Abstract
Rationale
Acute naloxone-precipitated morphine withdrawal (MWD) produces a conditioned place aversion (CPA) in rats even after one or two exposures to high-dose (20 mg/kg, sc) morphine followed 24-h later by naloxone (1 mg/kg, sc). However, the somatic withdrawal reactions produced by acute naloxone-precipitated MWD in rats have not been investigated. A recently discovered fatty acid amide, N-oleoylglycine (OlGly), which has been suggested to act as a fatty acid amide hydrolase (FAAH) inhibitor and as a peroxisome proliferator-activated receptor alpha (PPARα) agonist, was previously shown to interfere with a naloxone-precipitated MWD-induced CPA in rats.
Objectives
The aims of these studies were to examine the somatic withdrawal responses produced by acute naloxone-precipitated MWD and determine whether OlGly can also interfere with these responses.
Results
Here, we report that following two exposures to morphine (20 mg/kg, sc) each followed by naloxone (1 mg/kg, sc) 24 h later, rats display nausea-like somatic reactions of lying flattened on belly, abdominal contractions and diarrhea, and display increased mouthing movements and loss of body weight. OlGly (5 mg/kg, ip) interfered with naloxone-precipitated MWD-induced abdominal contractions, lying on belly, diarrhea and mouthing movements in male Sprague–Dawley rats, by both a cannabinoid 1 (CB1) and a PPARα mechanism of action. Since these withdrawal reactions are symptomatic of nausea, we evaluated the potential of OlGly to interfere with lithium chloride (LiCl)-induced and MWD-induced conditioned gaping in rats, a selective measure of nausea; the suppression of MWD-induced gaping reactions by OlGly was both CB1 and PPARα mediated.
Conclusion
These results suggest that the aversive effects of acute naloxone-precipitated MWD reflect nausea, which is suppressed by OlGly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute naloxone-precipitated morphine withdrawal (MWD) occurs even after one or two exposures to high-dose morphine followed several hours later by naloxone in humans (Heishman et al. 1990; June et al. 1995) and other animals (Eisenberg 1982; Martin and Eades 1964). Using the acute naloxone-precipitated MWD paradigm, it has been demonstrated that the aversive properties of naloxone are dramatically enhanced when preceded 24–48 h by a high dose of morphine (20 mg/kg, subcutaneous [sc]; Parker and Joshi 1998; Parker et al. 2002; Shoblock and Maidment 2006; Wills et al. 2016). Rats injected with naloxone 24 h after saline did not display a conditioned place aversion (CPA). On the other hand, rats injected with naloxone 24 h after morphine displayed a dramatic naloxone-induced CPA, presumably because morphine treatment promotes constitutive activity of the mu opioid receptors uncovering the aversive inverse agonist property of naloxone (Shoblock and Maidment 2006). Indeed, Wills et al. (2016) used this paradigm to investigate the role of the endocannabinoid system in the regulation of the affective properties of MWD.
We recently reported that N-oleoylglycine (OlGly), an endogenous fatty acid amide mediator structurally similar to N-acylethanolamines, which includes the endocannabinoid, anandamide (AEA), as well as the endogenous peroxisome proliferator-activated receptor alpha (PPARα) agonists N-oleoylethanolamide (OEA) and N-palmitoylethanolamide (PEA), interferes with nicotine reward and dependence in mice (Donvito et al. 2019) and with acute naloxone precipitated MWD-induced conditioned place aversion (CPA) in rats (Petrie et al. 2019). OlGly does not bind cannabinoid 1 (CB1) or cannabinoid 2 (CB2) receptors in vitro and does not produce the typical CB1 receptor tetrad (Martin et al. 1991) of behaviors (antinociception, hypothermia, catalepsy, and hypomobility) characteristic of CB1 receptor agonists, but it weakly inhibits fatty acid amide hydrolase (FAAH: IC50 8.65 μM; 89% inhibition of FAAH) and also binds PPARα (assessed by a modeling experiment) and behaved as a PPARα agonist in a specific luciferase assay for functional activity (Donvito et al. 2019). It is of interest to note that, while the potential of OlGly to interfere with nicotine reward in mice was prevented by pretreatment with a PPARα antagonist (MK866; Donvito et al. 2019) and the potential of OlGly to interfere with naloxone-precipitated MWD-induced CPA was instead prevented by pretreatment with a CB1 receptor antagonist (AM251; Petrie et al. 2019), suggesting that its action as a FAAH inhibitor may have been sufficient to elevate AEA levels and reduce the aversive withdrawal reactions via CB1 receptors (Ramesh et al. 2011).
Although Petrie et al. (2019) reported that OlGly interfered with the aversive properties of acute naloxone-precipitated MWD, it has not been determined if this lipid mediator would also interfere with the somatic effects produced by acute naloxone-precipitated MWD, which are mediated by action at different neurobiological substrates (e.g., Koob et al. 1989; Maldonado et al. 1996; Stinus et al. 1990). There has been little investigation of the somatic behavioral effects produced by acute naloxone-precipitated MWD. In a single study in mice, Shoblock and Maidment (2006) reported that following a single exposure to morphine, naloxone produced only the somatic reaction of jumping. The present study first aimed to determine the nature of somatic symptoms of acute naloxone-precipitated MWD in rats. Following two trial cycles of morphine (20 mg/kg, sc) followed 24 h by naloxone (1 mg/kg), rats displayed the nausea-like behaviors of abdominal contractions, diarrhea, and the abnormal posture of lying on belly (a response seen in rats experiencing LiCl malaise; Contrearas et al. 2007; Parker et al. 1984; Meachum and Bernstein 1992; Tuerke et al. 2012), as well as the behavior of mouthing movements (chewing) and loss of body weight. Pretreatment with OlGly (5 mg/kg, intraperitoneal [ip]) interfered with these somatic withdrawal reactions, without having any behavioral effect on its own. The suppression of acute MWD by OlGly was reversed with either a CB1 receptor antagonist or a PPARα antagonist. Given that acute naloxone-precipitated MWD has been shown (McDonald et al. 1997) to produce conditioned gaping reactions (Grill and Norgren 1978) in rats, a selective measure of nausea-like responding (Parker et al., 2014), and that a subset of the somatic behaviors were indicative of nausea (abdominal contractions, LOB, diarrhea), a reported withdrawal reaction in humans (Jaffee and Martin 1990), we also determined that OlGly interfered with conditioned gaping reactions produced by LiCl, as well as with LiCl-induced vomiting in the Suncus murinus, an animal model of emesis. Finally, we determined that OlGly interfered with naloxone-precipitated MWD-induced conditioned gaping in rats, by both a CB1 and PPARα mechanism of action.
Materials and methods
Subjects
The subjects were male Sprague–Dawley rats weighing between 257 and 297 g on the first treatment day. They were obtained from Charles River QC and pair-housed (for CPA experiments) or individually housed (for conditioned gaping response experiment) in a shoebox cage with ad lib access to food and water. The colony room was on a 24-h light dark cycle (7 AM; lights off; 7 PM lights on) such that behavioral testing was conducted during the dark phase of the light cycle. The Suncus murinus (house musk shrews) used in experiment 5 were bred and raised in the University of Guelph colony and ranged from 123 to 376 days old at the time of testing. Naive male (n = 7; 39.4–42.2 g) and female (n = 5; 25.9–26.5 g) were individually housed in opaque white mouse cages in the colony room at an ambient temperature of 21 °C on a 10/14-h light-dark schedule (lights off at 7 PM). Shrews were tested in their light cycle. They were provided with an open-ended plastic cylinder (4 × 8 cm) containing enviro-paper and maintained on Medical/Royal Canin Feline Maintenance mixed with Harlan Ferret dry chow and had ad libitum access to water. All animal procedures were approved by the Animal Care Committee of the University of Guelph and adhere to the guidelines of the Canadian Council of Animal Care.
Drugs
Morphine and naloxone were prepared in saline at a concentration of 20 mg/ml and 1 mg/ml, respectively, and administered subcutaneously (sc). Oleoyl glycine (OlGly) was dissolved in a vehicle (VEH) mixture of ethanol, Tween 80, and physiological saline in a 1:1:18 ratio. OlGly was first dissolved in ethanol, Tween 80 was then added to the solution, and the ethanol was evaporated off with a nitrogen stream, after which, the saline was added. The final VEH consisted of 1:9 (Tween80/saline). OlGly was prepared at a concentration of 5 mg/ml and was administered intraperitoneally (ip). MK886 (MK; Cayman Chemicals) and AM251 (Cayman Chemicals) were prepared in the same VEH as the OlGly at a concentration of 1 mg/ml and given ip at a dose of 1 mg/kg (Rock et al. 2017; Wills et al. 2014). LiCl (Sigma) was prepared as a 0.15 M solution with sterile water and administered ip at a volume of 20 ml/kg (for rats) and 60 ml/kg (for shrews; the dose necessary to establish vomiting, Parker et al. 2004).
Synthesis of Oleoyl glycine
A solution of oleic acid (1 g, 3.54 mmol) and N,N-dimethylformamide (266 μl, 3.64 mmol) in dry methylene chloride (10 ml) was added dropwise oxalyl chloride (2.0 M solution in methylene chloride, 3.5 ml, 7 mmol) under nitrogen atmosphere. The reaction mixture was stirred for 1 h, and then, the solvent was evaporated under a nitrogen flow. The crude material in methylene chloride (10 ml) was added to a solution of glycine (800 mg, 10.62 mmol) and 2 N potassium hydroxide in an ice bath. Then, the reaction mixture was stirred for 1 h, water (10 ml) was added, and the mixture was acidified to pH 3 with 1 N HCl. The product was extracted with ether (3 × 50 ml) and dried (MgSO4), and solvent was evaporated under reduced pressure. The crude material was chromatographed on silica gel (eluting with chloroform/methanol) to yield a crystalline solid. Melting point 93–94 C (degradation); LC-MS: (M-H)+ = 339 m/z; NMR (CD3OH, ppm): 5.35–5.32 (m,2H), 4.45 (s,2H), 2.13–2.18 (m, 6H), 1.58 (m, 2H), 1.32–1.29 (m, 20H), 0.88 (t, 3H).
Apparatus
For investigation of the acute naloxone-precipitated MWD somatic reactions in experiments 1 and 2, the observation chamber consisted of four black Plexiglas boxes (22.5 × 26 × 20 cm) with an opaque lid, sitting on top of a clear glass-topped table. There was a close circuit Panasonic WV-CP484 video camera placed below each of the chambers to video-record somatic withdrawal behaviors that was fire-wired to a computer for later scoring using “The Observer” Event recording software (Noldus Information Technology Inc, Leesburg, VA).
For investigation of conditioned gaping reactions in experiments 3 and 5, the rats were placed in taste reactivity (TR; Grill and Norgren, 1978) chambers with their cannula attached to an infusion pump (Model KDS100, KD Scientific, Holliston, MA, USA) for fluid delivery. The TR chamber was in a dark room next to a 25 W light source. The TR chambers used were made of clear Plexiglas (22.5 × 26 × 20 cm) and sat on a table with a clear glass top. A mirror beneath the chamber at a 45° angle facilitated viewing of the ventral surface of the rat to observe orofacial responses. A Sony video camera (Handycam, Henry’s Camera Waterloo ON) with a fire-wire connection to computer was focused on the mirror and used to record the rats from the mirror beneath the chamber. The videos were later scored using “The Observer” Event recording software (Noldus Information Technology Inc, Leesburg, VA).
For investigation of shrew vomiting in experiment 4, the clear Plexiglas observation chambers (22.5 × 26 × 20 cm) were placed on a table with a clear glass top. A mirror at a 45° angle beneath the chamber facilitated viewing of the ventral surface of each shrew.
Procedure
Experiment 1: effect of OlGly on acute naloxone-precipitated MWD somatic reactions.
Upon arrival in the laboratory, the rats were handled for 3 days prior to treatments. There were a total of six groups (n = 8/group, S = saline, M = morphine, V = VEH, OG = OlGly, N = naloxone): S-VN, S-VS, M-VN, M-VS, S-OGS, M-OGN. The rats received two trial cycles 24 h apart to evaluate the potential of naloxone to promote somatic withdrawal 24 h following morphine. On the first day of each cycle, all rats were weighed and received either a 1-ml/kg sc injection of saline or morphine (20 mg/kg, sc) and were placed in an empty shoebox cage in the colony room. The rats were monitored for signs of respiratory distress and returned to their home cage once fully ambulatory. Two hours after morphine, the rats were weighed again to determine potential weight loss. On the second day of each cycle, 24 h after the morphine or saline injections, all rats were weighed before they were injected ip (1 ml/kg) with VEH or OlGly (5 mg/kg, sc) and 10 min later were injected sc (1 ml/kg) with saline or naloxone (1 mg/kg, sc). Ten minutes later, they were placed in the observation chamber for 30 min and monitored for presence of diarrhea (yes/no). On the final trial, the rats’ somatic behaviors were videotaped from below the chambers. Two hours after the sc naloxone or saline injection, the rats were weighed again. The video tapes were later scored using The Observer for the behaviors listed in Table 1 which are typically produced by withdrawal from chronic exposure to morphine (Gellert and Sparber, 1977; Koob 2009; Maldonado et al. 1996).
Experiment 2: effect of a PPARα antagonist and a CB1 antagonist on OlGly inhibition of acute naloxone-precipitated MWD somatic reactions
We evaluated if the interference with somatic withdrawal by OlGly was PPARα-mediated, using the PPARα antagonist, MK886, or CB1 receptor mediated, using the CB1 receptor antagonist, AM251 with rats assigned to one of 6 groups: M-VN (n = 8), M-OGN (n = 8), M-MKVN (n = 8), M-MKOGN (n = 7), M-AMVN (n = 7), and M-AMOGN (n = 8). Rats were treated as in experiment 1, but all rats were injected with morphine (20 mg/kg, sc) 24 h prior to naloxone (1 mg/kg, sc). To determine the mechanism of the suppression of the somatic effects of MWD by OlGly, the rats were pretreated with MK886 (1 mg/kg, sc) or AM251 (1 mg/kg sc) 15 min prior to receiving an injection of OlGly (5 mg/kg, sc) or VEH 10 min prior to naloxone. The videotapes of the final trial were scored for the acute naloxone-precipitated MWD responses found in experiment 1.
Experiment 3: effect of OlGly on LiCl-induced conditioned gaping in rats
All rats were surgically implanted with an intraoral cannula under isofluorane anesthesia according to the procedure described by Limebeer et al. (2010). Following 3-day recovery from surgery, the rats received an adaptation trial in which they were placed in the TR chamber with their cannula attached to an infusion pump (Model KDS100, KD Scientific, Hollliston, MA, USA) for fluid delivery. Water was infused into their intraoral cannuale for 2 min at the rate of 1 ml/min. The conditioning groups included V-LiCl (n = 7), OlGly-Saline (n = 8), and OlGly-LiCl (n = 8). During conditioning, the rats were injected with OlGly (5 mg/kg, ip) or VEH 20 min prior to being individually placed in the TR chamber and intraorally infused with 0.1% saccharin solution for 2 min at the rate of 1 ml/min while the orofacial responses were video recorded. Immediately after the saccharin infusion, the rats were injected with 20 ml/kg of 0.15 M LiCl or equivolume saline and returned to their home cage. Seventy-two hours later, the rats were tested drug-free. They were again intraorally infused with 0.1% saccharin solution for 2 min at the rate of 1 ml/min while the orofacial reactions were video recorded. The videotapes were later scored by an observer blind to the experimental conditions using The Observer for the behavior of gaping (large openings of the mouth and jaw, with lower incisors exposed).
Experiment 4: effect of OlGly on LiCl-induced vomiting in shrews
Male (n = 7) and female (n = 5) shrews were transferred from the colony room to an empty cage in the experimental room that contained four meal worms. After 15 min, they were injected with VEH (3M/3F) or 5 mg/kg, ip, OlGly (4M/2F). Twenty minutes later, they were injected with LiCl and put into an observation chamber for 45 min. An observer recorded the frequency of vomiting episodes using The Observer.
Experiment 5: effect of OlGly on naloxone-precipitated MWD-induced conditioned gaping reactions in rats and mechanism of action
Experiment 5 was conducted in a similar manner as was experiment 3, except that the rats received two naloxone-precipitated MWD conditioning trials, to be consistent with the procedures of Petrie et al. (2019) and those of experiments 1 and 2. Three days after intraoral cannulation surgery, the rats were given an adaptation trial to the TR procedures (as in experiment 3). Twenty-four hours later, conditioning began. They were assigned to one of six groups: M-VN (n = 7), M-OGN (n = 8), M-MKVN (n = 8), M-MKOGN (n = 8), M-AMVN (n = 8), and M-AMOGN (n = 8). We have previously demonstrated that naloxone alone does not produce conditioned gaping in rats (McDonald et al. 1997; Parker and Rennie 1992). On the first day of each cycle, all rats received morphine (20 mg/kg, sc) and were placed in an empty shoebox cage in the colony room. The rats were monitored for signs of respiratory distress and returned to their home cage once fully ambulatory. On the second day of each cycle, 24 h after the morphine injection, all rats were injected ip (1 ml/kg) with VEH or OlGly (5 mg/kg, sc) and 20 min later were individually placed in the TR chamber and intraorally infused with 0.1% saccharin solution for 2 min at the rate of 1 ml/min. Immediately after the saccharin infusion, the rats were injected with naloxone (1 mg/kg) and returned to their home cage. Additional rats were pretreated with MK886 (1 mg/kg) or AM251 (1 mg/kg) 15 min prior to administration of OlGly or VEH.
Seventy-two hours after the second conditioning trial, the rats were tested drug-free. They were again placed in the TR chamber and intraorally infused with 0.1% saccharin solution for 2 min at the rate of 1 ml/min while the orofacial reactions were video recorded. The videotapes were later scored by an observer blind to the experimental conditions using The Observer for the behavior of gaping (large openings of the mouth and jaw, with lower incisors exposed).
Analysis
In experiments 1 and 2, each measure described in Table 1 was entered into a one-way analysis of variance (ANOVA), with subsequent Bonferroni post hoc tests. In experiments 3 and 5, the number of gaping reactions during the drug-free TR test were entered into a one-way ANOVA with subsequent Bonferroni post hoc tests. In experiment 4, the number of LiCl-induced vomiting reactions displayed by male and female shrews pre-treated with VEH or OlGly were entered into a 2 × 2 between factors ANOVA. Significance is defined as p < 0.05.
Results
Experiment 1: effect of OlGly on acute naloxone-precipitated MWD somatic reactions
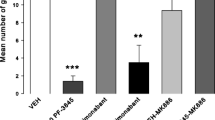
Only the behaviors that revealed evidence of acute naloxone-precipitated withdrawal are reported here. These behaviors included mouthing movements (probably reflecting chewing), abdominal contractions, lying on belly (LOB), instances of diarrhea (yes or no), and percentage body weight loss. OlGly interfered with naloxone-precipitated MWD-induced mouthing movements, abdominal contractions, lying on belly, and diarrhea, but not body weight loss. Figure 1 presents each of these behaviors for the various groups. The single factor ANOVA for each of these behaviors revealed a significant main effect: mouthing movement, F(5, 42) = 15.7; p < 0.001; lying on belly, F(5, 42) = 13.6; p < 0.001; abdominal contractions, F(5, 42) = 47.5; p < 0.001; percent body weight loss, F(5, 42) = 4.2; p = 0.004. Subsequent Bonferroni post hoc tests revealed that group M-VN displayed significantly more mouthing movements (p < 0.001), lying on belly (p < 0.001), abdominal contractions (p < 0.001) than all groups, including M-OGN. For the percentage of body weight loss, group M-VN displayed more weight loss than all groups (p < 0.025) except group M-OGN (which did not significantly differ from any other groups).
Mean (±SEM) number of mouthing movements, number of abdominal contractions, seconds of lying on belly, number of rats displaying diarrhea, and percent body weight loss of rats in the various groups in experiment 1. Rats in group M-VN displayed significantly more of these withdrawal behaviors than any other group, except for body weight loss in which rats in group M-VN did not differ from rats in group M-OGN signified by 1. ***p < 0.001; *p < 0.05
Experiment 2: effect of a PPARα antagonist and a CB1 antagonist on OlGly inhibition of acute naloxone-precipitated MWD somatic reactions
The effect of OlGly on acute naloxone-precipitated MWD somatic reactions was prevented by both the PPARα antagonist and by the CB1 antagonist, whereas neither antagonist per se produced any effect. Figure 2 presents the mean number of each of the acute naloxone-precipitated MWD somatic reactions in experiment 2. The one-way ANOVAs revealed a significant effect among groups for mouthing movements, F(5, 40) = 9.4; p < 0.001, abdominal contractions, F(5, 50) = 17.4; p < 0.001, and for lying on belly, F(5, 40) = 9.1; p < 0.001, and for instances of diarrhea, F(5, 40) = 3.5, p = 0.01. Bonferroni post hoc comparisons among groups revealed that group MOGN displayed fewer mouthing movements, abdominal contractions, and spent less time lying on belly than all other groups (p < 0.001), which did not differ from one another. For the measure of instances of diarrhea, Bonferroni tests revealed that groups M-AMVN and M-AMOGN differed significantly (p < 0.05) from group M-OGN, and less conservative least significant difference tests revealed that all groups differed significantly from M-OGN.
Mean (±SEM) number of mouthing movements, number of abdominal contractions, seconds of lying on belly, and number of rats displaying diarrhea in the various groups in experiment 2. MK = MK886, AM = AM251. Rats in group M-OGN displayed fewer of these withdrawal behaviors than any other group. ***p < 0.001, *p < 0.05
Experiment 3: effect of OlGly on LiCl-induced conditioned gaping in rats
OlGly interfered with LiCl-induced gaping reactions. Figure 3 presents the mean number of gapes displayed by LiCl-conditioned rats. The one-way ANOVA revealed a significant effect of group, F(2, 20) = 25.0; p < 0.001; subsequent Bonferroni tests revealed that group VEH-LiCl gaped significantly (p < 0.001) more than any other group.
Experiment 4: effect of OlGly on LiCl-induced vomiting in shrews
OlGly reduced LiCl-induced vomiting in the S. murinus. Figure 4 presents the mean number of vomiting episodes displayed by the male or female S. murinus following VEH or OlGly (5 mg/kg) pretreatment injections. The number of vomiting episodes was entered into a two-factor between analysis of variance (ANOVA) with the factors of pretreatment group and sex. The ANOVA revealed a significant group effect, F(1, 8) = 29.7; p = 0.001 for vomiting, and a non-significant sex effect F(1, 8) = 0.7; p > 0.05, and a non-significant group × sex interaction, F(1, 8) = 0.1; p > 0.05.
Experiment 5: effect of OlGly on acute naloxone-precipitated MWD-induced conditioned gaping in rats and mechanism of action
OlGly interfered with MWD-induced conditioned gaping in rats in a manner mediated by both a CB1 receptor and a PPARα mechanism. Figure 5 presents the mean number of gaping reactions displayed by the various groups during the final TR test trial. A one-way ANOVA revealed a significant effect of group, F(5, 41) = 3.5; p = 0.01. Subsequent Bonferroni post hoc comparison tests revealed that group M-OGN displayed significantly fewer conditioned gapes at test than did all other groups (p’s < 0.05).
Discussion
This was the first study to examine the somatic withdrawal reactions displayed by male Sprague–Dawley rats in the acute naloxone-precipitated withdrawal paradigm. Among the myriad of withdrawal reactions reported in the literature following withdrawal from chronic exposure to morphine, the only responses evident from acute naloxone-precipitated MWD were the nausea-like behaviors of lying on belly, abdominal contractions, and diarrhea, as well as mouthing movements (chewing) and body weight loss. These reactions were not simply a response to naloxone, because the S-VN group showed none of these behaviors. Shoblock and Maidment (2006) previously demonstrated in mice that the predominant reaction to acute naloxone-precipitated MWD was jumping; however, in rats, we found no evidence of jumping behavior in any group. This may have been due to the size of our chambers which may have limited the display of this response, or due to species differences in responding to acute naloxone-precipitated MWD.
Similar to the capability of OlGly to interfere with the affective properties of acute naloxone-precipitated MWD-induced CPA (Petrie et al. 2019), OlGly also interfered with the nausea-like somatic withdrawal reactions (as well as mouthing movements) of acute MWD. The suppression of these MWD reactions by OlGly was reversed by both the CB1 receptor antagonist, AM251, and the PPARα antagonist, MK886. Petrie et al. (2019) previously reported that OlGly interfered with acute naloxone-precipitated MWD-induced CPA in rats by a CB1 receptor mechanism of action, whereas Donvito et al. (2019) previously reported that OlGly interfered with nicotine-induced CPP in mice by a PPARα mechanism of action. The present results suggest that OlGly acts by both mechanisms to interfere with the somatic properties of naloxone-precipitated MWD. OlGly both inhibits FAAH and activates PPARα (Donvito et al. 2019). Therefore, it is likely that OlGly is acting as a FAAH inhibitor, thereby elevating AEA, PEA, and OEA. The action of elevated AEA at CB1 receptors was therefore prevented with the CB1 receptor antagonist (AM251), whereas the action of the PPARα agonists, PEA and OEA, as well as the direct action of OlGly as a PPARα agonist were prevented by the PPARα antagonist (MK886). It is interesting that both mechanisms appear to mediate the somatic MWD behavioral effects and MWD-induced nausea, suggesting cross-talk between these two systems in the regulation of nausea. Indeed, we have previously reported that FAAH inhibition inhibits acute nausea by its action on PPARα (Rock et al. 2015, 2017, 2019), but anticipatory nausea by its action on CB1 receptors (Rock et al. 2015). On the other hand, MAGL inhibition inhibits both acute and anticipatory nausea by its action on CB1 receptors (Parker et al. 2015; Sticht et al. 2016).
The somatic withdrawal responses elicited by acute naloxone-precipitated MWD are reminiscent of those elicited unconditionally by the nausea-inducing drug LiCl in rats (Contreras et al. 2007; Parker et al. 1984; Tuerke et al. 2012). That is, pretreatment with LiCl in rats can produce the abnormal posture of lying on belly and abdominal contractions. These reactions can be suppressed by the anti-emetic serotonin (5-HT) 3 antagonist, ondansetron (Tuerke et al. 2012). Acute naloxone-precipitated MWD has been shown to produce the nausea-induced behavior of conditioned gaping in rats (McDonald et al. 1997). Nausea is a primary symptom reported by humans experiencing MWD (Jaffee and Martin 1990).
Conditioned gaping reactions (Grill and Norgren 1978) in rats are only produced by emetic drugs and are selectively suppressed by antiemetic drugs (see Parker 2014 for review). The potential of OlGly to interfere with the somatic effects of acute naloxone-precipitated MWD may therefore reflect its potential to interfere with nausea. Accordingly, we found here that OlGly interfered with LiCl-induced conditioned gaping reactions in rats and with LiCl-induced vomiting in the Suncus murinus. Additionally, OlGly interfered with acute naloxone-precipitated MWD-induced conditioned gaping reactions in rats. This latter effect of the mediator, like its effect on somatic withdrawal elicited by acute naloxone-precipitated MWD, was again attenuated by both CB1 and PPARα receptor antagonists, thus supporting our hypothesis that the inhibitory effect of OlGly on the somatic responses might be a consequence of its anti-nausea effects.
Our investigation of the potential of OlGly to interfere with the aversive components of MWD has been limited to the acute naloxone-precipitated MWD paradigm because of the precision in timing of inducing the effect for the use in place conditioning/nausea studies. A single exposure to high-dose morphine (20 mg/kg, sc) is sufficient to demonstrate the aversive effects of naloxone-precipitated MWD 24 h (and even 48 h; Parker et al. 2002) later, while multiple exposures produce yet greater conditioned effects. However, it will also be important in future studies to investigate the potential of OlGly to interfere with withdrawal from chronic exposure to morphine, which has greater translational appeal. Understanding the neural substrates of both the positive and negative reinforcement of motivational withdrawal from opiates is critical for comprehending the chronic, relapsing compulsive use of opiates which produce a significant dysregulation of brain hedonic systems (Koob 2009). We have demonstrated that endogenous OlGly is elevated in the nucleus accumbens in rat brains undergoing acute naloxone precipitated MWD (Petrie et al. 2019). The nucleus accumbens is a region within the extended amygdala that has been implicated in regulation of the negative reinforcement of motivational withdrawal from opiates, coined as the “dark side of addiction” (Koob 2009; Koob et al. 1989) which drives drug seeking. OlGly may be released to regulate the deviation from homeostasis produced by acute naloxone-precipitated MWD. In future studies we will extend this investigation to determine if OlGly or other protective fatty acid amides are released in the nucleus accumbens, amygdala, and bed nucleus of the stria terminalis (extended amygdala) in rats experiencing withdrawal from chronic exposure to morphine.
Abbreviations
- AEA:
-
anandamide
- AM:
-
AM251
- ANOVA:
-
analysis of variance
- CB1 :
-
cannabinoid 1
- CB2 :
-
cannabinoid 2
- CPA:
-
conditioned place aversion
- FAAH:
-
fatty acid amide hydrolase
- ip:
-
intraperitoneal
- LiCl:
-
lithium chloride
- LOB:
-
lying on belly
- M:
-
morphine
- MK:
-
MK886
- MWD:
-
morphine withdrawal
- N:
-
naloxone
- OEA:
-
N-oleoylethanolamide
- OlGly:
-
N-oleoylglycine
- PEA:
-
N-palmitoylethanolamide
- PPARα:
-
peroxisome proliferator-activated receptor alpha
- S:
-
saline
- sc:
-
subcutaneous
- TR:
-
taste reactivity
- VEH:
-
vehicle
References
Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 26:655–658. https://doi.org/10.1126/science.1145590
Donvito G, Piscitelli F, Muldoon P, Jackson A, Vitale RM, D’Aniello E et al (2019) N-Oleoyl-glycine reduces nicotine reward and withdrawal in mice. Neuropharmacology 148:320–331. https://doi.org/10.1016/j.neuropharm.2018.03.020
Eisenberg RM (1982) Further studies on the acute dependence produced by morphine in opiate naive rats. Life Sci 31:1531–1540. https://doi.org/10.1016/0024-3205(82)90043-1
Gellert VF, Sparber SB (1977) A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol ExpTher 201:44–54
Grill HJ, Norgren R (1978) The taste reactivity test: 1 Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279. https://doi.org/10.1016/0006-8993(78)90568-1
Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1990) Acute opioid physical dependence in humans: effect of naloxone at 6 and 24 hours postmorphine. Pharmacol Biochem Behav 36:393–399. https://doi.org/10.1016/0091-3057(90)90421-D
Jaffee JH, Martin WR (1990) Opioid analgesics and antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P (eds) The pharmacological basis of therapeutics (8th ed), Pergamon, New York pp 485-521
June HL, Stitzer ML, Cone E (1995) Acute physical dependence: time course and relation to human plasma morphine concentrations. Clin Pharmacol Ther 57:270–280. https://doi.org/10.1016/0009-9236(95)90152-3
Koob GF (2009) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42:S32–S41. https://doi.org/10.1055/s-0029-1216356
Koob GF, Wall TL, Bloom FE (1989) Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology 98:530–534
Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A et al (2010) Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol 161:336–349. https://doi.org/10.1111/j.1476-5381.2010.00885.x
Maldonado R, Stinus L, Koob GF (1996) Neurobiological mechanisms of opiate withdrawal. R.G. Landes Company, Austin
Martin WR, Eades CG (1964) A comparison between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther 146:385–394
Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK et al (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40:471–478. https://doi.org/10.1016/0091-3057(91)90349-7
McDonald RV, Parker LA, Siegel S (1997) Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav 58:1003–1008. https://doi.org/10.1016/S0091-3057(97)00313-4
Meachum C, Bernstein IL (1992) Behavioral conditioned responses to contextual andodor stimuli paired with LiCl administration. Physiol Behav 52:895–899. https://doi.org/10.1016/0031-9384(92)90368-C
Parker LA (2014) Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol 722:122–133. https://doi.org/10.1016/j.ejphar.2013.09.070
Parker LA, Cyr JA, Santi AN, Burton PD (2002) The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine: evaluation by taste and place conditioning. Pharmacol Biochem Behav 72:87–92. https://doi.org/10.1016/S0091-3057(01)00724-9
Parker LA, Hills K, Jensen K (1984) Behavioral CRs elicited by a lithium- or an amphetamine-paired contextual test chamber. Anim Learn Behav 12:307–315. https://doi.org/10.3758/BF03199972
Parker LA, Joshi A (1998) Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav 61:331–333. https://doi.org/10.1016/S0091-3057(98)00104-X
Parker LA, Kwiatkowska M, Burton P, Mechoulam R (2004) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 171:156–161. https://doi.org/10.1007/s00213-003-1571-2
Parker LA, Niphakis MJ, Downey R, Limebeer CL, Rock EM, Sticht MA, Morris H, Abdullah R, Lichtman AH, Cravatt BF (2014) A new MAGL inhibitor, MJN110, reduces acute and anticipatory nausea in rats and vomiting in Suncus murinus. Psychopharmacology 232:583–593. https://doi.org/10.1007/s00213-016-4277-y
Parker LA, Rennie M (1992) Naltrexone-induced aversions: Assessment by the placeConditioning, taste reactivity and taste avoidance paradigms. Pharmacol Biochem Behav 41:559–565. https://doi.org/10.1016/0091-3057(92)90373-n
Petrie GN, Wills KL, Piscitelli F, Smoum R, Limebeer CL, Rock EM et al (2019) Oleoyl glycine: interference with the aversive effects of acute naloxone-precipitated MWD, but not morphine reward, in male Sprague-Dawley rats. Psychopharmacology 236:2623–2633. https://doi.org/10.1007/s00213-019-05237-9
Ramesh D, Ross GR, Schlosburg JE, Owens R, Abdullah R, Kinsey SG et al (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339:173–185. https://doi.org/10.1124/jpet.111.181370
Rock EM, Limebeer CL, Ward JM, Cohen A, Grove K, Niphakis MJ, Cravatt BF, Parker LA. (2015).Fatty acid amide hydrolase (FAAH) inhibition interferes with acute nausea by a PPARα mechanism and anticipatory nausea by a CB1 receptor mechanism in a double dissociation. Psychopharmacology, 232: 3841-3848. https://doi.org/10.1007/s00213-015-4050-7
Rock EM, Moreno-Sanz G, Limebeer CL, Petrie G, Angelini R, Piomelli D et al (2017) Suppression of acute and anticipatory nausea by peripherally restricted FAAH inhibitor in animal models: role of PPARa and CB1 receptors. Br J Pharmacol 174:3837–3847. https://doi.org/10.1111/bph.13980
Rock EM, Limebeer CL, Aliasi-Sanai L, Parker LA (2019) The ventral pallidum as a critical region regulating fatty acid amide hydrolase inhibition of nausea-induced conditioned gaping in male Sprague-Dawley rats. Neuropharmacology 155:142–149. https://doi.org/10.1016/j.neuropharm.2019.05.031
Shoblock JR, Maidment NT (2006) Constitutively active mu opioid receptors mediate the enhanced conditioned aversvie effect of naloxone in morphine-dependent mice. Neuropsychopharmacology 31:171–177. https://doi.org/10.1038/sj.npp.1300782
Sticht MA, Limebeer CL, Rafla BR, Abdullah RA, Poklis JL, Ho W, Niphakis MJ, Cravatt BF, Sharkey KA, Lichtman AH, Parker LA (2016) Endocannabinoid regulation of nausea is mediated by 2-arachidonolyglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology 102:92–102. https://doi.org/10.1016/j.neuropharm.2015.10.039
Stinus L, Le Moal M, Koob GF (1990) Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience 37:767–773. https://doi.org/10.1016/0306-4522(90)90106-E
Tuerke KJ, Winters BD, Parker LA (2012) Ondansetron interferes with unconditioned lying-on belly and acquisition of conditioned gaping induced by LiCl as models of nausea-induced behaviors in rats. Physiol Behav 105:856–860. https://doi.org/10.1016/j.physbeh.2011.10.017
Wills KL, Petrie GN, Millett G, Limebeer CL, Rock EM, Niphakis MJ et al (2016) Double dissociation of monoacylglycerol lipase inhibition and CB1 antagonism in the central amygdala, basolateral amygdala, and the interoceptive insular cortex on the affective properties of acute naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 41:1865–1873. https://doi.org/10.1038/npp.2015.356
Wills KL, Vemuri K, Kalmar A, Lee A, Limebeer CL, Makriyannis A et al (2014) CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology 231:4291–4300. https://doi.org/10.1007/s00213-014-3575-5
Funding
The research described here was funded by research grants from the Natural Sciences and Engineering Council of Canada (NSERC: 03629), Canadian Institutes of Health Research (CIHR:388239) and a research contract from PlantExt to Linda A Parker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rock, E.M., Ayoub, S.M., Limebeer, C.L. et al. Acute naloxone-precipitated morphine withdrawal elicits nausea-like somatic behaviors in rats in a manner suppressed by N-oleoylglycine. Psychopharmacology 237, 375–384 (2020). https://doi.org/10.1007/s00213-019-05373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05373-2