specific OH-PAHs, the results were provided as the sum of the two compounds (Tables 1 and 2).
Analytical performances
The challenge mainly consisted in obtaining a sensitive and selective analytical method compatible with the low concentrations of the analytes, the complexity of the hair matrix (containing 1–9 % lipids, 65–95 % protein, and 0.1–5 % melanin), and the physicochemical properties of OH-PAHs (hydrophobic compounds). Table 1 details the LOD, limit of quantification (LOQ), calibration curve equations, recovery results, accuracy, and imprecision (relative standard deviation) for all the analytes. A calibration curve was constructed using hair specimens supplemented with the following concentrations of OH-PAHs: 0, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500, and 1,000 pg mg−1. In accordance with several method validation guidelines, four replicates were analyzed for each concentration [63, 64]. Linearity was evaluated by linear regression and expressed by the coefficient of determination (R 2). The calibration curve was linear from the LOQ to 1,000 pg mg−1 for all the OH-PAHs tested (Table 1). The coefficient of determination (R 2) was above 0.997 for all the analytes, with the exception of 6-hydroxyindeno[1,2,3-c,d]pyrene (0.990) and 3-hydroxydibenz[a,h]anthracene (0.986).
Blank samples were analyzed to determine the initial concentration of OH-PAHs in the hairs of rats. Only naphthols were detected, and the recovery values were corrected by subtracting the concentration initially detected in blank samples. Recovery was evaluated by supplementing hair samples at three concentrations (1, 10, and 100 pg mg−1 hair) and by comparing the peak area with the peak area of samples treated identically but supplemented with the same concentrations of OH-PAHs just before the derivatization step. At the highest concentration, the recovery ranged from 4 to 69 % for all the OH-PAHs investigated in this work (Table 1). These results are in line with the recoveries for the OH-PAHs obtained from human hair samples that were mostly above 42 % with the GC–NCI-MS method [32]. Among the 52 OH-PAHs analyzed, only five compounds had recoveries below 25 %.
Regarding the specificity of the method, one or two confirmation transitions were used to ensure the presence of each target compound (Table S1). The variability in the ratio of the quantification transition to the confirmation transition was expected to be within 20 % to confirm the presence of the analytes.
Sensitivity was evaluated by the LOD and LOQ. The LOD was determined for each analyte as the lowest concentration with a signal-to-noise ratio of at least 3:1 for the quantification transition (Table 1). The LOQ was defined as the lowest concentration for each analyte that can be measured using supplemented hair samples with variability within (±) 25 % (n = 4) based on the quantification transition (Table 1). Clearer separation of isomer analytes as well as a higher analytical response were obtained by means of MTBSTFA derivatization and EI–triple quadrupole detection compared with (S,R)-HFBOPCl derivatization and GC–NCI-MS detection [65]. The present method allowed us to achieve LOQs that were from 15 to 30 times lower than those previously obtained for the same OH-PAHs derivatized with (S,R)-HFBOPCl and detected by GC–NCI-MS. The LOD ranged from 0.05 to 10 pg mg−1 hair and the LOQ ranged from 0.2 to 50 pg mg−1 depending on the analyte investigated (Table 1). The LOD and LOQ determined for hydroxynaphthalenes were probably overestimated owing to the presence of these compounds in the blank sample (see Table 1). The sensitivity determined for 9-hydroxyfluorene in hair was clearly below that evaluated for all the other OH-PAHs. This might be explained by poor derivatization due to steric hindrance which might hamper the MTBSTFA derivatization and/or by the fact that the proton from the hydroxyl group is less labile than in other OH-PAHs, for which the negative charge is stabilized by the aromaticity. In contrast, the negative charge of oxygen resulting from the loss of hydrogen is delocalized all around the ring for all the other OH-PAHs, thereby increasing alkoxide stability and improving its reactivity regarding the derivatization agent. As previously described by Schummer et al. [65], MTBSTFA derivatization on a standard solution facilitates the separation of OH-PAH isomer analytes, whereas N,O-bis(trimethylsilyl)trifluoroacetamide seems more suitable for sterically hindered compounds such as 9-hydroxyfluorene. Nevertheless, in previous work we observed that the sensitivity obtained using MTBSTFA derivatization for the analysis of 9-hydroxyfluorene in brain was acceptable (LOQ of 0.6 pg mg−1 brain tissue and LOD of 0.2 pg mg−1 brain tissue) [57], leading us to suppose that the lack of sensitivity observed here for the analysis of this compound in hair might be due to the presence of impurities specific to this matrix.
Precision and accuracy were evaluated with hair samples spiked at three different concentrations (1, 10, and 100 pg mg−1) for each compound (Table 1). The results are expressed as a percentage of the target value and the relative standard deviation. Except for hydroxyindeno[1,2,3-c,d]pyrenes, intraday precision (n = 4) was always below 15 % (and often below 10 %) for all the compounds. Interday precision (n = 30) was in most cases below 20 % and was always below 25 %. Except for 1-hydroxyindeno[1,2,3-c,d]pyrene and 8-hydroxyindeno[1,2,3-c,d]pyrene, intraday accuracy (n = 4) and interday accuracy (n = 30) evaluated at an OH-PAH dose of 100 pg mg−1 in hair were 95–105 % and 91–106 % (Table 1).
Although the present method exhibits satisfactory separation of most of the metabolites investigated here, 23 of the 75 possible monohydroxylated metabolites of PAHs were not analyzed because standards were not available from suppliers. As a consequence, it cannot be ruled out that the latter analytes were coeluted with the ones analyzed with the present method and possibly led to overestimation of the amount determined for some of the target compounds.
Application to hair samples from treated rats
The main disadvantages concerning hair analysis in the biomonitoring of human exposure to pollutants lie in (1) the possibility of external deposition of chemicals on the hair surface and (2) the possible influence of hair pigmentation (i.e., the melanin content of hair) [50]. To avoid misinterpretation due to external contamination, the method developed here focuses on the analysis of OH-PAHs. Being produced during the metabolic processes, these compounds are not present in air and are unlikely to be deposited via air. Naphthols are an exception, since these compounds were detected in air in areas where coal combustion is an important source of energy production [51–54]. Our in vivo study reports on the quantitative analysis of 52 OH-PAHs in hair samples obtained from Lister hooded rats exposed to a PAH mixture at doses ranging from 0.01 to 1 mg kg−1 for 28 days. The results of this study confirm that these metabolites can be incorporated in hair after intraperitoneal administration of the corresponding parent compounds (Figs. 2 and 3). The 52 OH-PAHs investigated here were metabolites of the 12 PAHs administered to the rats. Only 20 of the 52 metabolites were actually detected in hair samples. These 20 metabolites corresponded to nine parent PAHs (Table 2, Fig. 2). No metabolites of fluorene, benzo[k]fluoranthene, and dibenzo[a,h]anthracene were detected.
Chromatograms obtained from the analysis of all the investigated OH-PAHs in a supplemented hair sample, a blank hair sample, and a treated rat hair sample (rat exposed to polycyclic aromatic hydrocarbons at a dose of 1 mg kg−1 by intraperitoneal administration for 28 days). [1] 1-hydroxynaphthalene-d 7, [2] 1-hydroxynaphthalene, [3] 2-hydroxynaphthalene, [4] 9-hydroxyfluorene, [5] 3-hydroxyfluorene, [6] 2-hydroxyfluorene, [7] 4-hydroxyphenanthrene, [8] 9-hydroxyphenanthrene, [9] 3-hydroxyphenanthrene, [10] 1-hydroxyphenanthrene, [11] 2-hydroxyphenanthrene, [12] 3-hydroxyfluoranthene-13 C 6, [13] 3-hydroxyfluoranthene, [14] 1-hydroxypyrene-d 9, [15] 1-hydroxypyrene, [16] 2-hydroxybenzo[c]phenanthrene, [17] hydroxybenzo[a]anthracene-13 C 6, [18] 1-hydroxybenzo[a]anthracene, [19] 4-hydroxychrysene, [20] 6-hydroxychrysene and 11-hydroxybenzo[a]anthracene, [21] 2-hydroxybenzo[a]anthracene and 5-hydroxybenzo[a]anthracene, [22] 3-hydroxybenzo[c]pyrene, [23] 8-hydroxybenzo[a]anthracene, [24] 3-hydroxychrysene, [25] 1-hydroxychrysene and 4-hydroxybenzo[a]anthracene, [26] 10-hydroxybenzo[a]anthracene, [27] 3-hydroxybenzo[a]anthracene and 9-hydroxybenzo[a]anthracene, [28] 2-hydroxychrysene, [29] 8-hydroxybenzo[b]fluoranthene, [30] 11-hydroxy[a]pyrene, [31] 2-hydroxybenzo[b]fluoranthene, [32] 1-hydroxybenzo[b]fluoranthene and 7-hydroxybenzo[b]fluoranthene, [33] 12-hydroxybenzo[b]fluoranthene and 8-hydroxybenzo[k]fluoranthene, [34] 10-hydroxybenzo[a]pyrene, [35] 12-hydroxybenzo[a]pyrene and 6-hydroxybenzo[a]pyrene, [36] 5-hydroxybenzo[a]pyrene, [37] 11-hydroxybenzo[b]fluoranthene, [38] 10-hydroxybenzo[b]fluoranthene, [39] 4-hydroxybenzo[a]pyrene and 3-hydroxybenzo[k]fluoranthene, [40] 9-hydroxybenzo[k]fluoranthene and 7-hydroxybenzo[a]pyrene, [41] 9-hydroxybenzo[a]pyrene, [42] 2-hydroxybenzo[a] and 1-hydroxybenzo[a]pyrene, [43] 3-hydroxybenzo[a]pyrene, [44] 8-hydroxybenzo[a]pyrene, [45] 6-hydroxyindeno[1,2,3-c,d]pyrene, [46] 1-hydroxyindeno[1,2,3-c,d]pyrene, [47] 2-hydroxyindeno[1,2,3-c,d]pyrene, [48] 8-hydroxyindeno[1,2,3-c,d]pyrene, [49] 3-hydroxydibenzo[a,h]anthracene
Concentration of OH-PAHs in white hair of rats exposed to polycyclic aromatic hydrocarbons (0.01–1 mg kg−1, intraperitoneal administration, 28 days). The results are expressed as the mean ± the standard deviation. OH-naphthalene hydroxynaphthalene, OH-phenanthrene, hydroxyphenanthrene, 3-OH-fluoranthene 3-hydroxyfluoranthene, 1-OH-pyrene 1-hydroxypyrene, OH-chrysene hydroxychrysene, OH-B[a]A hydroxybenzo[a]anthracene, OH-B[b]F hydroxybenzo[b]fluoranthene, 3-OH-B[a]P 3-hydroxybenzo[a]pyrene, 6-OH-I[1,2,3-c,d]P 6-hydroxyindeno[1,2,3-c,d]pyrene
Although frequently encountered in human hairs, 9-hydroxyfluorene, 2-hydroxyfluorene, and 3-hydroxyfluorene, were not detected in rat hairs regardless of the dose analyzed. These results suggest that 2-hydroxyfluorene and 3-hydroxyfluorene were probably minor metabolites, and that other monohydoxlated or dihydroxylated metabolites could be produced in higher quantities. This is certainly the case for 9-hydroxyfluorene, which is the most abundant metabolite of fluorene detected in human hair [55]. The high LOQ determined for this compound did not allow the detection of concentrations below 50 pg mg−1 hair. The absence of positive detection in hair samples could also be explained by the fact that the level of exposure of humans to fluorene was higher than the controlled exposure of rats.
In parallel, three hypotheses may account for the absence of hydroxybenzo[k]fluoranthene and hydroxydibenzo[a,h]anthracene in hairs. Firstly, the metabolites of hydroxybenzo[k]fluoranthene and hydroxydibenzo[a,h]anthracene investigated here might not be produced or incorporated in hair. This assumption is supported by the low bioavailability of high molecular mass PAHs [66] and by recent findings of the absence of these metabolites in urine of control and asphalt workers and poor recovery in urine of coke oven workers [67]. Secondly, the high LOQ obtained for hydroxybenzo[k]fluoranthene and hydroxydibenzo[a,h]anthracene as compared with the other metabolites could also explain why these five-ring metabolites were not detected in rat hairs. Finally, rat metabolism may produce monohydroxylated metabolites different from those (3-hydroxybenzo[k]fluoranthene, 8-hydroxybenzo[k]fluoranthene, 9-hydroxybenzo[k]fluoranthene, and 3-hydroxydibenzo[a,h]anthracene) investigated in this study (standards unavailable from suppliers). Chromatographic analysis seems to confirm the latter hypothesis. On comparison of chromatograms of blank (n = 4) and supplemented (1, 10, and 100 pg mg−1) hair samples with hair samples from rats exposed to 1 mg kg−1 (n = 4) (data not shown), two peaks were detected at retention times of 18.15 and 18.31 min. Both peaks corresponded to the transitions common to hydroxybenzo[a]pyrene, hydroxybenzo[k]fluoranthene, or hydroxybenzo[b]fluoranthene with a precursor ion at m/z = 382.6 and a product ion at m/z = 325.2. Given that the retention time was identified for all the hydroxybenzo[a]pyrene isomers, these two peaks likely to correspond to isomers of hydroxybenzo[k]fluoranthene and/or hydroxybenzo>[b]fluoranthene.
The concentrations of the OH-PAHs in white hair samples of rats exposed to PAHs are presented in Table 2 and Fig. 3. Surprisingly, the levels of 1-hydroxynapthalene and 2-hydroxynaphthalene in the control group were considerably higher than in the other treated groups. The concentrations of naphthols detected in the hairs of the control group probably result from environmental exposure to naphthalene. Moreover, no statistical difference was observed between the control group and the treated groups, suggesting that for the naphthols, the contribution of the controlled exposure (0.01–1 mg kg−1 for 28 days) was negligible in comparison with environmental exposure.
In contrast, 3-hydroxyphenanthrene and 1-hydroxypyrene, although not detected in blank samples, were detected above the LOQ in rats which received the 0.1 and 1 mg kg−1 doses for 28 days. The method developed here did not allow the detection of most of the OH-PAH metabolites in rats which received the two lowest doses of PAHs (0.01 and 0.1 mg kg−1 for 28 days). Two hypotheses may explain these results: (1) PAH doses of 0.01 and 0.1 mg kg−1 were below the thresholds at which the metabolism starts to be induced (Fig. 3) and/or (2) the method is not sensitive enough. These results are in accordance with previous data obtained from rat brain after exposure of rats to PAHs at concentrations ranging between 0.01 and 1 mg kg−1 [57]. Except for 6-hydroxyindeno[1,2,3-c,d]pyrene, which was detected only in hair samples, all the OH-PAHs found in hairs were also detected in brain [57]. For example, 1-hydroxypyrene is the most abundant metabolite in both matrices (26.5 ± 22.7 pg mg−1 in brain tissue versus 6.7 ± 1.0 pg mg−1 in hairs of rats that received PAHs at a dose of 1 mg kg−1 for 28 days). These data are also in accordance with recent work that reported the presence of two- to four-ring OH-PAHs (1-hydroxynapthalene, 2-hydroxynaphthalene, 2-hydroxyfluorene, 9-hydroxyfluorene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 3-hydroxyphenanthrene, 4-hydroxyphenanthrene, 9-hydroxyphenanthrene, 1-hydroxypyrene, 2-hydroxybenzo[c]phenanthrene, and 6-hydroxychrysene) in the hairs of humans exposed to environmental levels of PAHs [55].
The influence of hair pigmentation has also been mentioned among the possible limitations to the use of hair as a biomarker of exposure [50]. For instance, rats which were received methadone in their drinking water exhibited significantly higher concentrations in pigmented hair than in white hair in a ratio of 21:1 [68]. For this reason, the 52 OH-PAHs investigated in this work were analyzed in both black and white hairs that were separately collected from each rat. Although the concentration was significantly higher in black hair for 4-hydroxyphenanthrene and 1-hydroxypyrene (p < 0.05), the difference between black and white hair was not significant for the other metabolites (Table 2). For 1-hydroxypyrene, the concentrations were significantly higher in pigmented hair for exposure at both 0.1 mg kg−1 (p = 0.105) and 1 mg kg−1 (p < 0.05). Nevertheless, the black hair–white hair concentration ratios of 4-hydroxyphenanthrene and 1-hydroxypyrene remain weakly affected (1.3:1 and 1.7:1, respectively). In addition, taking into account the sum of the five hydroxyphenanthrenes, we found that the difference observed between black and white hairs for 4-hydroxyphenanthrene was not significant (Fig. 4). Similar differences were observed for dimethylphosphate between pigmented and white hairs of bicolor rabbits after long-term exposure to dimethoate (an organophosphate used as a pesticide) [46]. Although the concentration of this metabolite was higher in pigmented hairs after 4 and 6 months’ exposure and for the two levels tested, the pigmented hair–white hair concentration ratio did not exceed 1.8 [46]. Margariti et al. [46] expressed some reservations about their results (such as the metabolic difference between species and the limited number of rabbits) and concluded that hair pigmentation may affect the incorporation of dimethylphosphate into hair. Thus, our results demonstrate that, at least for rats, hair pigmentation has no influence on the concentration of most OH-PAHs and only a limited influence on the concentrations of 4-hydroxyphenanthrene and 1-hydroxypyrene. These results still have to be confirmed in human hair.
Concentration of OH-PAHs in white and black hair of rats exposed to polycyclic aromatic hydrocarbons (1 mg kg−1, intraperitoneal administration, 28 days). The results are expressed as the mean ± the standard deviation. Asterisk P < 0.05 statistically significant differences from control rats (Dunn’s procedure for post hoc comparisons)
Conclusions
This study has described the development of a sensitive method for the simultaneous determination of OH-PAHs in hair by GC–EI-MS/MS. We have presented a validated, highly sensitive, and selective method for the determination of very low concentrations of 52 OH-PAHs in rat hairs ranging between 0.2 and 50 pg mg−1. This rat experiment confirmed the incorporation of PAH metabolites in hairs and demonstrated that the method was sensitive enough to highlight low levels of exposure to PAHs. These results confirm the usefulness of hair analysis for the biomonitoring of human exposure to PAHs.
References
Chetiyanukornkul T, Toriba A, Kameda T, Tang N, Hayakawa K (2006) Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Anal Bioanal Chem 386(3):712–718. doi:10.1007/s00216-006-0628-6
Elovaara E, Mikkola J, Makela M, Paldanius B, Priha E (2006) Assessment of soil remediation workers’ exposure to polycyclic aromatic hydrocarbons (PAH): biomonitoring of naphthols, phenanthrols, and 1-hydroxypyrene in urine. Toxicol Lett 162(2–3):158–163. doi:10.1016/j.toxlet.2005.09.028
Grainger J, Huang W, Patterson DG Jr, Turner WE, Pirkle J, Caudill SP, Wang RY, Needham LL, Sampson EJ (2006) Reference range levels of polycyclic aromatic hydrocarbons in the US population by measurement of urinary monohydroxy metabolites. Environ Res 100(3):394–423. doi:10.1016/j.envres.2005.06.004
Vaananen V, Elovaara E, Nykyri E, Santonen T, Heikkila P (2006) Road pavers’ occupational exposure to asphalt containing waste plastic and tall oil pitch. J Environ Monit 8(1):89–99. doi:10.1039/b513505b
Bouchard M, Normandin L, Gagnon F, Viau C, Dumas P, Gaudreau E, Tremblay C (2009) Repeated measures of validated and novel biomarkers of exposure to polycyclic aromatic hydrocarbons in individuals living near an aluminum plant in Quebec, Canada. J Toxicol Environ Health A 72(23):1534–1549. doi:10.1080/15287390903129481
Bouchard M, Thuot R, Carrier G, Viau C (2002) Urinary excretion kinetics of 1-hydroxypyrene in rats subchronically exposed to pyrene or polycyclic aromatic hydrocarbon mixtures. J Toxicol Environ Health A 65(16):1195–1209. doi:10.1080/152873902760125408
Bouchard M, Viau C (1996) Urinary excretion kinetics of pyrene and benzo(a)pyrene metabolites following intravenous administration of the parent compounds or the metabolites. Toxicol Appl Pharmacol 139(2):301–309. doi:10.1006/taap.1996.0169
Chahin A, Guiavarc’h YP, Dziurla MA, Toussaint H, Feidt C, Rychen G (2008) 1-Hydroxypyrene in milk and urine as a bioindicator of polycyclic aromatic hydrocarbon exposure of ruminants. J Agric Food Chem 56(5):1780–1786
Chien YC, Yeh CT (2010) Amounts and proportion of administered pyrene dose excreted as urinary 1-hydroxypyrene after dietary exposure to polycyclic aromatic hydrocarbons. Arch Toxicol 84(10):767–776. doi:10.1007/s00204-010-0570-4
Chien YC, Yeh CT (2012) Excretion kinetics of urinary 3-hydroxybenzo[a]pyrene following dietary exposure to benzo[a]pyrene in humans. Arch Toxicol 86(1):45–53. doi:10.1007/s00204-011-0727-9
Heredia-Ortiz R, Bouchard M, Marie-Desvergne C, Viau C, Maitre A (2011) Modeling of the internal kinetics of benzo(a)pyrene and 3-hydroxybenzo(a)pyrene biomarker from rat data. Toxicol Sci 122(2):275–287. doi:10.1093/toxsci/kfr135
Hollender J, Koch B, Dott W (2000) Biomonitoring of environmental polycyclic aromatic hydrocarbon exposure by simultaneous measurement of urinary phenanthrene, pyrene and benzo[a]pyrene hydroxides. J Chromatogr B: Biomed Sci Appl 739(1):225–229
Jacob J, Grimmer G (1996) Metabolism and excretion of polycyclic aromatic hydrocarbons in rat and in human. Cent Eur J Public Health 4(Suppl):33–39
Marie C, Bouchard M, Heredia-Ortiz R, Viau C, Maitre A (2010) A toxicokinetic study to elucidate 3-hydroxybenzo(a)pyrene atypical urinary excretion profile following intravenous injection of benzo(a)pyrene in rats. J Appl Toxicol 30(5):402–410. doi:10.1002/jat.1511
Ariese F, Verkaik M, Hoornweg GP, van de Nesse RJ, Jukema-Leenstra SR, Hofstraat JW, Gooijer C, Velthorst NH (1994) Trace analysis of 3-hydroxy benzo[a]pyrene in urine for the biomonitoring of human exposure to polycyclic aromatic hydrocarbons. J Anal Toxicol 18(4):195–204
Forster K, Preuss R, Rossbach B, Bruning T, Angerer J, Simon P (2008) 3-hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup Environ Med 65(4):224–229. doi:10.1136/oem.2006.030809
Gendre C, Lafontaine M, Morele Y, Payan JP, Simon P (2002) Relationship between urinary levels of 1-hydroxypyrene and 3-hydroxybenzo[a]pyrene for workers exposed to ploycyclic aromatic hydrocarbons. Polycycl Aromat Compd 22:761–769
Hansen AM, Mathiesen L, Pedersen M, Knudsen LE (2008) Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies—a review. Int J Hyg Environ Health 211:471–503
Viau C, Diakite A, Ruzgyte A, Tuchweber B, Blais C, Bouchard M, Vyskocil A (2002) Is 1-hydroxypyrene a reliable bioindicator of measured dietary polycyclic aromatic hydrocarbon under normal conditions? J Chromatogr B Anal Technol Biomed Life Sci 778(1–2):165–177
Nethery E, Wheeler AJ, Fisher M, Sjodin A, Li Z, Romanoff LC, Foster W, Arbuckle TE (2012) Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J Expo Sci Environ Epidemiol 22(1):70–81. doi:10.1038/jes.2011.32
Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG Jr (2008) Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res 107(3):320–331. doi:10.1016/j.envres.2008.01.013
Romanoff LC, Li Z, Young KJ, Blakely NC 3rd, Patterson DG Jr, Sandau CD (2006) Automated solid-phase extraction method for measuring urinary polycyclic aromatic hydrocarbon metabolites in human biomonitoring using isotope-dilution gas chromatography high-resolution mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 835(1–2):47–54. doi:10.1016/j.jchromb.2006.03.004
Gmeiner G, Geisendorfer T, Kainzbauer J, Nikolajevic M, Tausch H (2002) Quantification of ephedrines in urine by column-switching high-performance liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci 768(2):215–221
Gmeiner G, Krassnig C, Schmid E, Tausch H (1998) Fast screening method for the profile analysis of polycyclic aromatic hydrocarbon metabolites in urine using derivatisation–solid-phase microextraction. J Chromatogr B: Biomed Sci Appl 705(1):132–138
Grimmer G, Dettbarn G, Jacob J (1993) Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols). Int Arch Occup Environ Health 65(3):189–199
Yang M, Koga M, Katoh T, Kawamoto T (1999) A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol 36(1):99–108
Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu R, Waidyanatha S (2009) Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr B Anal Technol Biomed Life Sci 877(11–12):1117–1125. doi:10.1016/j.jchromb.2009.02.067
Ramsauer B, Sterz K, Hagedorn HW, Engl J, Scherer G, McEwan M, Errington G, Shepperd J, Cheung F (2011) A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal Bioanal Chem 399(2):877–889. doi:10.1007/s00216-010-4355-7
Xu X, Zhang J, Zhang L, Liu W, Weisel CP (2004) Selective detection of monohydroxy metabolites of polycyclic aromatic hydrocarbons in urine using liquid chromatography/triple quadrupole tandem mass spectrometry. Rapid Commun Mass Spectrom 18(19):2299–2308. doi:10.1002/rcm.1625
Benowitz NL, Jacob P 3rd, Bernert JT, Wilson M, Wang L, Allen F, Dempsey D (2005) Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev 14(6):1376–1383. doi:10.1158/1055-9965.EPI-04-0667
Jacob P 3rd, Wilson M, Benowitz NL (2007) Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem 79(2):587–598. doi:10.1021/ac060920l
Schummer C, Appenzeller BM, Millet M, Wennig R (2009) Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in human hair by gas chromatography-negative chemical ionization mass spectrometry. J Chromatogr A 1216(32):6012–6019. doi:10.1016/j.chroma.2009.05.068
Toriba A, Kuramae Y, Chetiyanukornkul T, Kizu R, Makino T, Nakazawa H, Hayakawa K (2003) Quantification of polycyclic aromatic hydrocarbons (PAHs) in human hair by HPLC with fluorescence detection: a biological monitoring method to evaluate the exposure to PAHs. Biomed Chromatogr 17(2–3):126–132. doi:10.1002/bmc.222
Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS (2007) Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J 21(1):108–116. doi:10.1096/fj.06-6848com
Uchida K, Araki T, Toiyama Y, Yoshiyama S, Inoue M, Ikeuchi H, Yanagi H, Miki C, Yamamura T, Kusunoki M (2006) Preoperative steroid-related complications in Japanese pediatric patients with ulcerative colitis. Dis Colon Rectum 49(1):74–79. doi:10.1007/s10350-005-0213-7
Kintz P (2007) Analytical and practical aspects of drug testing in hair. CRC, Boca Raton
Pragst F, Balikova MA (2006) State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370(1–2):17–49. doi:10.1016/j.cca.2006.02.019
Tsatsakis A, Tutudaki M (2004) Progress in pesticide and POPs hair analysis for the assessment of exposure. Forensic Sci Int 145(2–3):195–199. doi:10.1016/j.forsciint.2004.04.036
Appenzeller BM, Agirman R, Neuberg P, Yegles M, Wennig R (2007) Segmental determination of ethyl glucuronide in hair: a pilot study. Forensic Sci Int 173(2–3):87–92. doi:10.1016/j.forsciint.2007.01.025
Margariti MG, Tsatsakis AM (2009) Analysis of dialkyl phosphate metabolites in hair using gas chromatography-mass spectrometry: a biomarker of chronic exposure to organophosphate pesticides. Biomarkers 14(3):137–147. doi:10.1080/13547500902792912
Barbounis EG, Tzatzarakis MN, Alegakis AK, Kokkinaki A, Karamanos N, Tsakalof A, Tsatsakis AM (2012) Assessment of PCBs exposure in human hair using double focusing high resolution mass spectrometry and single quadrupole mass spectrometry. Toxicol Lett 210(2):225–231. doi:10.1016/j.toxlet.2011.07.031
Nakao T, Aozasa O, Ohta S, Miyata H (2002) Assessment of human exposure to PCDDs, PCDFs and Co-PCBs using hair as a human pollution indicator sample I: development of analytical method for human hair and evaluation for exposure assessment. Chemosphere 48(8):885–896. doi:10.1016/S0045-6535(02)00156-X
Nakao T, Aozasa O, Ohta S, Miyata H (2005) Survey of human exposure to PCDDs, PCDFs, and coplanar PCBs using hair as an indicator. Arch Environ Contam Toxicol 49(1):124–130. doi:10.1007/s00244-004-0059-3
Ohgami T, Nonaka S, Irifune H, Watanabe M, Tsukazaki N, Tanaka K, Yano M, Yoshida H, Murayama F, Rikioka Y (1991) A comparative study on the concentrations of polychlorinated biphenyls (PCBs) and polychlorinated quaterphenyls (PCQs) in the blood and hair of “Yusho” patients and inhabitants of Nagasaki Prefecture. Fukuoka Igaku Zasshi 82(5):295–299
Margariti MG, Tsakalof AK, Tsatsakis AM (2007) Analytical methods of biological monitoring for exposure to pesticides: recent update. Ther Drug Monit 29(2):150–163. doi:10.1097/FTD.0b013e31803d3509
Margariti MG, Tsatsakis AM (2009) Assessment of long-term subacute exposure to dimethoate by hair analysis of dialkyl phosphates DMP and DMTP in exposed rabbits: the effects of dose, dose duration and hair colour. Environ Res 109(7):821–829. doi:10.1016/j.envres.2009.07.009
Tutudaki M, Tsakalof AK, Tsatsakis AM (2003) Hair analysis used to assess chronic exposure to the organophosphate diazinon: a model study with rabbits. Hum Exp Toxicol 22(3):159–164
Tutudaki M, Tsatsakis AM (2005) Pesticide hair analysis: development of a GC-NCI-MS method to assess chronic exposure to diazinon in rats. J Anal Toxicol 29(8):805–809
Hasei T, Ohno T, Inoue T, Watanabe T (2011) Determination of 3,6-dinitrobenzo[e]pyrene in tea leaves as a possible exposure source and in human hair as a biomarker using a two-dimensional HPLC system. J Health Sci 57(1):53–59
Appenzeller BMR, Tsatsakis AM (2012) Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: state of the art, critical review and future needs. Toxicol Lett 210(2):119–140. doi:10.1016/j.toxlet.2011.10.021
Kishikawa N, Morita S, Wada M, Ohba Y, Nakashima K, Kuroda N (2004) Determination of hydroxylated polycyclic aromatic hydrocarbons in airborne particulates by high-performance liquid chromatography with fluorescence detection. Anal Sci 20(1):129–132
Kishikawa N, Wada M, Ohba Y, Nakashima K, Kuroda N (2004) Highly sensitive and selective determination of 9, 10-phenanthrenequinone in airborne particulates using high-performance liquid chromatography with pre-column derivatization and fluorescence detection. J Chromatogr A 1057(1–2):83–88
Simoneit BR, Bi X, Oros DR, Medeiros PM, Sheng G, Fu J (2007) Phenols and hydroxy-PAHs (arylphenols) as tracers for coal smoke particulate matter: source tests and ambient aerosol assessments. Environ Sci Technol 41(21):7294–7302
Wang G, Kawamura K (2005) Molecular characteristics of urban organic aerosols from Nanjing: a case study of a mega-city in China. Environ Sci Technol 39(19):7430–7438
Appenzeller BM, Mathon C, Schummer C, Alkerwi A, Lair ML (2012) Simultaneous determination of nicotine and PAH metabolites in human hair specimen: a potential methodology to assess tobacco smoke contribution in PAH exposure. Toxicol Lett. doi:10.1016/j.toxlet.2011.11.022
Martins LF, Yegles M, Chung H, Wennig R (2006) Sensitive, rapid and validated gas chromatography/negative ion chemical ionization-mass spectrometry assay including derivatisation with a novel chiral agent for the enantioselective quantification of amphetamine-type stimulants in hair. J Chromatogr B Anal Technol Biomed Life Sci 842(2):98–105. doi:10.1016/j.jchromb.2006.04.024
Grova N, Salquebre G, Schroeder H, Appenzeller BM (2011) Determination of PAHs and OH-PAHs in rat brain by gas chromatography tandem (triple quadrupole) mass spectrometry. Chem Res Toxicol 24(10):1653–1667. doi:10.1021/tx2003596
World Health Organization (1998) International Program on Chemical Safety. Environmental health criteria 202. Selected non-heterocyclic aromatic hydrocarbons. World Health Organization, Geneva, pp 540–543
Menzie CA, Potocki BB, Santodonato J (1992) Exposure to carcinogenic PAHs in the environment. Environ Sci Technol 26:1278–1284
Han E, Yang W, Lee S, Kim E, In S, Choi H, Chung H, Song JM (2011) Establishment of the measurement uncertainty of 11-nor-D9-tetrahydrocannabinol-9-carboxylic acid in hair. Forensic Sci Int 206(1–3):e85–92. doi:10.1016/j.forsciint.2010.11.028
Han E, Park Y, Kim E, Lee S, Choi H, Chung H, Song JM (2010) The dependence of the incorporation of methamphetamine into rat hair on dose, frequency of administration and hair pigmentation. J Chromatogr B Anal Technol Biomed Life Sci 878(28):2845–2851. doi:10.1016/j.jchromb.2010.08.040
Grova N, Monteau F, Le Bizec B, Feidt C, Andre F, Rychen G (2005) Determination of phenanthrene and hydroxyphenanthrenes in various biological matrices at trace levels using gas chromatography-mass spectrometry. J Anal Toxicol 29(3):175–181
Peters FT, Drummer OH, Musshoff F (2007) Validation of new methods. Forensic Sci Int 165(2–3):216–224. doi:10.1016/j.forsciint.2006.05.021
Hartmann C, Smeyers-Verbeke J, Massart DL, McDowall RD (1998) Validation of bioanalytical chromatographic methods. J Pharm Biomed Anal 17(2):193–218. doi:10.1016/S0731-7085(97)00198-2
Schummer C, Delhomme O, Appenzeller BM, Wennig R, Millet M (2009) Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 77(4):1473–1482. doi:10.1016/j.talanta.2008.09.043
Chang LH (1943) The fecal excretion of polycyclic hydrocarbons following their administration to the rat. J Biol Chem 151:93–99
Campo L, Fustinoni S, Bertazzi P (2011) Quantification of carcinogenic 4-to 6-ring polycyclic aromatic hydrocarbons in human urine by solid-phase microextraction gas chromatography-isotope dilution mass spectrometry. Anal Bioanal Chem 401(2):625–634. doi:10.1007/s00216-011-5110-4
Green SJ, Wilson JF (1996) The effect of hair color on the incorporation of methadone into hair in the rat. J Anal Toxicol 20(2):121–123
Acknowledgments
We are most grateful to Henri Schroeder and Marie France Schoën for their technical assistance. This work was supported by the Luxembourg Ministère de l’Enseignement Supérieur et de la Recherche.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 24 kb)
Rights and permissions
About this article
Cite this article
Grova, N., Salquèbre, G. & Appenzeller, B.M.R. Gas chromatography–tandem mass spectrometry analysis of 52 monohydroxylated metabolites of polycyclic aromatic hydrocarbons in hairs of rats after controlled exposure. Anal Bioanal Chem 405, 8897–8911 (2013). https://doi.org/10.1007/s00216-013-7317-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7317-z