Abstract
Rationale
Atypical antipsychotic drugs (APDs), many of which are direct or indirect serotonin (5-HT)1A agonists, and tandospirone, a 5-HT1A partial agonist, have been reported to improve cognition in schizophrenia.
Objectives and methods
We tested the effect of 5-HT1A agonism, alone, and in combination with other psychotropic agents, including the atypical APD, lurasidone, in reversing the deficit in novel object recognition (NOR) induced by subchronic treatment with the non-competitive NMDA receptor antagonist, phencyclidine (PCP) (2 mg/kg, b.i.d., for 7 days).
Results
Subchronic treatment with PCP induced a persistent NOR deficit. Lurasidone (0.1 mg/kg), a potent 5-HT1A partial agonist, 5-HT2A antagonist, and weaker D2 antagonist, tandospirone (0.6 mg/kg), and the selective post-synaptic 5-HT1A agonist, F15599 (0.16 mg/kg), ameliorated the subchronic PCP-induced-NOR deficit. The 5-HT1A antagonist, WAY100635 (0.6 mg/kg), blocked the ameliorating effects of tandospirone and lurasidone. The combination of sub-effective doses of tandospirone (0.2 mg/kg) and lurasidone (0.03 mg/kg) also reversed the PCP-induced NOR-deficit. Buspirone, a less potent partial 5-HT1A agonist than tandospirone, was less effective. Co-administration of tandospirone (0.2 mg/kg) and pimavanserin (3 mg/kg), a relatively selective 5-HT2A receptor inverse agonist, did not reverse the effect of sub-chronic PCP on NOR. The D2 antagonist, haloperidol, blocked the ameliorating effect of tandospirone on the PCP-induced deficit in NOR.
Conclusions
These results indicate that 5-HT1A agonism is adequate to ameliorate the PCP-induced impairment in NOR and suggest further study of utilizing the combination of a 5-HT1A agonist and an atypical APD to ameliorate some types of cognitive impairment in schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moderate–severe deficits in multiple domains of cognition, including declarative memory, are present in schizophrenia (Kenny and Meltzer 1991; Saykin et al. 1991; Meltzer and McGurk 1999; Stone and Hsi 2011). Stimulation of 5-HT1A receptors has been identified as a target for improving cognitive impairment in schizophrenia (Meltzer 1999; Bantick et al. 2001). Post-mortem studies report increased density of 5-HT1A receptors in frontal and temporal cortices in schizophrenic patients (Burnet et al. 1996; 1997; Gurevich and Joyce 1997; Hashimoto et al. 1991; Simpson et al. 1996; Sumiyoshi et al. 1996). This is consistent with positron emission tomographic studies demonstrating increased cortical 5-HT1A receptor binding in schizophrenia (Kasper et al. 2002; Tauscher et al. 2002). Sumiyoshi et al. (2000; 2001a; 2001b) reported that the addition of tandospirone, a 5-HT1A partial agonist, to ongoing treatment with typical antipsychotic drugs (APDs) improved executive function, verbal learning, and memory, suggesting that the upregulation of 5-HT1A receptor density noted above may be a compensatory phenomenon to schizophrenia pathology. Sub-chronic treatment with phencyclidine (PCP), a noncompetitive N-methyl-d-aspartate receptor (NMDA-R) antagonist, has been reported to increase 5-HT1A receptor binding in the medial–prefrontal and dorsolateral frontal cortex in the absence of changes in 5-HT2A receptor binding in any brain region (Choi et al. 2009). This, too, may represent a compensatory process. Further, 5-HT1A agonists, e.g., F15599, a preferential post-synaptic 5-HT1A agonist (Maurel et al. 2007; Newman-Tancredi et al. 2009), improves cognition in rodents (see “Discussion”).
There is conflicting evidence that atypical APDs are more effective than typical APDs in attenuating some cognitive deficits in schizophrenia (Hagger et al. 1993; Meltzer and McGurk 1999; Woodward et al. 2005; Keefe et al. 2007). Some atypical APDs, including aripiprazole, clozapine, and lurasidone, are 5-HT1A partial agonists in vivo (Newman-Tancredi 2010). The increased dopamine (DA) release in medial prefrontal cortex (mPFC) induced by atypical APDs has been suggested to be due to 5-HT1A receptor agonism as WAY100635, a 5-HT1A antagonist, blocks the effect of atypical APDs to increase cortical DA release; 5-HT1A agonists themselves enhance rat cortical DA efflux (Rollema et al. 1997; Ichikawa et al. 2001; 2002), leading to the suggestion that 5-HT1A agonism-induced cortical DA release may play a role in the disputed ability of atypical APDs to improve cognitive function in schizophrenia (Ichikawa et al. 2002).
Hypoglutamatergic function in the frontal cortex and hippocampus has also been suggested to underlie the cognitive impairment in schizophrenia (Coyle 2006). The main evidence for such a deficit in glutamatergic function is that the NMDA-R antagonists, PCP, MK-801, and ketamine induce cognitive impairment in healthy subjects (Javitt and Zukin 1991; Krystal et al. 1999; Lahti et al. 2001; Newcomer et al. 1999) and increase psychosis in patients with schizophrenia (Lahti et al. 1995; 2001). Acute or subchronic administration of PCP and MK-801 has been reported to produce impairments in visual and learning memory, attention, reasoning and problem solving, working memory, and social cognition in rodents (see Neill et al. 2010 for review). Novel object recognition (NOR) is a possible analog of declarative memory in humans (Neill et al. 2010). Atypical APDs, but not the typical APD haloperidol, have been reported to reverse cognitive deficits induced by sub-chronic PCP treatment in NOR (see Meltzer et al. 2011 for review). We have recently reported that the ability of sub-effective doses of atypical APDs to reverse the NOR deficit in sub-chronic PCP-treated rats is potentiated by the 5-HT2A inverse agonists, as well as the mGluR2/3 agonist, LY379268 (Snigdha et al. 2010; Horiguchi et al. 2011a). Moreover, the effect of atypical APDs to improve the PCP-induced NOR deficit is blocked by haloperidol, a D2 receptor antagonist (Snigdha et al. 2010).
The aim of this study was to examine the effect of 5-HT1A agonism on cognitive impairment in NOR induced by sub-chronic PCP treatment and its interaction with a variety of other mechanisms of interest for NOR. Experiments were carried out using the 5-HT1A partial agonist tandospirone, alone and in combination with the selective 5-HT1A antagonist WAY100635, the 5-HT2A inverse agonist pimavanserin, the novel atypical APD lurasidone (which is itself a 5-HT1A partial agonist), and the D2 antagonist, haloperidol. We also studied the effect of another 5-HT1A partial agonist, buspirone, and a preferential post-synaptic 5-HT1A agonist, F15599, to reverse the effects of sub-chronic PCP.
Materials and methods
Animals
Eighty-six female Long–Evans (LE) rats from two separate batches (8–9 weeks old) (Harlan Sprague Dawley, Inc, Indianapolis, IN, USA) were used in the NOR experiment. The first 43 rats (rat group 1) were used for NOR experiments 1 to 4 and the second 43 rats (rat group 2) were used for NOR experiments 5 to 7. LE rats were housed in groups of three or four on a 12-h light/dark cycle. All experiments were conducted during the light phase. Food and water were available ad libitum. All experiments were conducted in accordance with Vanderbilt animal committee regulations.
Drugs
Lurasidone and tandospirone were provided by Dainippon Sumitomo Pharma (Osaka, Japan). Pimavanserin was provided by Acadia Pharmaceuticals (Torrence, CA, USA). Buspirone and haloperidol were obtained from Sigma-Aldrich (St Louis, MO, USA). PCP was supplied as a generous gift from the National Institute of Drug Abuse. F15599 was obtained from Pierre Fabre (Castres, France). WAY100635 was a gift from Wyeth Laboratories (Philadelphia, PA, USA). Lurasidone and F15599 were solubilized in 0.5 % methylcellulose, 0.2 % Tween80. The other drugs were dissolved in distilled water. All drugs or vehicle were administered intraperitoneally (i.p.) at a volume of 1 mL/kg body weight.
Drug treatment
LE rats in group 1 were randomly assigned to two treatment groups: nine were treated with vehicle (saline, i.p.) and the remainder were treated with PCP (2 mg/kg i.p.) twice daily for 7 days. Rats in group 2 were randomly assigned to two treatment groups: 19 were treated with vehicle (saline, i.p.) and the remainder were treated with PCP (2 mg/kg i.p.) twice a day for 7 days. Subsequently, animals were given a 7-day washout period prior to NOR testing (Grayson et al. 2007; Snigdha et al. 2010). Each rat was tested four times in the NOR paradigm with a 7-day washout period between each of the test sessions. This multiple-treatment regimen has previously been shown to not effect results when compared to testing rats only once and is preferred on humane and economic grounds. The criterion for continuing to test rats was exploration time in the acquisition and retention phases to either of two objects ≥ 5 s. If a rat did not explore at least 5 s in either of these two phases, its data were excluded from analysis. This rarely occurred and did not affect the ability to complete the analysis using data from the remaining animals of that group. All experimental groups consisted of six to nine rats.
NOR test
Testing was carried out according to a previously validated method (Snigdha et al. 2010). All rats were habituated for 1 h to the test environment and NOR arena for three consecutive days prior to the first NOR test. Rats were given a further 3-min habituation on the day of testing. After the 3-min habituation period, the rats were given two 3-min trials (an acquisition trial and a retention trial), separated by a 1-min intertrial return to their home cage. During the acquisition trial, the animals were allowed to explore two identical objects (A1 and A2). During the retention trial, the animals explored a familiar object (A) from the acquisition trial and a novel object (B). Behavior was recorded on video for blind scoring of objects exploration. Object exploration was defined by animal’s licking, sniffing, or touching the object with the forepaws while sniffing. The exploration time(s) of each object in each trial was recorded manually by the use of two stopwatches. The discrimination index (DI) [(time spent exploring the novel object − time spent exploring the familiar object) / total exploration time] was calculated for the retention trials.
Data analysis
All data are expressed as mean ± S.E.M. (n = 6–9 per group). Exploration data were analyzed by a repeated-measures two-way ANOVA followed by pair-wise comparison when a significant effect was detected by ANOVA. DI data were analyzed by one-way ANOVA followed by Bonferroni test when a significant effect was detected by ANOVA.
Results
Effect of tandospirone in subchronic PCP-treated rats (experiment 1)
In the acquisition trial, no significant differences in time spent exploring the two identical objects were observed in any group (Fig. 1a). There were no significant effects of drugs during the acquisition trial period in any of the experiments (“Electronic supplementary material”, Figs. S1, S2, S3, S4 and S5). In the retention phase, vehicle-treated rats explored the novel object significantly longer than the familiar objects (p < 0.01; Fig. 1b). The ability to discriminate novel and familiar objects was abolished by subchronic PCP treatment (Fig. 1b). Tandospirone 0.6 mg/kg, but not 0.2 mg/kg, significantly attenuated the NOR deficit (p < 0.01; Fig. 1b). Co-administration of tandospirone (0.6 mg/kg) with WAY100635 (0.6 mg/kg) abolished the ability of tandospirone to reverse the PCP-induced deficit (Fig. 1b). The DI was significantly reduced following subchronic PCP treatment (p < 0.01; Fig. 1c). Tandospirone 0.6 mg/kg, but not 0.2 mg/kg, significantly reversed the PCP-induced reduction in DI (p < 0.01; Fig. 1c). Futhermore, the effect of tandospirone (0.6 mg/kg) was significantly antagonized by 0.6 mg/kg WAY100635 (p < 0.01; Fig. 1c). Tandospirone 0.6 mg/kg significantly increased the total exploration time in the acquisition and retention phase in the PCP-treated rats (p < 0.05 and p < 0.01, respectively; Fig. 1a, b).
Effect of tandospirone (TAN, 0.2, 0.6 mg/kg) and TAN (0.6 mg/kg) plus WAY100635 (WAY, 0.6 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of TAN (0.2, 0.6 mg/kg) and TAN (0.6 mg/kg) plus WAY (0.6 mg/kg) on exploration of two identical objects in the aquisition trial. Data are shown as mean ± S.E.M. (n = 7–9 per group). b Effect of TAN (0.2, 0.6 mg/kg) and TAN (0.6 mg/kg) plus WAY (0.6 mg/kg) on exploration of a novel and a familiar object in the retention trial. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. c Effect of TAN (0.2, 0.6 mg/kg) and TAN (0.6 mg/kg) plus WAY (0.6 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, significant decrease in DI compared with the vehicle. ## p < 0.01, significant reversal in DI compared with PCP group. $$ p < 0.01, significant decrease in DI compared with PCP and 0.6 mg/kg TAN-treated group
Effect of buspirone and F15599 in subchronic PCP-treated rats (experiment 2)
In the retention trial, vehicle-treated rats showed preference for the novel object (p < 0.01; Fig. 2a). PCP-treated rats did not show preference for the novel object (Fig. 2a). Buspirone (1 mg/kg) did not affect the exploration times of the PCP-treated rats. However, 0.3 mg/kg buspirone increased the amount of time the rats spent exploring the novel object (p < 0.01; Fig. 2a). F15599 (0.16 mg/kg) significantly reversed the PCP-induced NOR deficit (p < 0.01; Fig. 2a). Subchronic PCP treatment significantly reduced the DI compared with control rats (p < 0.01, Fig. 2b). Buspirone (1 mg/kg) did not affect the PCP-induced reduction in DI (Fig. 2b). Buspirone (0.3 mg/kg) produced a higher DI score in the vehicle-treated group as compared to the PCP-treated group (0.343 ± 0.121 vs. 0.129 ± 0.060), but statistical analysis showed no significance difference in DI between buspirone (0.3 mg/kg) versus vehicle administered to PCP-treated rats (Fig. 2b). A dose of 0.16 mg/kg F15599 significantly improved the PCP-induced DI reduction (p < 0.05; Fig. 2b). Buspirone 1 mg/kg significantly enhanced the total exploration time compared with the PCP-treated rats in the acquisition and retention ephase (p < 0.05; Fig. 2b; “Electronic supplementary material”, Fig. S1).
Effect of buspirone (BUS, 0.3, 1 mg/kg) and F15599 (0.16 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of BUS (0.3, 1 mg/kg) and F15599 (0.16 mg/kg) on exploration of a novel and a familiar object in the retention trial. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, *p < 0.05, significant difference in time spent exploring the novel compared with the familiar object. b Effect of BUS (0.3, 1 mg/kg) and F15599 (0.16 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. # p < 0.05, significant reversal in DI compared with PCP group
Effect of lurasidone and WAY100635 in subchronic PCP-treated rats (experiment 3)
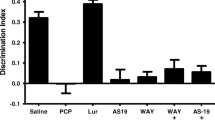
In the retention trial, vehicle-treated rats showed exploratory preference for the novel object (p < 0.01) and subchronic PCP treatment abolished the preference (Fig. 3a). Lurasidone (0.1 mg/kg) significantly reversed the PCP-induced NOR deficit (p < 0.01; Fig. 3a). However, lurasidone (0.1 mg/kg) plus WAY100635 did not improve the NOR deficit (Fig. 3a). WAY100635 (0.6 mg/kg), by itself, did not affect the exploration times of the PCP-treated rats (Fig. 3a). The DI was significantly reduced following subchronic PCP treatment (p < 0.01), and 0.1 mg/kg lurasidone significantly improved the DI reduction (p < 0.01; Fig. 3b). Co-administration of 0.1 mg/kg lurasidone with 0.6 mg/kg WAY100635 or 0.6 mg/kg WAY100635 alone did not improve DI reduction (Fig. 3b).
Effect of lurasidone (LUR, 0.1 mg/kg), LUR (0.1 mg/kg) plus WAY100635 (WAY, 0.6 mg/kg), and WAY (0.6 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LUR (0.1 mg/kg), LUR (0.1 mg/kg) plus WAY (0.6 mg/kg), and WAY (0.6 mg/kg) on exploration of a novel and a familiar object in the retention trial. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. b Effect of LUR (0.1 mg/kg), LUR (0.1 mg/kg) plus WAY (0.6 mg/kg), and WAY (0.6 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. ## p < 0.01, significant reversal in DI compared with PCP group. LUR (0.1 mg/kg) plus WAY (0.6 mg/kg)-treated group tend to reduce the DI compared with LUR (0.1 mg/kg)-treated group (p = 0.050)
Effect of tandospirone in combination with lurasidone or pimavanserin in subchronic PCP-treated rats (experiment 4)
In the retention trial, vehicle-treated rats showed exploratory preference for the novel object (p < 0.01) and this preference was abolished in the PCP-treated rats (Fig. 4a). Tandospirone (0.2 mg/kg) plus 0.03 mg/kg lurasidone, but not 0.03 mg/kg lurasidone alone, reversed the PCP-induced deficit (p < 0.01; Fig. 4a). A dose of 0.2 mg/kg tandospirone plus 3 mg/kg pimavanserin did not affect the exploration times of the PCP-treated rats (Fig. 4a). Subchronic PCP treatment significantly reduced the DI (p < 0.01; Fig. 4b). Lurasidone (0.03 mg/kg) did not reverse the reduction of the DI. However, 0.2 mg/kg tandospirone in combination with 0.03 mg/kg lurasidone, but not with 3 mg/kg pimavanserin, improved the subchronic PCP-induced DI reduction (p < 0.01; Fig. 4b).
Effect of lurasidone (LUR, 0.03 mg/kg), tandospirone (TAN, 0.2 mg/kg) plus LUR (0.03 mg/kg), and TAN (0.2 mg/kg) plus pimavanserin (PIM, 3 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LUR (0.03 mg/kg), TAN (0.2 mg/kg) plus LUR (0.03 mg/kg), and TAN (0.2 mg/kg) plus PIM (3 mg/kg) on exploration of a novel and a familiar object in the retention trial. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. b Effect of LUR (0.03 mg/kg), TAN (0.2 mg/kg) plus LUR (0.03 mg/kg), and TAN (0.2 mg/kg) plus PIM (3 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. ## p < 0.01, significant reversal in DI compared with PCP group
Effect of buspirone in combination with lurasidone in subchronic PCP-treated rats (experiment 5)
In the retention trial, vehicle-treated rats showed preference for the novel object (p < 0.01; Fig. 5a). WAY100635 did not affect the exploration times of the control rats (Fig. 5a). PCP-treated rats did not show preference for the novel object and buspirone (0.1 mg/kg) did not affect the exploration times of the PCP-treated rats (Fig. 5a). Moreover, 0.1 mg/kg buspirone in combination with 0.03 mg/kg lurasidone did not improve the PCP-induced NOR deficit (Fig. 5a). WAY100635 did not affect the DI in control rats (Fig. 5b). Subchronic PCP significantly reduced the DI (p < 0.01; Fig. 5b). Buspirone (0.1 mg/kg) alone or with 0.03 mg/kg lurasidone did not affect the PCP-induced DI reduction (Fig. 5b).
Effect of WAY100635 (WAY, 0.6 mg/kg) on control rats and buspirone (BUS, 0.1 mg/kg) and BUS (0.1 mg/kg) plus lurasidone (LUR, 0.03 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of WAY (0.6 mg/kg) to control rats and BUS (0.1 mg/kg) and BUS (0.1 mg/kg) plus LUR (0.03 mg/kg) on exploration of a novel and a familiar object in the retention trial. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. b Effect of WAY (0.6 mg/kg) to control rats and BUS (0.1 mg/kg) and BUS (0.1 mg/kg) plus LUR (0.03 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 6–8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group
Effect of tandospirone or lurasidone in combination with haloperidol in subchronic PCP-treated rats (experiment 6)
In the retention trial, vehicle-treated rats explored the novel significantly more than the familiar object (p < 0.01) and subchronic PCP treatment abolished the preference (Fig. 6a). Lurasidone (0.1 mg/kg) or tandospirone (0.6 mg/kg) in combination with 0.1 mg/kg haloperidol did not improve the NOR deficit (Fig. 6a). The DI was significantly reduced following subchronic PCP treatment (p < 0.01), and lurasidone (0.1 mg/kg) or tandospirone (0.6 mg/kg) in combination with 0.1 mg/kg haloperidol did not improve the PCP-induced reduction in DI (Fig. 6b).
Effect of lurasidone (LUR, 0.1 mg/kg) plus haloperidol (HAL, 0.1 mg/kg) and tandospirone (TAN, 0.6 mg/kg) plus HAL (0.1 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LUR (0.1 mg/kg) plus HAL (0.1 mg/kg) and TAN (0.6 mg/kg) plus HAL (0.1 mg/kg) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. b Effect of LUR (0.1 mg/kg) plus HAL (0.1 mg/kg) and TAN (0.6 mg/kg) plus HAL (0.1 mg/kg) on the DI. Data are shown as mean ± S.E.M. (n = 7–9 per group). **p < 0.01, significant decrease in DI compared with the vehicle group
Discussion
The major findings of this study are that the 5-HT1A partial agonist, tandospirone, and the preferential post-synaptic 5-HT1A agonist, F15599, significantly reversed the subchronic PCP-induced deficit in NOR. Pretreatment with WAY100635, a 5-HT1A antagonist, blocked the ability of tandospirone and lurasidone, a novel atypical APD with potent 5-HT1A partial agonist properties (Ishibashi et al. 2010), to reverse the deficit in NOR produced by subchronic PCP. Co-administration of sub-effective doses of tandospirone and lurasidone also reversed the impairment in PCP-treated rats, but no such synergy was noted with the combination of sub-effective doses of buspirone, a less efficacious 5-HT1A agonist than tandospirone. The combination of sub-effective doses of tandospirone with pimavanserin, a selective 5-HT2A inverse agonist, did not ameliorate the PCP-induced NOR deficit. On the other hand, co-administration of 0.1 mg/kg haloperidol, a D2 antagonist, blocked the ameliorating effect of both 0.6 mg/kg tandospirone and 0.1 mg/kg lurasidone, consistent with previous evidence that blockade of D2 receptors beyond a limited extent has negative consequences on reversing the effect of atypical APDs on the PCP-induced deficit (Snigdha et al. 2010).
Deficits in declarative memory are among the most common and severe of all cognitive impairments in schizophrenia (Stone and Hsi 2011). The subchronic PCP-induced deficit in NOR has been suggested to provide a useful animal model of declarative memory (see Meltzer et al. 2011 for review). Some clinical studies indicate that atypical APDs which are 5-HT2A/D2 antagonists and direct or indirect 5-HT1A agonists improve declarative memory in schizophrenic patients, not due to a practice effect (Meltzer and Sumiyoshi 2008). It has been reported that atypical APDs, but not the typical APD, haloperidol, reverse the NOR deficit produced by the subchronic PCP treatment employed here (Grayson et al. 2007; Snigdha et al. 2010; Horiguchi et al. 2011a; 2011b). The findings of the present study, along with these previous findings, provide additional support for the conclusion that this model can differentiate between typical and atypical APDs and may be valuable for identifying the mechanisms relevant to the etiology and treatment of cognitive dysfunction in schizophrenia. A potential limitation of this study is that the same animals were used for up to four times, separated by a 7-day washout period, raising the possibility of a carryover effect. The 7-day interval should be sufficient to wash out any residual drug. This design, which has been used in previous studies, requires the use of fewer animals for research purposes and is, thus, both more humane and cost-effective. The procedure has previously been validated by conducting the same experiment as the first in one group of animals and the last in another group of animals and finding no difference in results (Snigdha et al. 2010). This indicates that this subchronic PCP treatment regimen (2 mg/kg, i.p., twice a day for 7 days) induces a stable and persistent impairment in NOR and that single doses of agents only temporarily reverse the adverse effects of subchronic PCP (Grayson et al. 2007; Snigdha et al. 2010).
This is the first report describing the effects of 5-HT1A partial agonists on the subchronic PCP-induced NOR deficit. Both tandospirone and F15599 significantly improved the PCP-induced NOR deficit. Furthermore, the efficacy of tandospirone was antagonized by the 5-HT1A antagonist, WAY100635, suggesting that stimulation of 5-HT1A receptors is the likely basis for the improvement produced by 5-HT1A agonists in the PCP-induced NOR deficit. Our results are in accordance with other studies showing that 5-HT1A agonists improve PCP- or amphetamine-induced cognitive impairment (Stevens et al. 2006; Winstanley et al. 2003). Depoortère et al. (2010) reported that F15599 partially alleviated PCP-induced impairment in working and reference memory and cognitive flexibility in a rat reversal learning task and the post-synaptic preference of F15599 could be important for its procognitive profile. Further studies will be needed to clarify the effect of pre- and post-synaptic 5-HT1A receptors on the cognitive impairment of schizophrenia. The atypical APDs, aripiprazole and perospirone, both 5-HT1A receptor partial agonists, improved the PCP-induced NOR deficit in ICR mice, and the ameliorating effect of these atypical APDs was also blocked by WAY100635 (Hagiwara et al. 2008; Nagai et al. 2009). Moreover, Snigdha and Neill (2008) reported that PCP-induced deficits in social behaviors were reversed by aripiprazole and WAY100635 prevented the reversal of social behavior deficits observed with aripiprazole. F15063, a compound with 5-HT1A agonism, attenuated PCP-induced social interaction impairments owing to its 5-HT1A agonist activity (Depoortère et al. 2007). Our results support the view that 5-HT1A agonism is an effective means to attenuate the action of PCP (Depoortère et al. 2007). The clinical relevance of our findings is supported by previous studies from this laboratory (see “Introduction”). By contrast, tandospirone has been reported to impair explicit memory function in demented patients (Yasuno et al. 2003). The results reported here suggest that the combined administration of tandospirone with lurasidone may be a means to potentiate their ability to improve cognitive impairment in patients with schizophrenia.
Lurasidone, a novel atypical APD which is a 5-HT1A partial agonist (Ishibashi et al. 2010; Meyer et al. 2009), has been previously shown to improve the PCP-induced NOR deficit in female rats (Horiguchi et al. 2011a; 2011b). We have now found that WAY100635 blocked the effect of lurasidone to improve the PCP-induced NOR deficit. Moreover, a sub-effective dose of tandospirone in combination with a sub-effective dose of lurasidone, but not with the 5-HT2A inverse agonist, pimavanserin, improved the PCP-induced NOR deficit. The dose of pimavanserin used in these studies, a dose that achieves nearly complete 5-HT2A receptor occupancy (Vanover et al. 2006), was based on previous microdialysis experiments showing the potentiation of DA and ACh efflux in the rat brain when administered in combination with subthreshold doses of APDs (Kuroki et al. 1999; Ichikawa et al. 2002; Li et al. 2005). These results demonstrate that 5-HT1A agonism contributes to the ability of lurasidone to ameliorate the subchronic PCP-induced deficit on NOR. Clinically, the combination of a sub-effective dose of lurasidone, or perhaps other atypical APDs, with a 5-HT1A partial agonist may permit lower doses of the atypical APD, thereby decreasing the side effect burden associated with a full dose.
It has been reported that 5-HT1A agonism produces functional effects similar to those produced by 5-HT2A antagonism (Meltzer and Huang 2008 for review). The results of this study indicate that the activation of 5-HT1A receptors is sufficient to the subchronic PCP-induced deficits in NOR. In contrast, we have recently reported that 5-HT2A inverse agonists do not improve the PCP-induced NOR deficits by itself but can restore the ability of a sub-effective dose of atypical APDs to attenuate these deficits (Snigdha et al. 2010). These results are in accordance with the microdialysis study. Tandospirone and F15599 enhanced cortical DA efflux which may contribute to improve cognition (Kuroki et al. 1999; Yoshino et al. 2002; Lladó-pelfort et al. 2010). On the other hand, 5-HT2A inverse agonists alone have no effect on basal DA efflux but can regulate DA efflux when combined with the blockade of D2 (Bonaccorso et al. 2002; Li et al. 2005). As previously mentioned, subchronic treatment with PCP increased 5-HT1A receptor binding in the medial–prefrontal and dorsolateral–frontal cortex and decreased D1 receptor density in the medial and lateral caudate-putamen, with no changes in any other brain regions nor any changes in 5-HT2A or D2 receptors in any brain region (Choi et al. 2009). The 5-HT1A effect, which parallels the post-mortem data cited in the “Introduction”, suggest the possibility that subchronic PCP treatment impairs cortical 5-HT release, leading to an upregulation of post-synaptic 5-HT1A receptors, which may contribute to the efficacy of 5-HT1A agonists in this model. It is noteworthy that the efficacy of clozapine to prevent PCP-induced desynchronization of PFC is dependent upon 5-HT1A activation, but not 5-HT2A blockade (Kargieman et al. 2011), a further evidence for the important role of 5-HT1A agonism to ameliorate hypoglutamatergic deficits. The results reported here also indicate that the combination of 5-HT1A partial agonism and 5-HT2A antagonism is insufficient to reverse the PCP-induced NOR deficit. This indicates that some other component of atypical APDs other than 5-HT1A partial agonism is needed to reverse the effect of subchronic PCP. We previously reported that haloperidol, 0.1 mg/kg, blocked the ability of risperidone to reverse the PCP-induced deficit in NOR (Snigdha et al. 2010). Similarly, the same dose of haloperidol also blocked the effect of lurasidone and tandospirone to improve NOR, indicating the importance of avoiding excessive blockade of D2 receptors with 5-HT1A agonists or atypical APDs that rely on serotonergic mechanisms for their efficacy.
While tandospirone clearly improved the PCP-induced NOR deficit, buspirone, 0.3 mg/kg, partially improved this deficit but a 1-mg/kg dose did not. Moreover, 0.1 mg/kg buspirone in combination with a sub-effective dose of lurasidone did not ameliorate the NOR deficit. These results parallel those from clinical studies in patients with schizophrenia, which reported that tandospirone (Sumiyoshi et al. 2001a; 2001b), but not buspirone, enhanced some cognitive domains, e.g., verbal learning and memory and executive function (Sumiyoshi et al. 2007; Piskulić et al. 2009). The differences between tandospirone and buspirone to affect the PCP-induced deficit in NOR may be related to differences in intrinsic activity as 5-HT1A receptor agonists. Tandospirone and F15599 are nearly full agonists at 5-HT1A receptors (Tanaka et al. 1995; Newman-Tancredi et al. 1998; Maurel et al. 2007; Newman-Tancredi et al. 2009), whereas buspirone is a weaker 5-HT1A partial agonist (Newman-Tancredi et al. 2009).
High doses of tandospirone (0.6 mg/kg) and buspirone (1 mg/kg) increased the total exploration time in both trials. A similar effect has been reported with pimavanserin and LY379268, a mGluR2/3 agonist (Snigdha et al. 2010; Horiguchi et al. 2011a). Additional studies are necessary to clarify the effect of 5-HT1A agonists, 5-HT2A inverse agonists, and mGluR2/3 agonists on exploration time in subchronic PCP-treated rodents.
The present study also evaluated the effect of the 5-HT1A antagonist WAY100635 on NOR, by itself, in vehicle- and PCP-treated rats. WAY100635 alone did not affect the exploration times of vehicle-treated rats and did not improve the NOR deficit induced by PCP. Previous studies of the effects of WAY100635 in other animal models of cognitive function have produced conflicting results. Consistent with the findings of the present study, WAY100635 had no effect on PCP-induced NOR deficit in ICR mice and female rats (Hagiwara et al. 2008; McLean et al. 2009; Nagai et al. 2009). On the other hand, some previous animal studies have indicated that 5-HT1A antagonists have beneficial effects on cognitive impairment induced by MK-801 or scopolamine (Pitsikas et al. 2003; Boast et al. 1999; Wedzony et al. 2000; Hirst et al. 2008). These conflicting results could be due to differences in mechanisms of MK-801 and scopolamine versus PCP and/or the acute administration of scopolamine and MK-801 in contrast to the subchronic PCP regimen of this study.
In conclusion, these results indicate that 5-HT1A agonism is required for the amelioration of the PCP-induced impairment of NOR by some, but not all, atypical APDs which are 5-HT2A/D2 antagonists. Further, some 5-HT1A agonists may be useful for treating cognitive deficits in schizophrenia.
References
Bantick RA, Deakin JF, Grasby PM (2001) The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics? J Psychopharmacol 15(1):37–46
Boast C, Bartolomeo AC, Morris H, Moyer JA (1999) 5HT antagonists attenuate MK801-impaired radial arm maze performance in rats. Neurobiol Learn Mem 71(3):259–271
Bonaccorso S, Meltzer HY, Li Z, Dai J, Alboszta AR, Ichikawa J (2002) SR46349-B, a 5-HT(2A/2 C) receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology 27(3):430–441
Burnet PW, Eastwood SL, Harrison PJ (1996) 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology 15(5):442–455
Burnet PW, Eastwood SL, Harrison PJ (1997) [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int 30(6):565–574
Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI (2009) Subchronic effects of phencyclidine on dopamine and serotonin receptors: implications for schizophrenia. J Mol Neurosci 38(3):227–235
Coyle JT (2006) Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 26(4–6):365–384
Depoortère R, Auclair AL, Bardin L, Bruins Slot L, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A (2007) F15063, a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. III. Activity in models of cognition and negative symptoms. Br J Pharmacol 151(2):266–277
Depoortère R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A (2010) F15599, a preferential post-synaptic 5-HT(1A) receptor agonist: activity in models of cognition in comparison with reference 5-HT(1A) receptor agonists. Eur Neuropsychopharmacol 20:641–654
Grayson B, Idris NF, Neill JC (2007) Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 184(1):31–38, 22
Gurevich EV, Joyce JN (1997) Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol Psychiatry 42(7):529–545
Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY (1993) Improvement in cognitive functions and psychiatric symptoms in treatment-refractory schizophrenic patients receiving clozapine. Biol Psychiatry 34:02–712
Hagiwara H, Fujita Y, Ishima T, Kunitachi S, Shirayama Y, Iyo M, Hashimoto K (2008) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antipsychotic drug perospirone: role of serotonin 5-HT1A receptors. Eur Neuropsychopharmacol 18(6):448–454
Hashimoto T, Nishino N, Nakai H, Tanaka C (1991) Increase in serotonin 5-HT1A receptors in prefrontal and temporal cortices of brains from patients with chronic schizophrenia. Life Sci 48(4):355–363
Hirst WD, Andree TH, Aschmies S, Childers WE, Comery TA, Dawson LA, Day M, Feingold IB, Grauer SM, Harrison BL, Hughes ZA, Kao J, Kelly MG, van der Lee H, Rosenzweig-Lipson S, Saab AL, Smith DL, Sullivan K, Rizzo SJ, Tio C, Zhang MY, Schechter LE (2008) Correlating efficacy in rodent cognition models with in vivo 5-hydroxytryptamine1a receptor occupancy by a novel antagonist, (R)-N-(2-methyl-(4-indolyl-1-piperazinyl)ethyl)-N-(2-pyridinyl)-cyclohexane carboxamide (WAY-101405). J Pharmacol Exp Ther 325(1):134–145
Horiguchi M, Huang M, Meltzer HY (2011a) Interaction of mGlu(2/3) agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology (Berl) 217:13–24
Horiguchi M, Huang M, Meltzer HY (2011b) The role of 5-HT7 receptors in the phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. J Pharmacol Exp Ther 338:605–614
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY (2001) 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76(5):1521–1531
Ichikawa J, Li Z, Dai J, Meltzer HY (2002) Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res 956(2):349–357
Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-HT7 and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148(10):1301–1308
Kargieman L, Riga MS, Artigas F, Celada P (2011) Clozapine reverses phencyclidine-induced desynchronization of prefrontal cortex through a 5-HT(1A) receptor-dependent mechanism. Neuropsychopharmacology. doi:10.1038/npp.2011.249
Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Küfferle B, Barnas C, Stastny J, Praschak-Rieder N, Pezawas L, de Zwaan M, Quiner S, Pirker W, Asenbaum S, Podreka I, Brücke T (2002) Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette's disorder—implications for psychopharmacology. World J Biol Psychiatry 3(3):133–146
Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA, CATIE Investigators, Neurocognitive Working Group (2007) Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry 64(6):633–647
Kenny JT, Meltzer HY (1991) Attention and higher cortical functions in schizophrenia. J Neuropsychiatry Clin Neurosci 3(3):269–275
Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S (1999) NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7(3):125–143
Kuroki T, Meltzer HY, Ichikawa J (1999) Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 288:774–781
Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995) Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13:9–19
Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25(4):455–467
Li Z, Ichikawa J, Huang M, Prus AJ, Dai J, Meltzer HY (2005) ACP-103, a 5-HT2A/2 C inverse agonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Psychopharmacology (Berl) 183(2):144–153
Lladó-Pelfort L, Assié MB, Newman-Tancredi A, Artigas F, Celada P (2010) Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br J Pharmacol 160(8):1929–1940
Maurel JL, Autin JM, Funes P, Newman-Tancredi A, Colpaert F, Vacher B (2007) High-efficacy 5-HT1A agonists for antidepressant treatment: a renewed opportunity. J Med Chem 50(20):5024–5033
McLean SL, Woolley ML, Thomas D, Neill JC (2009) Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology (Berl) 206(3):403–414
Meltzer HY (1999) The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21(2 Suppl):106S–115S
Meltzer HY, Huang M (2008) In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 172:177–197
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255
Meltzer HY, Sumiyoshi T (2008) Does stimulation of 5-HT(1A) receptors improve cognition in schizophrenia? Behav Brain Res 195(1):98–102
Meltzer HY, Horiguchi M, Massey BW (2011) The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology 213(2–3):289–305
Meyer JM, Loebel AD, Schweizer E (2009) Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs 18(11):1715–1726
Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T (2009) Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology (Berl) 202(1–3):315–328
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128(3):419–432
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW (1999) Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20(2):106–118
Newman-Tancredi A (2010) The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Investig Drugs 11(7):802–812
Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verrièle L, Audinot V, Millan MJ (1998) Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur J Pharmacol 355(2–3):245–256
Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, Heusler P, Bruins Slot L, Colpaert FC, Vacher B, Cussac D (2009) Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol 156(2):338–353
Piskulić D, Olver JS, Maruff P, Norman TR (2009) Treatment of cognitive dysfunction in chronic schizophrenia by augmentation of atypical antipsychotics with buspirone, a partial 5-HT(1A) receptor agonist. Hum Psychopharmacol 24(6):437–446
Pitsikas N, Rigamonti AE, Cella SG, Muller EE (2003) The 5-HT 1A receptor antagonist WAY 100635 improves rats performance in different models of amnesia evaluated by the object recognition task. Brain Res 983(1–2):215–222
Rollema H, Lu Y, Schmidt AW, Zorn SH (1997) Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol 338(2):R3–R5
Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P (1991) Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry 48(7):618–624
Simpson MD, Lubman DI, Slater P, Deakin JF (1996) Autoradiography with [3H]8-OH-DPAT reveals increases in 5-HT(1A) receptors in ventral prefrontal cortex in schizophrenia. Biol Psychiatry 39(11):919–928
Snigdha S, Neill JC (2008) Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav Brain Res 191(1):26–31
Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY (2010) Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 332(2):622–631
Stevens KE, O'Neill HC, Rose GM, Luthman J (2006) The 5-HT(1A) receptor active compounds (R)-8-OH-DPAT and (S)-UH-301 modulate auditory evoked EEG responses in rats. Amino Acids 31(4):365–375
Stone WA, Hsi X (2011) Declarative memory deficits and schizophrenia: problems and prospects, Neurobiol Learning Memory online 20 April 2011
Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY (1996) Serotonin1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res 708(1–2):209–214
Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Uehara T, Kurachi M, Meltzer HY (2000) Effect of adjunctive treatment with serotonin-1A agonist tandospirone on memory functions in schizophrenia. J Clin Psychopharmacol 20(3):386–388
Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, Jayathilake K, Meltzer HY (2001a) Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry 158(10):1722–1725
Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, Sumiyoshi S, Sumiyoshi C, Meltzer HY (2001b) The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol Psychiatry 49(10):861–868
Sumiyoshi T, Park S, Jayathilake K, Roy A, Ertugrul A, Meltzer HY (2007) Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 95(1–3):158–168
Tanaka H, Tatsuno T, Shimizu H, Hirose A, Kumasaka Y, Nakamura M (1995) Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain. Gen Pharmacol 26(8):1765–1772
Tauscher J, Kapur S, Verhoeff NP, Hussey DF, Daskalakis ZJ, Tauscher-Wisniewski S, Wilson AA, Houle S, Kasper S, Zipursky RB (2002) Brain serotonin 5-HT(1A) receptor binding in schizophrenia measured by positron emission tomography and [11 C]WAY-100635. Arch Gen Psychiatry 59(6):514–520
Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf BR, Brann MR, Davis RE (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317(2):910–918
Wedzony K, Maćkowiak M, Zajaczkowski W, Fijał K, Chocyk A, Czyrak A (2000) WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology 23(5):547–559
Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167(3):304–314
Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8(3):457–472
Yasuno F, Suhara T, Nakayama T, Ichimiya T, Okubo Y, Takano A, Ando T, Inoue M, Maeda J, Suzuki K (2003) Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am J Psychiatry 160(2):334–340
Yoshino T, Nisijima K, Katoh S, Yui K, Nakamura M (2002) Tandospirone potentiates the fluoxetine-induced increases in extracellular dopamine via 5-HT(1A) receptors in the rat medial frontal cortex. Neurochem Int 40(4):355–360
Acknowledgements
This research was supported, in part, by a grant from Dainippon Sumitomo Pharma Co. Ltd. We thank the National Institute of Drug Abuse for providing PCP for these studies.
Conflicts of interest
Herbert Y. Meltzer: consultant to Cypress, Dainippon Sumitomo Pharma, Janssen, Merck, and Pfizer and shareholder of SureGene, Bio Vail, and ACADIA. Masakuni Horiguchi: employed as a research scientist by Dainippon Sumitomo Pharma.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horiguchi, M., Meltzer, H.Y. The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology 221, 205–215 (2012). https://doi.org/10.1007/s00213-011-2561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2561-4