Abstract
A first gas chromatography–tandem mass spectrometry (GC–MS/MS) method was designed for analysis of four tetrahydroxylated benzo[a]pyrene metabolites (benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol, benzo[a]pyrene-r-7,t-8,t-9,t-10-tetrahydrotetrol, benzo[a]pyrene-r-7,t-8,c-9,c-10-tetrahydrotetrol, and benzo[a]pyrene-r-7,t-8,c-9,t-10-tetrahydrotetrol) in hair. Hair powder extract was submitted to liquid–solid extraction, followed by C18 solid-phase purification. The analytes were derivatized with use of N-methyl-N-(trimethylsilyl)trifluoroacetamide and then analyzed by GC–MS/MS in negative chemical ionization mode. The calibration curve was linear from the limit of quantification (LOQ) to 20 pg/mg in hair. The coefficient of determination of the calibration curve was more than 0.975 for all the analytes investigated. The LOQs ranged from 0.075 to 0.2 pg/mg in hair. The method was afterward applied to the analysis of hair of 16 rats randomly allocated to experimental groups receiving 16 polycyclic aromatic hydrocarbons solubilized in oil at 0 or 0.8 mg/kg body weight by oral administration three times per week for 90 days. The analysis of monohydroxylated and dihydroxylated benzo[a]pyrenes was conducted in parallel by GC–MS/MS on the same samples. All tetrahydroxylated benzo[a]pyrene isomers were detected in hair samples collected from rats exposed to polycyclic aromatic hydrocarbons. Benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol, the most abundant isomer in hair of treated rats, was also the principal isomer released in DNA adduct hydrolysis in humans. Moreover, the benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol concentrations in hair were significantly greater than those of 2-hydroxybenzo[a]pyrene, 1-hydroxybenzo[a]pyrene, 7-hydroxybenzo[a]pyrene, and 4-hydroxybenzo[a]pyrene and similar to those of 9-hydroxybenzo[a]pyrene and 3-hydroxybenzo[a]pyrene. The method was also sufficiently sensitive to monitor environmental levels of exposure because two hair specimens in the eight analyzed from smokers were above the LOQ for benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol and benzo[a]pyrene-r-7,t-8,c-9,t-10-tetrahydrotetrol. This study therefore demonstrated that tetrahydroxylated benzo[a]pyrenes in hair might be a useful biomarker for the assessment of both the general population and occupationally exposed workers.

GC-NCI-MS/MS analysis of tetrahydroxylated benzo[a]pyrene metabolites in hair of rat under controlled exposure to benzo[a]pyrene (10 mg/kg, 28 days)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since exposure to polycyclic aromatic hydrocarbons (PAHs) is suspected to be responsible for several health issues (such as cancer, cardiovascular diseases, and neurological disorders), significant efforts have gone into the development of efficient strategies for the assessment of human exposure to these ubiquitous pollutants. Alongside the analysis of urinary metabolites and DNA adducts, which are traditional biomarkers of PAH exposure, the possibility of detecting PAHs as well as their metabolites in hair has also recently been demonstrated [1–3]. In addition to the easiness of its sampling, hair offers wider windows of detection than most biological fluids (up to several months depending on sample length) and therefore provides an average value of the individual level of exposure. Moreover, in contrast to urine, the persistence of chemicals in hair even after exposure has stopped increases the likelihood of positive detection, which limits the influence of sampling time on the results. Although the risk of result misinterpretation due to possible external deposition of chemicals on the hair surface has been pointed out [3], it has been overcome by the development of methods focused on PAH metabolites [1, 4], which are for the most part absent from air [5] and therefore are only incorporated in hair through biological pathways. Naphthols are an exception, because they were detected in the air in areas where coal combustion is an important source of energy production [5–8]. In this context, a method based on gas chromatography (GC)–tandem mass spectrometry (MS/MS) was recently developed for the analysis of 52 monohydroxylated PAHs in hair [4]. The analysis of hair collected from animals exposed to PAHs confirmed the incorporation of PAH metabolites in hair and demonstrated that the method was sensitive enough to highlight low levels of exposure to PAHs [4]. The separate analysis of white and black hair collected from the same animals also demonstrated that unlike other for chemicals [3], PAH metabolite incorporation was not affected by pigmentation. In addition to the latter results, the relevance of hair analysis for human biomonitoring of exposure to PAHs was highlighted in preliminary work in which exposure to PAHs was assessed simultaneously with the smoking status of 105 individuals by the determination of 12 monohydroxylated PAHs and nicotine [9]. All the hair samples tested positive for nicotine, with an estimated median concentration of 0.5 ng/mg for nonsmokers and 10.7 ng/mg for smokers. In the analysis of hydroxylated PAHs in hair, 70 % of the samples tested positive. The commonest metabolite was 2-naphthol (61 %), with concentrations significantly higher in smokers than in nonsmokers (median 111 pmol/g vs 70 pmol/g, p < 0.01). 2-Hydroxybenzo[c]phenanthrene and 6-hydroxychrysene were only detected once, in a hair from a nonsmoker. Only six samples tested positive for more than two different metabolites [9]. To obtain even more comprehensive information on individuals’ global exposure, the range of hydroxlated PAHs used as biomarkers of human exposure was widened so as to include the study of tetrahydroxylated PAH isomers in hair. Under the assumption that these metabolites allow quantitative evaluation of the internal dose, they appear more relevant than monohydroxylated forms for the measurement of heavy compounds (more than four aromatic rings) and supply information about the toxicity of the parent compound linked to the individuals’ own metabolism; they could constitute suitable biomarkers for the assessment of human exposure to PAHs. In this context, recent work by Yuan et al. [10] has indeed underlined the interest in the analysis of r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (tetra-OH-Phe) in urine as a new indicator of the internal dose of PAHs and of metabolism. Because the urinary levels of tetra-OH-Phe were greater than those measured for the tetrahydrotetrols derived from benzo[a]pyrene (B[a]P), Zhong et al. [11] suggested that tetra-OH-Phe could be a pertinent biomarker of PAH exposure and metabolic activation through the formation pathway of diol epoxide, which is known to be involved in DNA adduct formation. Interest in urinary tetra-OH-Phe has also been highlighted by correlations with urinary tetrahydroxylated B[a]P and with an increase in lung cancer risk in smokers [10]. The concentrations of tetrahydroxylated B[a]Ps measured in these smokers were similar to those recently evaluated for 3-OH-B[a]P in urine of smokers in another cohort and were around 0.02 nmol per mole of creatinine [12]. Although urinary tetrahydroxylated B[a]P has been used for the biomonitoring of PAH exposure, no study has yet been undertaken to measure the concentrations of these tetrahydroxylated isomers in hair.
The present study was therefore aimed at developing a method for the determination of tetrahydroxylated B[a]Ps in hair as new biomarkers of long-term exposure to B[a]P. The applicability of the method was firstly tested on hair samples collected from rats under controlled exposure to B[a]P alone or to a mixture of PAHs. To evaluate the suitability of analysis of tetrahydroxylated B[a]P in hair as a biomarker of B[a]P exposure, the concentrations of the target analytes were compared with those obtained for monohydroxylated and dihydroxylated B[a]Ps in the same hair samples. Finally, the method was tested on hair collected from human volunteers.
Material and methods
Chemicals
(±)-Benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol (B[a]P-RTTC), (±)-benzo[a]pyrene-r-7,t-8,t-9,t-10-tetrahydrotetrol (B[a]P-RTTT), (±)-benzo[a]pyrene-r-7,t-8,c-9,c-10-tetrahydrotetrol (B[a]P-RTCC), and (±)-anti-r-7,t-8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene-D 8 [(±)anti-B[a]PDE-D 8] were obtained from MRIGlobal (Kansas City, MO, USA). (±)-Benzo[a]pyrene-r-7,t-8,c-9,t-10-tetrahydrotetrol (B[a]P-RTCT) was obtained from Toronto Research Chemicals (North York, Canada). The purity of B[a]P-RTTC and B[a]P-RTTT was more than 99 %, whereas the purity of B[a]P-RTCC and B[a]P-RTCT was 91 % and 92.3 %, respectively. The level of purity was taken into consideration for the preparation of the standard solutions. Tetrahydroxylated B[a]P standard stock solutions were prepared by dissolving 2 mg of each compound in 2 mL of a 60:40 acetonitrile–dimethyl sulfoxide (DMSO) solution. The hydrolysis of anti-B[a]PDE-D 8 (0.5 mg) was performed with 1 mL methanol–water–DMSO (80:5:15) as previously described [13]. The reaction mixture at 10 mg/L was diluted by successive tenfold dilutions in acetonitrile. This hydrolysis allows the formation of two compounds used as internal standards: (1) B[a]P-RTTC-D 8 used for the quantification of B[a]P-RTTC and B[a]P-RTCC and (2) B[a]P-RTTT-D 8 used for the quantification of B[a]P-RTTT. The percentage of each internal standard was determined by use of the calibration curve of the respective standard and was estimated at 29.6 % for B[a]P-RTTC-D 8 and 70.4 % for B[a]P-RTTT-D 8. All B[a]P metabolites, including 1-OH-B[a]P, 2-OH-B[a]P, 3-OH-B[a]P, 4-OH-B[a]P, 5-OH-B[a]P, 6-OH-B[a]P, 7-OH-B[a]P, 8-OH-B[a]P, 9-OH-B[a]P, 10-OH-B[a]P, 11-OH-B[a]P, and 12-OH-B[a]P, and 1-hydroxybenz[a]anthracene-13 C 6 were purchased in powder form from MRIGlobal (Kansas City, MO, USA). Working solutions were prepared in acetonitrile by successive tenfold dilutions at concentrations ranging from 1000 μg/L to 10 μg/L and were stored at -20 °C.
Bond Elut C18 (50 mg) and ENVI-Chrom P (100 mg) solid-phase extraction columns were purchased from Agilent Technologies (Diegem, Belgium) and Chromoptic (Courtaboeuf, France), respectively. The derivatization agents N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA; purity 96 % or greater) and N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (MTBSTFA; purity 97 % or greater) containing 1 % tert-butyldimethylchlorosilane were obtained from Macherey-Nagel (Filterservice, Eupen, Belgium) and Sigma-Aldrich (Diegem, Belgium), respectively. Sodium dodecyl sulfate was also supplied by Sigma-Aldrich. The quality “Dioxins, Pesti-S” was chosen for methanol, DMSO, ethyl acetate, and cyclohexane, and the quality “ULC-MS” was selected for acetonitrile and water. All solvents were supplied by Biosolve (Dieuze, France). Ultrapure water was produced by means of an AFS-8 system from Millipore (Brussels, Belgium).
Animal experimental designs
The development of the extraction and purification procedure was firstly conducted with hair specimens collected from rats exposed to B[a]P alone. The experimental design was approved by the institutional ethics committee of the University of Lorraine in France (authorization number B54–547–13). The latter experiment consisted in exposure of ten rats (Wistar, males, Harlan, Gannat, France) to a high concentration of B[a]P (daily intraperitoneal injection of B[a]P at 10 mg/kg body weight for 28 days) plus ten control rats which received the vehicle only.
Secondly, the applicability of the method was evaluated with hair specimens collected from Long Evans female rats exposed to a mixture of 16 PAHs at a level of exposure more representative of human PAH exposure [14]. Sixteen adult female Long Evans rats were randomly allocated to experimental groups receiving a mixture of 16 PAHs solubilized in fresh vegetable oil (Isio 4, Lesieur, Coudekerque-Branche, France) at 0 or 0.8 mg/kg body weight by oral administration three times per week for 90 days. This oil was a combination of sunflower, rapeseed, and grape seed oils and had been checked previously for the absence of contamination with PAHs [15]. Control rats received the vehicle only. The animal experiment protocols were approved by both the Luxembourg Ministry of Health and the Ministry of Agriculture, and the official agreement was received on October 2, 2012.
In both studies, rats were housed under controlled conditions (22 ± 3 °C; relative humidity 50 ± 20 %; 12-h light–dark cycle, light on at 7:00 p.m., food and water provided ad libitum). They were shaved before the beginning of the experiment to ensure that the hair collected at the end of the experiment represented only the period of exposure. Hair was collected from both exposed and control rats and stored in aluminum foil at -20 °C until analysis. To avoid external contamination of hydroxylated PAHs in hair by urine excretion, special bedding with high absorption capacity (7099 TEK-Fresh, Harlan) was used and was replaced every 2 days. This bedding is known to be nontoxic if eaten by rats and was tested to be PAH free. All animal experimental procedures were conducted in compliance with the rules of the European Union (Directive 2010/63/EU) and the French Government (Decree 2013-118, February 1, 2013).
Human hair collection
The subjects in this study consisted of four children (younger than 14 years) and 12 adults (age ranging from 21 to 62 years), including six nonsmokers and six smokers (more than ten cigarettes per day). The volunteers or the guardians (in the case of children) were fully informed about the procedure and the objectives of the study and signed an informed consent form. Before any sampling was performed, the protocol was reviewed and approved by the National Research Ethics Committee (CNER; agreement no. 201303/06). A strand of hair (100 mg) was collected from each volunteer and stored in aluminum foil at -20 °C until analysis.
GC–MS/MS analysis of monohydroxylated B[a]Ps in hair
Decontaminated hair specimens were supplemented with 20 μL of 1-hydroxybenz[a]anthracene-13 C 6 (at 1 mg/mL) as an internal standard and hydrolysis was performed with 1 mL of 1 N NaOH overnight at 40 °C. The extraction and purification were performed in accordance with the method described by Grova et al. [4]. Hair extracts were reconstituted in 50 μL of MTBSTFA. Derivatization of target analytes was completed after 30 min at 60 °C, and 2 μL of the extract was injected into the GC–MS/MS system. Analyses were performed with an Agilent 7890A gas chromatograph equipped with an HP-5 ms capillary column (30 m, 0.25-mm inner diameter, 0.25-μm film thickness) coupled to an Agilent 7000B triple quadrupole mass spectrometer operating in electron impact ionization mode and a CTC PAL autosampler. The analytical conditions used for chromatography and MS/MS detection were as previously described [4]. Calibration curves were obtained with hair specimens supplemented with increasing concentrations of hydroxylated B[a]P from the limit of quantification (LOQ) to 1 ng/mg for all the hydroxylated B[a]Ps tested. The LOQs ranged from 3 to 30 pg/mg for the 12 hydroxylated B[a]Ps.
GC–MS/MS analysis of tetrahydroxylated B[a]Ps in hair
Extraction and purification
To eliminate the dust present on the hair, a strand of hair was washed with two successive 5-min baths of a solution of sodium dodecyl sulfate at 5 % and of methanol at 100 % as described by Duca et al. [16]. The sample was then dried with absorbent paper before being pulverized for 5 min in a mixer mill (MM200, Retsch, Aarselaar, Belgium). The development of the extraction method for tetrahydroxylated B[a]Ps in hair was based on the one implemented for the analysis of B[a]P adducts in DNA isolated from white blood cells [13]. Briefly, 50 mg of hair powder (homogenized at approximately 10 μm) was placed into a 4-mL screw-cap glass vial supplemented with 10 μL of a solution of each internal standard B[a]P-RTTC-D 8 and B[a]P-RTTT-D 8 at 0.1 mg/L. The liquid–solid extraction was performed by addition of 2 mL of an acetonitrile–water mixture (50:50, v/v) and stirring of the resultant mixture at 350 rpm overnight at 40 °C. The day after, the sample was centrifuged for 10 min at 2800 g. The supernatant was collected and then evaporated under a nitrogen flow. Phosphate buffer (0.1 M) at pH 7.1 (2 mL) was added to the dry residue. The sample was then laid on a previously conditioned (1 mL of methanol, 1 mL of water) C18 solid-phase extraction column. The tubes were rinsed and the columns were washed successively twice with 1 mL of water. The elution of the analytes was conducted twice with 0.5 mL of methanol. The eluate was collected in a glass tube. The solvent was removed by evaporation under a nitrogen flow, and then the dry extract was diluted in 80 μL of the same solvent, transferred to a vial with an insert, and then evaporated again under nitrogen. Before analysis, the extracts containing the tetrahydroxylated B[a]Ps were derivatized by means of MSTFA, with a view to obtaining the trimethylsilyls of tetrahydrotetrols of B[a]P, which are analyzable by GC. The derivatization was achieved at 60 °C for 2 h by addition of 10 μL of MSTFA. The sample was then analyzed by GC–negative chemical ionization (NCI) MS/MS. The chromatograms obtained for a mixture of tetrahydroxylated B[a]P standards and their respective internal standards added to a hair matrix are shown in Fig. 2.
Instrumentation
Analyses were performed with an Agilent 7890B gas chromatograph coupled to an Agilent 7000A triple quadrupole mass spectrometer and a CTC PAL autosampler.
Gas chromatography
The GC conditions were similar to those described by Grova et al. [13]. Briefly, the inlet was at 260 °C. Two microliters of extract was injected in pulsed splitless mode with a pressure of 47 psi for 1.5 min. Chromatographic separation was done with an HP-5 ms capillary column (30 m, 0.25-mm inner diameter, 0.25-μm film thickness). Helium was used as the carrier gas at 1.2 mL/min. The oven temperature program was as follows: the temperature was kept at 100 °C for 2 min, increased to 235 °C at a rate of 40 °C/min, raised to 280 °C at 10 °C/min and maintained at this temperature for 3 min, and finally after the temperature had been increased at 10 °C/min, it was maintained at 300 °C for 3 min. After each run, the temperature was set at 300 °C for an additional 4 min in backflush mode to remove high-boiling-point compounds through the split vent.
Mass spectrometry
The spectrometer was operated in NCI mode with multiple reaction monitoring. The source temperature was set at 230 °C. Helium and nitrogen were used as the quench gas in the collision cell (2.25 mL/min) and the collision-induced-dissociation gas (1.5 mL/min), respectively. Methane was used as the reagent gas at a flow of 40 % (2 mL/min).
The fragmentation pattern and relative intensity in the daughter ion spectrum of m/z 446 obtained with the NCI source were similar to those previously described [11, 13]. The transition m/z 446 → m/z 284 (corresponding to the loss of [HO-trimethylsilyl + trimethylsilyl]) was used as the quantification transition for the analysis of tetrahydroxylated B[a]P isomers, whereas the transitions m/z 446 → m/z 255 and m/z 446 → m/z 89 were used as confirmation transitions (MS/MS detection parameters are given in Table S1).
Selectivity
Detection by MS/MS allows great selectivity because in addition to compound identification by the retention time, the presence is confirmed by multiple reaction monitoring transitions, which are much more compound specific than single ion monitoring transitions. Regarding the specificity of the method presented here, in addition to the quantification transition, two confirmation transitions were used to ensure the presence of each target compound (Table S1). To confirm the presence of the target compound, the ratio of the “quantification transition” to the “confirmation transition” had to be less than 20 % different from the ratio obtained with standard compounds. Finally, the specificity of the method is also increased by use of NCI mode, allowing particularly low background noise and therefore clearly reducing the potential interferent peaks.
Validation parameters
Peaks were identified by the retention time and quantifier and qualifier ion peak area ratios. The transition yielding the highest signal-to-noise ratio for each analyte was used for quantification; the second and third transitions were used as qualifiers. Details on the chromatography and mass spectrometry parameters were provided previously [13]. Quantification was based on the ratio of the target analyte peak area to the peak area of internal standards.
For each analytical procedure, matrix-matched calibration curves were obtained by supplementation of hair samples (50 mg) free of tetrahydroxylated B[a]Ps (not detected) with increasing concentrations of tetrahydroxylated B[a]Ps: 0, 0.075, 0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 pg/mg hair. The latter concentration range was based on the concentrations detected in the analysis of monohydroxylated B[a]Ps in human hair and in hair of animals under controlled exposure to B[a]P [1, 4, 9]. Six replicates were analyzed at each concentration. Interday variability and accuracy were determined on hair samples supplemented with all tetrahydroxylated B[a]Ps at 0.5, 5, and 20 pg/mg hair. The accuracy was calculated as the percentage of the relative error from the target concentration. Interday accuracy was determined on six replicates of each concentration. Considering the complex composition of hair or other biological matrices [17], 25 % of the target concentration was considered as acceptable accuracy. Similarly to accuracy and for the same concentrations (0.5, 5, and 20 pg/mg hair), interday repeatability was measured for six repetitions per concentration. Repeatability was calculated as the ratio of the standard deviation of the values considered (n = 6) to the average of the said values. Repeatability (relative standard deviation) is expressed as a percentage.
The LOQ, which corresponds to the lowest concentration of the calibration curve, was established on the basis of the quantification transition by use of six supplemented hair samples and determination of the accuracy and repeatability (Table 1). Recovery was evaluated for three concentrations (0.5, 5, and 20 pg/mg) by comparison of five samples of hair supplemented and then treated as described in “Extraction and purification” and five samples treated similarly but supplemented before the derivatization step.
Data analysis
Statistical analysis was then performed on the concentrations detected for monohydroxylated and tetrahydroxylated B[a]Ps in the hair of the rats exposed to B[a]P and that of controls. Given that the data did not follow a Gaussian distribution, the concentrations of tetrahydroxylated and monohydroxylated B[a]Ps were analyzed by a nonparametric Kruskal–Wallis procedure with B[a]P metabolites as an independent factor, followed by Dunn’s procedure for post hoc comparisons. Differences were considered significant at the level of p < 0.05.
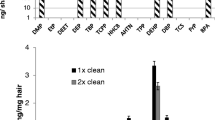
Pertaining to the choice of the extraction solvent, for which the data followed a Gaussian distribution, both the t test (Fig. 1b) and analysis of variance (Fig. 2b) were applied by use of the percentage of solvent as an independent factor. A Dunnett procedure for post hoc comparison was then performed by use of the condition acetonitrile–water (50:50, v/v) as a control. The statistical analysis was performed with SigmaPlot (Systat Software Erkrath, Germany).
Influence of the solvent on the tetrahydroxylated benzo[a]pyrene (B[a]P) extraction efficiency. a Comparison of two solid extractions with a mixture of methanol and water (50:50, v/v) and a mixture of acetonitrile (ACN) and water (50:50, v/v) conducted on a pool of hair supplemented with tetrahydroxylated B[a]Ps at 2 pg/mg. The results are expressed as the means of triplicates. Three asterisks p < 0.001, two asterisks p < 0.01, t p < 0.07 statistically significant difference between pairs of treatments (t test). b The percentage of acetonitrile added to the acetonitrile–water mixture to allow suitable extraction. The results are expressed as the means of quadruplicates. Given that the data follow a Gaussian distribution, the results were analyzed by the analysis of variance procedure with the percentage of solvent as an independent factor, followed by a Dunnett procedure for post hoc comparison by selection of the condition acetonitrile–water (50:50, v/v) as a control. Three asterisks p < 0.001, two asterisks p < 0.01, one asterisk p < 0.05 for the percentage of acetonitrile. B[a]P-RTCC (±)-benzo[a]pyrene-r-7,t-8,c-9,c-10-tetrahydrotetrol, B[a]P-RTCT (±)-benzo[a]pyrene-r-7,t-8,c-9,t-10-tetrahydrotetrol, B[a]P-RTTC (±)-benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydrotetrol, B[a]P-RTTT (±)-benzo[a]pyrene-r-7,t-8,t-9,t-10-tetrahydrotetrol
Chromatograms obtained from the analysis of tetrahydroxylated B[a]Ps in hairs of a rat supplemented with each isomer at 2 pg/mg, a hair sample collected from a control rat, and a hair sample collected from a B[a]P-treated rat (10 mg/kg body weight, intraperitoneal injection five times per week for 28 days)
Safety considerations
The general guidelines for working with organic solvents, acids, and alkalis were duly respected. All standard compounds were designated as chemical carcinogens. This does not necessarily imply that the sample is a known carcinogen, but merely that it is intended for use in research involving chemical carcinogens and that it should be treated as a carcinogen.
Results and discussion
Development and validation of the method
This study describes the first GC–MS/MS method allowing the quantification of tetrahydroxylated B[a]P isomers in hair samples collected from B[a]P- or PAH-treated rats as well as from human volunteers. The analytical sensitivity necessary to reach environmental concentrations was obtained by use of GC–MS/MS and NCI–triple quadrupole detection. The quantity of hair used (50 mg) for the measurement of tetrahydroxylated B[a]Ps was in accordance with the methods usually used and recommended for drugs of abuse in hair by the Society of Hair Testing [18]. To obtain a homogeneous sample aliquot, the hair was pulverized before extraction. Hydrolysis of the hair matrix was then evaluated by use of acidic (1 N HCl, overnight, at 40 °C), alkaline (1 N NaOH, overnight, at 40 °C [4]), and enzymatic (50 units of purified sulfatase and 635 units of purified glucuronidase from Helix pomatia juice at 37 °C [19]) conditions for specimens supplemented with tetrahydroxylated B[a]Ps at 2 pg/mg. The results demonstrated that acidic and alkaline conditions induce a change in the proportion of each isomer, unlike with enzymatic hydrolysis. Although the different hydrolysis conditions resulted in satisfactory responses (peak area) for most of the metabolites investigated, the results highlighted higher responses of tetrahydroxylated B[a]Ps in enzymatic hydrolysis conditions (see Fig. S1). A second test, with or without enzymatic hydrolysis, was then performed on hair samples (n = 5) collected from B[a]P-treated rats (10 mg/kg body weight) to evaluate the percentage of tetrahydroxylated B[a]Ps in conjugated forms. The results demonstrated that 100 % of these metabolites in hair were unconjugated because no difference was found between the two conditions tested (see Fig. S2). This hydrolysis step was therefore removed from the method.
The next step in the development of the method focused on solvent extraction. Due to the hydrophilic character of tetrahydroxylated B[a]Ps, two solid extractions with a mixture of methanol and water (50:50, v/v) and mixture of acetonitrile and water (50:50, v/v) were compared. The highest responses (peak area) and the highest signal-to-noise ratios were obtained for the four isomers investigated with an acetonitrile–water mixture (Fig. 1a). The percentage of water in the mixture was then tested; Fig. 1b shows that a 50:50 ratio allows suitable extraction. Regarding the purification and derivatization conditions, procedures similar to those developed for the analysis of tetrahydroxylated B[a]Ps released from DNA were applied, and the details are described by Grova et al. [13]. Since tetrahydroxylated B[a]Ps are hydrophilic compounds, they cannot be extracted in the organic layer, unlike monohydroxylated, dihydroxylated, and trihydroxylated B[a]Ps (the recovery determined for the four tetrahydroxylated B[a]Ps ranged from 10.8 to 16.1 %; see Fig. S3). Moreover, the extraction and purification procedures (enzymatic hydrolysis, C18 purification, and MSTFA derivatization) used for their analysis are incompatible with those used for the extraction and purification of all the other metabolites of B[a]Ps investigated (NaOH hydrolysis, ENVI-Chrom P purification, and derivatization with MTBSTFA containing 1 % tert-butyldimethylchlorosilane; see Fig. S3). The extraction procedure envisaged for monohydroxylated metabolites in hair was therefore not used for tetrahydroxylated analogs [4].
Fair linearity was observed along the entire calibration curve for each target compound (R 2 > 0.997). The validation parameters are given in Table 1. The method allows suitable degrees of sensitivity to be obtained for the four tetrahydroxylated B[a]Ps investigated because the LOQs were between 0.075 and 0.5 pg/mg (Table 1). Regardless of the compound and concentration tested, both interday accuracy and interday repeatability were systematically lower than 25 % for all concentrations evaluated (0.5, 5, and 20 pg/mg hair) (Table 1) [20]. The average recovery determined for the three concentrations tested was 61 % for B[a]P-RTCT, 67 % for B[a]P-RTTC, 64 % for B[a]P-RTTT, and 63 % for B[a]P-RTCC (Table 1).
Analysis of hair samples collected from rats and human volunteers
Hair samples from B[a]P- or PAH-treated rats
In hair analysis and on comparison of the chromatograms obtained from the analysis of blank and supplemented hair samples (tetrahydroxylated B[a]Ps at 2 pg/mg) and hair specimens collected from rats exposed to B[a]P, the four tetrahydroxylated B[a]P isomers investigated were detected (Fig. 1). B[a]P-RTTC was the most abundant isomer in the treated rats, followed by B[a]P-RTCT (Fig. 2; p < 0.01). B[a]P-RTTC was detected in all the rats exposed to B[a]Ps (n = 10; average concentration 7.8 ± 3.0 pmol/g) but in only two of the ten control ras (0.04 ± 0.02 pmol/g). The detection of the latter isomer in the control rats demonstrated that this method was sufficiently sensitive to highlight environmental levels of B[a]P exposure. Besides, the results obtained from the experiment conducted on PAH-treated rats were in line with those of the experiment relying on B[a]P exposure only (four isomers of tetrahydroxylated B[a]P detected with a similar tetrahydroxylated B[a]P distribution profile in hair matrix).
B[a]P-RTTC, which was the most abundant isomer in hair, was also proved to be the principal isomer released in DNA adduct hydrolysis in humans [13, 21]. In contrast, the analysis of tetrahydroxylated B[a]Ps in DNA isolated from white blood cells of the same rats demonstrated that B[a]P-RTCC was the most susceptible isomer released in DNA adduct hydrolysis, followed by B[a]P-RTTC. These results were supported by those obtained by Islam et al. [22], who also demonstrated that B[a]P-RTCC was the major isomer of tetrahydroxylated B[a]P released in albumin hydrolysis in rats following a single intraperitoneal injection of B[a]P at concentrations ranging from 40 to 100 mg/kg body weight.
The data obtained from the B[a]P-treated rats are linked to the short exposure (28 days), which allows the simultaneous detection of both monohydroxylated and tetrahydroxylated B[a]Ps in hair. The exposure time seems to play an important role in the incorporation of tetrahydroxylated B[a]Ps in hair because the rats that were treated with a mixture of PAHs at doses 12.5 times lower (10 mg/kg body weight vs 0.8 mg/kg body weight) over a period three times longer (28 days vs 90 days) exhibited concentrations similar to those obtained for the B[a]P-treated rats (Table 2). For instance, the median concentration of B[a]P-RTTC was 7 and 2.5 pmol/g in B[a]P- and PAH-treated-rats, respectively (Table 2).
The B[a]P-RTTC concentrations in hair were significantly greater than those of 2-OH-B[a]P and 1-OH-B[a]P and similar to those of 9-OH-B[a]P and 3-OH-B[a]P, the latter being classically analyzed in urine (Table 2). These results were also confirmed by those obtained from the experiment conducted on rats exposed to a mixture of PAHs because the statistical analysis revealed that the concentrations of B[a]P-RTTC were similar to those obtained for 9-OH-B[a]P and 3-OH-B[a]P and significantly greater than those obtained for all the other monohydroxylated and tetrahydroxylated forms (Table 2), supporting the relevance of B[a]P-RTTC as a biomarker for human exposure to B[a]P. A discrepancy related to the concentrations of 4-OH-B[a]P and 7-OH-B[a]P was nevertheless found between the two animal experimental designs. Both metabolites were detected in the rats treated with the mixture of PAHs and were absent from the rats exposed to B[a]P only. As these two metabolites were co-eluted with 3-hydroxybenzo[k]fluoranthene and 9-hydroxybenzo[k]fluoranthene, respectively, which exhibit the same quantification and confirmation transitions, the signals observed could be the result of benzo[k]fluoranthene’s metabolites. This hypothesis has been partially confirmed by treating two rats (0.8 mg/kg, 28 days) with benzo[k]fluoranthene and B[a]P separately since hair samples collected from rats treated with benzo[k]fluoranthene were positive for 3-hydroxybenzo[k]fluoranthene (0.53 ± 0.03 pmol/g of hair), whereas those exposed to B[a]P did not show any signal for both metabolites. Finally, the comparison of the chromatograms corresponding to the control rats, PAH-mixture-treated rats (PAHs at 0.8 mg/kg), and a hair sample supplemented with tetrahydroxylated B[a]Ps at 2 pg/mg allowed the determination of three unknown isomers (1, 2, and 3 in Fig. 3), which could correspond to tetrahydroxylated benzo[k]fluoranthene and/or tetrahydroxylated benzo[b]fluoranthene isomers. The analysis concomitantly conducted on hair of rats exposed to each compound (1 mg/kg, oral administration, 28 days) separately clearly allowed the identification of these unknown peaks as isomers of tetrahydroxylated benzo[k]fluoranthene (see Fig. S4).
Chromatograms obtained from the analysis of tetrahydroxylated B[a]Ps in hairs of a rat supplemented with each isomer at 2 pg/mg, a hair sample collected from a control rat, and a hair sample collected from a polycyclic aromatic hydrocarbon (PAH)-treated rat (0.8 mg/kg body weight, oral administration three times per week for 90 days)
Analysis of human hair
In an attempt to confirm the suitability of the method for the assessment of human exposure, the method was applied to the analysis of 16 hair samples collected from volunteers with no self-reported occupational exposure (Table 3). Only the smokers exhibited values above the LOQ for B[a]P-RTCT and B[a]P-RTTC. Before an attempt is made to interpret the findings, the difference between smokers and nonsmokers first remains to be confirmed with a greater number of analyses, the statistical power still being limited as it is by the limited size of the human samples considered. In the evaluation of the exposure of young children, although all the values were below the LOQs, three of the four hair samples analyzed were positive for at least two of the four isomers.
The presence of target compounds in hair was confirmed by the ratio of the “quantification transition” to the “confirmation transition,” which had to be less than 20 % different from the ratio obtained with standard compounds. There was therefore no doubt as to the presence of the target tetrahydroxylated B[a]Ps in hair of young children. The latter results demonstrated that these new biomarkers might be particularly interesting for the evaluation of exposure of young children, in whom metabolism specificity may favor the formation of diol epoxide B[a]Ps and consequently tetrahydrotetrols.
Conclusion
This study has described the development of a sensitive method for the determination of tetrahydroxylated B[a]Ps in hair by GC–NCI-MS/MS. We have reported a validated, highly sensitive, and selective method for the determination of very low concentrations of four tetrahydroxylated B[a]Ps in rat hair ranging from 0.2 to 50 pg/mg. The application of the method developed here to samples collected from rats proved the transfer of B[a]P metabolites in hair and supported the relevance of these new biomarkers of exposure. Moreover, their detection in hair samples collected from human volunteers demonstrated the possibility of highlighting environmental levels of exposure.
References
Schummer C, Appenzeller BM, Millet M, Wennig R. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in human hair by gas chromatography-negative chemical ionization mass spectrometry. J Chromatogr A. 2009;1216(32):6012–9. doi:10.1016/j.chroma.2009.05.068.
Toriba A, Kuramae Y, Chetiyanukornkul T, Kizu R, Makino T, Nakazawa H, et al. Quantification of polycyclic aromatic hydrocarbons (PAHs) in human hair by HPLC with fluorescence detection: a biological monitoring method to evaluate the exposure to PAHs. Biomed Chromatogr. 2003;17(2-3):126–32. doi:10.1002/bmc.222.
Appenzeller BMR, Tsatsakis AM. Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: state of the art, critical review and future needs. Toxicol Lett. 2012;210(2):119–40. doi:10.1016/j.toxlet.2011.10.021.
Grova N, Salquebre G, Appenzeller BM. Gas chromatography-tandem mass spectrometry analysis of 52 monohydroxylated metabolites of polycyclic aromatic hydrocarbons in hairs of rats after controlled exposure. Anal Bioanal Chem. 2013;405(27):8897–911. doi:10.1007/s00216-013-7317-z.
Wang G, Kawamura K. Molecular characteristics of urban organic aerosols from Nanjing: a case study of a mega-city in China. Environ Sci Technol. 2005;39(19):7430–8.
Kishikawa N, Morita S, Wada M, Ohba Y, Nakashima K, Kuroda N. Determination of hydroxylated polycyclic aromatic hydrocarbons in airborne particulates by high-performance liquid chromatography with fluorescence detection. Anal Sci. 2004;20(1):129–32.
Kishikawa N, Wada M, Ohba Y, Nakashima K, Kuroda N. Highly sensitive and selective determination of 9,10-phenanthrenequinone in airborne particulates using high-performance liquid chromatography with pre-column derivatization and fluorescence detection. J Chromatogr A. 2004;1057(1-2):83–8.
Simoneit BR, Bi X, Oros DR, Medeiros PM, Sheng G, Fu J. Phenols and hydroxy-PAHs (arylphenols) as tracers for coal smoke particulate matter: source tests and ambient aerosol assessments. Environ Sci Technol. 2007;41(21):7294–302.
Appenzeller BM, Mathon C, Schummer C, Alkerwi A, Lair ML. Simultaneous determination of nicotine and PAH metabolites in human hair specimen: a potential methodology to assess tobacco smoke contribution in PAH exposure. Toxicol Lett. 2012;210(2):211–9. doi:10.1016/j.toxlet.2011.11.022.
Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71(21):6749–57. doi:10.1158/0008-5472.can-11-0209.
Zhong Y, Wang J, Carmella SG, Hochalter JB, Rauch D, Oliver A, et al. Metabolism of [D10]phenanthrene to tetraols in smokers for potential lung cancer susceptibility assessment: comparison of oral and inhalation routes of administration. J Pharmacol Exp Ther. 2011;338(1):353–61. doi:10.1124/jpet.111.181719.
Barbeau D, Maitre A, Marques M. Highly sensitive routine method for urinary 3-hydroxybenzo[a]pyrene quantitation using liquid chromatography-fluorescence detection and automated off-line solid phase extraction. Analyst. 2011;136(6):1183–91. doi:10.1039/c0an00428f.
Grova N, Salquebre G, Hardy EM, Schroeder H, Appenzeller BM. Tetrahydroxylated-benzo[a]pyrene isomer analysis after hydrolysis of DNA-adducts isolated from rat and human white blood cells. J Chromatogr A. 2014;1364:183–91. doi:10.1016/j.chroma.2014.08.082.
Menzie CA, Potocki BB, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environ Sci Technol. 1992;26:1278–84.
Grova N, Valley A, Turner JD, Morel A, Muller CP, Schroeder H. Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology. 2007;28(3):630–6. doi:10.1016/j.neuro.2007.01.010.
Duca RC, Hardy E, Salquebre G, Appenzeller BM. Hair decontamination procedure prior to multi-class pesticide analysis. Drug Test Anal. 2014;6 Suppl 1:55–66. doi:10.1002/dta.1649.
Gray TR, Shakleya DM, Huestis MA. Quantification of nicotine, cotinine, trans-3'-hydroxycotinine, nornicotine and norcotinine in human meconium by liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2008;863(1):107–14. doi:10.1016/j.jchromb.2008.01.001.
Cooper GGA, Kronstrand R, Kintz P. Society of hair testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218:20–4. doi:10.1016/j.forsciint.2011.10.024.
Grova N, Salquebre G, Schroeder H, Appenzeller BM. Determination of PAHs and OH-PAHs in rat brain by gas chromatography tandem (triple quadrupole) mass spectrometry. Chem Res Toxicol. 2011;24(10):1653–67. doi:10.1021/tx2003596.
Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165(2-3):216–24. doi:10.1016/j.forsciint.2006.05.021.
Ragin AD, Crawford KE, Etheredge AA, Grainger J, Patterson Jr DG. A gas chromatography-isotope dilution high-resolution mass spectrometry method for quantification of isomeric benzo[a]pyrene diol epoxide hemoglobin adducts in humans. J Anal Toxicol. 2008;39(9):728–36.
Islam GA, Greibrokk T, Harvey RG, Ovrebo S. HPLC analysis of benzo[a]pyrene-albumin adducts in benzo[a]pyrene exposed rats. Detection of cis-tetrols arising from hydrolysis of adducts of anti- and syn-BPDE-III with proteins. Chem Biol Interact. 1999;123(2):133–48.
Acknowledgments
This work was supported by the Luxembourg Ministère de l’Enseignement Supérieur et de la Recherche (MESR), the Agence de l’Environnement et de la Maîtrise de l’Énergie (ADEME), and the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement du Travail (ANSES). We thank Henri Schroeder, who allowed the performance of animal experiments with benzo[a]pyrene alone in Nancy (URAFPA-INRA UC340). We are most grateful to Marie France Schoën, Chantal Courtois, Stephanie Sallai, and Anaïs Oudin for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 102 kb)
Rights and permissions
About this article
Cite this article
Grova, N., Hardy, E.M., Meyer, P. et al. Analysis of tetrahydroxylated benzo[a]pyrene isomers in hair as biomarkers of exposure to benzo[a]pyrene. Anal Bioanal Chem 408, 1997–2008 (2016). https://doi.org/10.1007/s00216-016-9338-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9338-x