Abstract
A method is presented for determining monohydroxy polycyclic aromatic hydrocarbons (OHPAHs) having 2-, 3- and 4-rings in human urine by using high-performance liquid chromatography with fluorescence detection. A urine sample containing conjugates of OHPAHs was hydrolysed in the presence of β-glucuronidase/aryl sulfatase and the solution was cleaned up with a solid-phase extraction (C18 and silica). Eight OHPAHs, namely 1- and 2-hydroxynaphthalenes, 2-hydroxyfluorene, 2-, 3- and 4-hydroxyphenanthrenes, 3-hydroxyfluoranthene and 1-hydroxypyrene, were separated and 1- and 9-hydroxyphenanthrenes co-eluted on an alkylamide-type reversed-phase column with fluorimetric detection. The urinary concentrations of OHPAHs were quantified by using deuterated 1-hydoxypyrene as an internal standard. The method showed good repeatability for inter- and intra-day precisions as well as good linearity of calibration curves (r 2 ranged from 0.996 to 0.999). The limits of detection (S/N=3) were in the range from 2.3 fmol to 2.2 pmol per injection. This method was successfully applied to urine samples from non-smoking taxi drivers, traffic policemen and rural villagers of Chiang Mai, Thailand. The results showed higher urinary concentrations of OHPAHs in rural villagers, consistent with higher respiratory exposure to PAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are formed and emitted into the environment as a result of incomplete combustion of organic materials from natural and human activities. Some PAHs are carcinogenic or co-carcinogenic compounds [1]. PAHs are also known to have endocrine-disrupting activity [2, 3]. Humans are exposed to PAHs from air, water and foods. Therefore, environmental pollution, human activity and/or life-style might affect the exposure level of PAHs. PAHs are absorbed into the human body through the skin, lungs and gastrointestinal tract, and are then metabolized to their monohydroxylated PAHs (OHPAHs) and finally to glucuronides and sulfates. Conjugates of OHPAHs are excreted in urine or bile.
Measurement of OHPAHs in human urine is a method to assess recent individual exposure to PAHs, in particular, when multiple routes of exposure have to be taken into account. Urinary metabolites of PAHs reflect a more accurate estimation of the quality of the actual PAH intake compared to ambient air measurements because they estimate the internal dose. In 1987, Jongeneelen et al. [4] developed an HPLC method for the determination of 1-hydroxypyrene (1-OHPyr), one of the major urinary metabolites of pyrene. Urinary 1-OHPyr has been used in various studies as a biological indicator of exposure to PAHs [5–7]. Workers occupationally exposed to high concentrations of PAHs (e.g. from coke plants, road paving, wood impregnation and aluminium smelting) have shown elevated excretion of 1-OHPyr. This biological monitoring method has the disadvantage that it does not measure the metabolites of PAHs other than pyrene. In other words, 1-OHPyr may sometimes be an indicator of the absorption of pyrene only. Therefore, analytical methods for simultaneously determining OHPAHs in human urine have been recently proposed. We recently developed a column-switching method that has a high selectivity for 2-hydroxyfluorene (2-OHFle) [8]. 2-OHFle was a suitable indicator of the exposure to PAHs in the vapour phase from smoking [9]. Thus, simultaneous determination of several urinary OHPAHs may provide more comprehensive information to estimate the individual exposure to PAHs.
Exposure to PAHs via the inhalation pathway is due to PAHs being present in ambient and indoor air. Most people spend more than 80% of their time indoors, so indoor air is a significant contributor to human exposure to PAHs. Other sources of PAHs besides ambient air include cigarette smoke [10, 11] and domestic heating [12]. In ambient air and indoor air, the concentrations of the two-, three- and four-ringed PAHs, which are predominantly in the vapour phase, were significantly higher than those of five-ringed or larger PAHs that are primarily associated with the particulate phase [13–15]. In particular, several findings indicated that concentrations of 2- to 4-ringed PAHs in indoor air always exceed those in ambient air [13–15]. Therefore, the metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene to which humans may be exposed at high concentrations were chosen as multiple biomarkers of exposure to representative PAHs having two to four rings in this study.
Many HPLC methods have been used to determine urinary OHPAHs such as hydroxynaphthalenes (OHNaps) and/or hydroxyphenanthrenes (OHPhes) and/or 1-OHPyr [16–19]. GC-MS has also been used to determine various OHPAHs, although it requires a derivatization step [20, 21]. HPLC methods seem to be easier than GC-MS methods and have been preferred as the method of choice for determining urinary OHPAHs. On the other hand, the use of an internal standard or standard addition method is desirable to accurately quantify an analyte in biological samples. The sample matrix and interferences may be different in different lots of the urine, originating from different subjects, and may therefore result in variation in the recovery of the pretreatment method. Use of an internal standard for the analysis of biological samples is highly recommended, since the standard addition method requires that samples be run at least three times. We previously developed HPLC methods for determining 1-OHPyr [22] and 2-OHFle [8] by using their deuterated internal standards with fluorescence detection and demonstrated the usefulness of the internal standards.
In this study, we developed an HPLC method for simultaneously determining ten urinary OHPAHs, including 1- and 2-OHNaps, 2-OHFle, 1-, 2-, 3-, 4- and 9-OHPhes, 3-hydroxyfluoranthene (3-OHFrt) and 1-OHPyr. Deuterated 1-OHPyr (1-OHPyr-d 9) was used for the quantification of the analytes as an internal standard. As an application, characteristics of urinary OHPAHs of Thai non-smoking subjects were investigated.
Experimental
Chemicals
2-OHFle and 1-OHPyr were purchased from Aldrich (Milwaukee, WI, USA). 1- and 2-OHNaps were purchased from Wako Pure Chemicals (Osaka, Japan). 1-, 2-, 3-, 4- and 9-OHPhes and 1-OHPyr-d 9 (internal standard) were obtained from Chiron AS (Trondheim, Norway). 3-OHFrt was purchased from the NCI Chemical Carcinogen Repository (MRI, Kansas City, MO, USA). HPLC-grade acetonitrile was obtained from Kanto Chemical (Tokyo, Japan) and water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals and solvents used were of analytical reagent grade and obtained from Wako. Stock solutions of OHPAHs were prepared in methanol. A working solution of ten OHPAHs was made by combining each stock solution and diluting further in methanol.
HPLC system and conditions
The HPLC system consisted of a Rheodyne sample injector with a 200-μL loop, two LC-10AD pumps, a DGU-14A degasser, a CTO-10AS column oven, a RF-10AXL fluorescence detector and an SCL-10A system controller (all from Shimadzu, Kyoto, Japan) controlled by Shimadzu CLASS-VP Ver.6.1 software. OHPAHs were separated on a Discovery RP-Amide C16, column (250×4.6-mm I.D., 5 μm, Supelco, Bellefonte, PA, USA) with a guard column (Discovery RP-Amide C16 20×4-mm I.D., 5 μm, Supelco). The following HPLC conditions were used: eluent A, 10 mM phosphate buffer (pH 7.0); eluent B, acetonitrile; gradient program, 0–20 min (eluent B composition, 45%), 20–37 min (B, 45–60%), 40–45 min (B 60%); detection wavelength program (excitation/emission (nm)), 0–16 min (227/355), 16–21 min (270/327), 21–36 min (256/370), 36–38 min (292/473), 39–45 min (240/387). The optimal excitation and emission wavelengths for each OHPAH are shown in Table 1. The flow rate was kept at 1.0 mL min−1 and column temperature was maintained at 40 °C. The columns were washed with the eluent (B composition, 90%) for 5 min after the analysis was finished at 45 min. After the wash, the mobile phase under the initial conditions was run for 15 min before the next sample was injected.
Sample preparation
To determine the ten OHPAHs in a urine sample, a 10-mL aliquot of urine sample was pretreated according to our previous methods used to determine urinary 1-OHPyr or 2-OHFle [8, 22] with some modifications. A urine sample was adjusted to pH 5.0 with 0.1 M HCl and then buffered with 20 mL of 0.1 M acetate buffer (pH 5.0). After 1-OHPyr-d 9 (internal standard) was added, the reaction mixture was incubated for 2 h with β-glucuronidase/aryl sulfatase (1,655 and 63 units, respectively) at 37 °C. Using a vacuum manifold holding 10 cartridges, the reaction mixture was then loaded onto a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) that had been primed with 5 mL methanol and 10 mL water. The cartridge was sequentially washed with 10 mL water and 10 mL of 20% methanol in water. The cartridge was completely dried under vigorous air flow, then connected to a Sep-Pak Silica Plus cartridge (Waters), which was conditioned with 20 mL n-hexane. After washing with 10 mL n-hexane, the trapped metabolites were eluted with 10 mL n-hexane/ethyl acetate (9:1, v/v) through the two cartridges. After the addition of 20 μL dimethylsulfoxide, the eluate was evaporated. The residue was redissolved in 180 μL of methanol, and sonicated. A 10-μL aliquot of the solution was then injected into the HPLC system.
Precision and accuracy
Precision and accuracy of intra-days (within-day) and inter-days (between-day) were studied by replicate analysis (n=5) of a pooled urine sample. The urine sample with the enzymatic hydrolysis was spiked at two concentrations of 144 and 720 nmol L−1 for 1-OHNap; 4.0 and 20 nmol L−1 for 2-OHNap and 3-OHFrt; 0.96 and 4.8 nmol L−1 for 2-OHFle, 1-OHPhe, 2-OHPhe, 3-OHPhe, 4-OHPhe and 9-OHPhe; 0.48 and 2.4 nmol L−1 for 1-OHPyr. The sample concentrations were quantified from the peak area ratio of the metabolites to 1-OHPyr-d 9. Precision is expressed as the percentage of relative standard deviation (RSD, %). Accuracy is expressed by the following formula: (mean observed concentration/spiked concentration)×100 (%).
Sample collection
First urine samples after getting up in the morning were collected from thirty Thai non-smoking male subjects (mean age, 42.2; range, 25–62) who lived in Chiang Mai, Thailand. The 30 subjects consisted of rural villagers (n=10) who live in the countryside and most of whom work as farmers in that area, taxi drivers (n=10) and traffic policemen (n=10), who worked mainly in the downtown area. Each subject filled out a questionnaire concerning the sources of PAHs, foods during the last 2 days, cigarette smoke and occupational PAH exposure. The urine specimens were kept at −20 °C until analysis. Urinary concentrations of OHPAHs were normalized to the creatinine concentration (mol/mol creatinine), which was determined with a test kit (Wako) according to Jaffe’s method [23]. Mean urinary OHPAH concentrations were compared statistically, using an unpaired Student’s t-test with StatView-J 5.0 (Nankodo, Tokyo, Japan), in which a value of P< 0.05 was considered to be significant.
Results and discussion
Chromatography
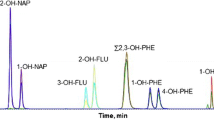
To separate 1-OHPyr and the internal standard (1-OHPyr-d 9), we chose an alkylamide-type reversed-phase column, because 1-OHPyr and the deuterated internal standard were well separated on this column, though not on an ODS column [22]. Figure 1 shows typical chromatograms of a standard mixture of the ten OHPAHs and a Thai non-smoking taxi driver’s urine. The latter chromatogram shows that ten kinds of OHPAHs, 1- and 2-OHNaps, 2-OHFle, 2-, 3- and 4-OHPhes, 1- and 9-OHPhes and 1-OHPyr, in human urine were quantitatively separated. 1-OHPyr-d 9 eluted prior to the non-deuterated compound with enough resolution (R s, 1.45). 1- and 9-OHPhes co-eluted and could not be quantified separately. Since the peak intensity of 1-OHPhe was almost the same as that of 9-OHPhe, it is possible to quantify the total concentration of these two OHPhes. Several groups have developed HPLC/fluorescence detection methods for simultaneously analysing one OHNap, five OHPhes and 1-OHPyr [16], three OHPhes and 1-OHPyr [17], five OHPhes and 1-OHPyr [18], and two OHNaps, five OHPhes and 1-OHPyr [19]. The elution order of OHPAHs in this study was similar to elution orders previously reported [16, 19]. In this study, we successfully developed an analytical method for simultaneous determination of 10 kinds of OHPAHs, and use of the deuterated compound as an internal standard allowed them to be accurately quantified.
Sample preparation
The efficient removal of substances that interfere with the detection of analytes is necessary for the pretreatment of biological samples. Human urine contains many fluorescent hydrophilic materials, and the retention of OHPAHs on a reversed-phase column decreases with decreasing ring number. In our preliminary study, it was difficult to detect and identify OHNaps and 2-OHFle in urine samples treated only with a C18 SPE cartridge due to the many interfering peaks in the chromatogram. Although we have successfully identified and quantified urinary 2-OHFle by using a column-switching technique [8], such a switching method seems to have difficulty in simultaneously analyzing a number of analytes with different retention times. Therefore, we developed a pretreatment method for urine samples using two different types of SPE cartridge (Sep-Pak C18 and Silica cartridges). Although various kinds of normal-phase SPE cartridges including aminopropyl (NH2), diol, cyanopropyl (CN) and silica were evaluated in combination with a Sep-Pak C18 cartridge (data not shown), the best recovery (95%) was obtained by a combination of C18 and silica cartridges. After loading of hydrolysed urine, the C18 cartridge was washed with milli-Q water followed by methanol/water (2:8, v/v). Washing the C18 cartridge with solutions containing more than 20% of methanol greatly decreased the recoveries of OHNaps. We previously showed that OHPAHs were retained with n-hexane on the silica cartridge, and the specific elution of OHPAHs is successfully achieved by the slight increase of the eluotropic strength of the eluting solvent (i.e. the addition of ethyl acetate to n-hexane) [24]. Almost all of the OHPAHs trapped on the C18 cartridge were eluted with n-hexane and were retained on the silica cartridge. A fraction of interfering materials was also eluted with n-hexane, and the solvent washed out the materials which are more hydrophobic than OHPAHs. After that, OHPAHs were eluted with n-hexane/ethyl acetate (9:1, v/v) through the two cartridges, whereas the interfering materials which are more hydrophilic than OHPAHs were trapped on the silica cartridge. Figure 1b shows a representative chromatogram of a Thai non-smoking taxi driver’s urine. After treatment with the SPE cartridges (C18 and silica), the peaks of the ten OHPAHs were free from any interfering peaks, though several interfering peaks were observed around the peaks of OHNaps. The vapour pressures of OHPAHs are one to three orders of magnitude lower than those of the parent PAHs [24]. However, 1-OHNap and 2-OHNap were easily lost during the evaporation step and decreased their recoveries in the pretreatment method. Their vapour pressures (0.19 and 0.22) are higher than those of other OHPAHs (6.1×10−4–3.9×10−6). The addition of dimethylsulfoxide (DMSO) to the eluate effectively prevented the loss of OHNaps, because DMSO is not volatilized during the evaporation, dissolves the analytes and is mixable with hydrophilic solvents such as methanol [25].
Detection limits, calibration curves, quantification limit and validation
As shown in Table 2, the limits of detection (S/N=3) were in the range from 2.3 fmol per injection (1-OHPyr) to 2.2 pmol per injection (1-OHNap). Good linearities of the calibration curves were obtained for all OHPAHs (0.996≤r 2≤0.999). Their slopes were almost identical to those of the working curves obtained by adding standard OHPAHs into human urine. The limit of quantification for OHPAHs ranged from 64 pmol L−1 urine (1-OHPyr) to 14.4 nmol L−1 urine (1-OHNap). The analytical intra-day (n=5) and inter-day (n=5) data of the ten urinary OHPAHs are acceptable with variation in the values from 1.6% (3-OHFrt) to 17% (2-OHPhe) for precision and 92.3% (3-OHFrt) to 119% (3-OHPhe) for accuracy (Table 3). These values indicate that 1-OHPyr-d 9 is an excellent internal standard and the proposed method is satisfactory for determining OHPAHs in human urine. The limit of quantification for OHPAHs in this study were lower than those of the reported methods with HPLC-fluorescence detection (0.7–6.9 nmol L−1 urine [16] and 0.8–28.0 nmol L−1 urine [19]).
Application of the method
By using the proposed method, OHPAHs were quantified and compared in urine samples of non-smoking male subjects who lived in Chiang Mai, Thailand. The subjects were divided into three groups, namely rural villagers, taxi drivers and traffic policemen. The taxi drivers and traffic policemen lived in the urban area of the city and were expected to be continually exposed to automobile exhaust. On the other hand, the rural villagers had little or no source of PAHs related to automobile exhaust. The mean concentrations of OHNaps (normalized to the concentration of creatinine) were much higher than those of 2-OHFle, OHPhes and 1-OHPyr in all groups (Table 4). PAH concentrations in outdoor and indoor air increase with decreasing ring number; in particular, the concentration of naphthalene is approximately 10 to 50 times higher than other PAHs [13–15]. The concentrations of urinary OHPAHs also increased with decreasing ring number. 3-OHFrt was not detected in any of the urine samples tested in this study. Grimmer et al. determined urinary total OHFrt at the mean concentration of 0.1 ng L−1 urine for unexposed volunteers [26]. Our finding that the urinary 3-OHFrt level was below the detection limit for all subjects in this study was also consistent with their result.
The most interesting result was that the concentrations of all detected metabolites, except for 1-OHNap, of rural villagers were significantly higher than those of the other two groups (Table 4). In contrast, no significant difference in any of the metabolites was found between the taxi drivers and the traffic policemen. The urinary 1-OHPyr level (1.2 μmol/mol creatinine) of the rural villagers was much higher than that of non-occupationally exposed non-smoking subjects from various countries (0.03–0.68 μmol/mol creatinine) and much higher than the proposed limit for 1-OHPyr (0.24 μmol/mol creatinine) for them [27]. The concentrations of other OHPAHs also exceeded post-shift OHPAH levels in workers exposed to diesel exhaust [16]. The villages were located in the countryside near forests and the traffic volume was much lower than that of the Chiang Mai downtown area. The high urinary levels of OHPAHs of rural villagers are thought to be mainly due to atmospheric PAHs produced by open-burning for agricultural purposes and by burning of biomass (wood and charcoal) for cooking and heating. Exposure to indoor air pollution from the combustion of solid fuels is a serious problem in developing countries [28]. Household members involved in cooking are exposed to biomass smoke including particulate matter (PM) with carcinogens such as PAHs in rooms without a ventilation system. Monitoring of personal exposures in biomass-burning households has shown PM concentrations many times higher than those in industrial countries [29]. It is interesting that the incidence of lung cancer is higher in Chiang Mai province than in other parts of Thailand and other Asian cities [30]. The high exposure to PAHs might be a major cause of morbidity and mortality of lung cancer in Chiang Mai. We are currently studying the relationship between urinary OHPAH levels and indoor air pollution of the rural villagers. Together, our results suggest that the proposed method is useful for the detailed analysis of urinary OHPAHs, which are related to PAH exposure.
Conclusions
A method was developed to determine ten OHPAHs in human urine by using HPLC with fluorescence detection. The use of deuterated 1-OHPyr as an internal standard allowed the OHPAHs to be accurately and precisely quantitated. Our method was used to estimate the exposure to PAHs from multiple routes in Thai non-smoking subjects. Significantly higher excretion of OHPAHs in the rural villagers suggests high-exposure to biomass smoke including PM with carcinogens such as PAHs. Thus, the urinary profile of OHPAHs can serve as multiple biomarkers which reflect the exposure to PAHs from the environment and human activities.
References
IARC; International Agency for Research on Cancer (1983) WHO 32(Part 1):155–161, 225–231
Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, Burnstein KL, Hayakawa K (2003) Arch Toxicol 77:335–343
Okamura K, Kizu R, Toriba A, Murahashi T, Mizokami A, Bunstein KL, Klinge CM, Hayakawa K (2004) Toxicology 195:243–254
Jongeneelen FJ, Anzion RB, Henderson PT (1987) J Chromatogr A 413:227–232
Strickland P, Kang D (1999) Toxicol Lett 108:191–199
Bouchard M, Viau C (1999) Biomarkers 4:159–187
Jacob J, Seidel A (2002) J Chromatogr B 778:31–47
Toriba A, Chetiyanukornkul T, Kizu R, Hayakawa K (2003) Analyst 128:605–610
Chetiyanukornkul T, Toriba A, Kizu R, Hayakawa K (2004) Polycycl Aromat Compd 24:474–476
Gmeiner G, Stehlik G, Tausch H (1997) J Chromatogr A 767:163–169
Hoffmann D, Hoffmann I (1997) J Toxicol Environ Health 50:307–364
Traynor GW, Apte MG, Sokol HA, Chuang JC, Tucker WG, Mumford JL (1990) Environ Sci Technol 24:1265–1270
Mitra S, Ray B (1995) Atmos Environ 29:3345–3356
Chuang JC, Callahan PJ, Lyu CW, Wilson NK (1999) J Expo Anal Environ Epidemiol 9:85–98
Ohura T, Sugiyama T, Amagai T, Fusaya M, Matsushita H (2002) J AOAC Int 85:188–202
Kuusimäki L, Peltonen Y, Mutanen P, Peltonen K, Savela K (2004) Int Arch Occup Environ Health 77:23–30
Lintelmann J, Hellemann C, Kettrup A (1994) J Chromatogr B 660:67–73
Angerer J, Mannschreck C, Gündel J (1997) Int Arch Occup Environ Health 69:323–331
Elovaara E, Väänänen V, Mikkola J (2003) Arch Toxicol 77:183–193
Serdar B, Waidyanatha S, Zheng Y, Rappaport SM (2003) Biomarkers 8:93–109
Smith CJ, Huang W, Walcott CJ, Turner W, Grainger J, Patterson DG (2002) Anal Bioanal Chem 372:216–220
Chetiyanukornkul T, Toriba A, Kizu R, Makino T, Nakazawa H, Hayakawa K (2002) J Chromatogr A 961:107–112
Bonsnes RW, Taussky HH (1945) J Biol Chem 158:581–591
Kamiya M, Toriba A, Onoda Y, Kizu R, Hayakawa K (2005) Food Chem Toxicol 43:1017–1027
Toriba A, Kuramae Y, Chetiyanukornkul T, Kizu R, Makino T, Nakazawa H, Hayakawa K (2003) Biomed Chromatogr 17:126–132
Grimmer G, Dettbarn G, Naujack KW, Jacob J (1991) Intern J Environ Anal Chem 43:177–186
Jongeneelen FJ (2001) Ann Occup Hyg 45:3–13
Ezzati M, Kammen DM (2002) Environ Health Perspect 110:1057–1068
Smith KR, Samet JM, Romieu L, Bruce N (2000) Thorax 55:518–532
Vatanasapt V, Martin N, Sriplung H, Chindavijak K, Sontipong S, Sriamporn H, Parkin DM, Ferlay J (1995) Cancer Epidemiol Biomarkers Prev 4:475–483
Acknowledgments
This study was supported, in part, by the Industrial Technology Research Grant Program in 2005 from New Energy and Industrial Technology Development Organization (NEDO) of Japan (ID: 05A21705a), and by the Kanazawa University 21-Century COE Program. We thank Assoc. Prof. Dr. Tippawan Prapamontol, Assoc. Prof. Dr. Prasak Thavornyutthikarn and Parinya Phanuwet, Chiang Mai University, for the sample collection in Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chetiyanukornkul, T., Toriba, A., Kameda, T. et al. Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Anal Bioanal Chem 386, 712–718 (2006). https://doi.org/10.1007/s00216-006-0628-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0628-6