Abstract
Rationale
Subchronic administration to rodents of the N-methyl-d-aspartate non-competitive antagonist, phencyclidine (PCP), impairs novel object recognition (NOR). Atypical antipsychotic drugs (APDs) reverse the effects of subchronic PCP on NOR. The effect of metabotropic glutamate2/3 receptor (mGlu2/3) agonists upon NOR is unknown.
Objectives and methods
We tested the hypotheses that the mGlu2/3 agonist, LY379268, by itself, or in combination with APDs or pimavanserin, a 5-HT2A inverse agonist, would reverse the deficit in NOR induced by subchronic treatment with PCP (2 mg/kg, b.i.d., for 7 days).
Results
The mGlu2/3 agonist LY379268 (1 or 3 mg/kg) did not attenuate the PCP-induced NOR deficit. However, together with sub-effective dose of the atypical APDs, clozapine (0.1 mg/kg) or lurasidone (0.03 mg/kg), but not the typical APD, haloperidol (0.1 mg/kg), or pimavanserin (3 mg/kg), LY379268, 1 mg/kg, significantly reversed the PCP-induced NOR deficit. Moreover, the effect of clozapine was blocked by the mGlu2/3 antagonist, LY341495 (1 mg/kg).
Conclusions
These results indicate that mGlu2/3 agonism can potentiate the ability of atypical, but not typical APDs, to ameliorate the effect of subchronic PCP on NOR, that mGlu2/3 agonism may contribute to the ability of atypical APDs to acutely reverse the effect of subchronic PCP on NOR, but that by itself, mGlu2/3 agonism, is ineffective in this model of cognitive impairment in schizophrenia. These results suggest that mGlu2/3 receptor agonism should be investigated as an adjunctive treatment of cognitive impairment in schizophrenia rather than as monotherapy, which may be effective for control of psychosis, but not for cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deficits in multiple domains of cognition, including visual learning and declarative memory, are present in most patients with schizophrenia (Palmer et al. 1997). There is controversial evidence that atypical antipsychotic drugs (APDs) which are more potent 5-HT2A than D2 antagonists, e.g., clozapine and risperidone (Meltzer et al. 1989; Schotte et al. 1996; Meltzer and Huang 2008), are more effective than typical APDs to attenuate some cognitive deficits (Hagger et al. 1993; Meltzer and McGurk 1999; Woodward et al. 2005; Keefe et al. 2007). Unless otherwise noted, atypical APDs mentioned in this article refer to the aforementioned class of atypical APDs, excluding drugs such as amisulpride which belong to other classes of atypical APDs. Although average mean effect sizes for the improvement in cognition by atypical APDs such as clozapine and risperidone are at best moderate for domains such as semantic and declarative memory (Woodward et al. 2005), there are substantial numbers of individual patients who have large, clinically significant improvements, beyond expected practice effects particularly in both of these domains, following a switch to atypical APDs (Hagger et al. 1993; Bilder et al. 2002). The discovery of novel adjunctive pharmacologic treatments which can improve at least some domains of cognition in schizophrenia, accompanied by functional improvement, is a major unmet need (Meltzer and McGurk 1999; Gray and Roth 2007).
Cortical hypodopaminergic, as well as hypoglutamatergic activity, have been postulated to be major causes of the cognitive impairment of schizophrenia (Goldman-Rakic and Selemon 1997; Coyle 2006). The main evidence for the deficit in glutamatergic function in schizophrenia is that non-competitive N-methyl-d-aspartate (NMDA) receptor antagonists, e.g., phencyclidine (PCP) and ketamine, induce schizophrenia-like cognitive impairments in healthy subjects (Javitt and Zukin 1991; Krystal et al. 1999), but the hypothesis is also supported by post-mortem and genetic studies (Gunduz-Bruce 2009). There is, however, conflicting evidence about the effects of acute and chronic ketamine on declarative memory in patients with schizophrenia and normal volunteers (LaPorte et al. 1996; Morgan et al. 2009; Stefanovic et al. 2009). Nevertheless, the effects of NMDA non-competitive antagonists to impair cognitive function in rodents and monkeys have been intensively studied as an animal model of the cognitive deficit in schizophrenia (Gunduz-Bruce 2009). Acute or subchronic administration of PCP and MK-801, another non-competitive NMDA antagonist, have been reported to produce impairments in visual learning and memory, attention, reasoning and problem solving, working memory, and social cognition in rodents (see Neill et al. (2010) for review).
Atypical APDs which are 5-HT2A/D2 antagonists, but not haloperidol, a typical APD, have been reported to reverse cognitive deficits induced by sub-chronic PCP treatment in novel object recognition (NOR) (Grayson et al. 2007; Hashimoto et al. 2005; Nagai et al. 2009; Hagiwara et al. 2008; McKibben et al. 2010; Snigdha et al. 2010), a possible analog of declarative memory in humans. Lurasidone is a novel atypical APD recently approved for schizophrenia (see Meyer et al. (2009) for review). Lurasidone has high binding affinities for the human 5-HT2A, 5-HT7, D2, and 5-HT1A receptors (K i = 0.47, 0.50, 0.99, 6.4 nM, respectively; Ishiyama et al. 2009). Lurasidone has been reported to have cognitive benefits in animal models: e.g., reversal of scopolamine- and MK-801-induced impairment in the passive avoidance, Morris water maze, and radial-arm maze tests in rats (Ishiyama et al. 2007; Enomoto et al. 2008).
We have recently reported that the ability of sub-effective doses of risperidone to reverse the deficit in NOR caused by subchronic PCP treatment is potentiated by the 5-HT2A inverse agonists, pimavanserin and M100907, although neither of the latter drugs attenuated this deficit by themselves (Snigdha et al. 2010). Microdialysis studies in rats have shown that atypical APDs preferentially enhance cortical and hippocampal dopamine (DA) efflux, which may contribute to their ability to improve cognition, at least in some schizophrenia patients, and that the effect on cortical DA is related to greater affinity for 5-HT2A than D2 receptors as well as 5-HT1A receptor agonism (Moghaddam and Bunney 1990; Kuroki et al. 1999; Ichikawa et al. 2002; Chung et al. 2004). The ability of sub-effective doses of typical and atypical APDs to increase DA efflux in the mPFC is enhanced by 5-HT2A inverse agonists (Liegeois et al. 2002; Meltzer and Huang 2008). This enhancement of DA efflux in the mPFC by the combination of atypical APDs and 5-HT2A inverse agonists may be related to the improved performance in the NOR test following the combined administration of these agents.
Metabotropic glutamate2/3 receptor (mGlu2/3) agonists are putative antipsychotic agents (Swanson and Schoepp 2002; Patil et al. 2007). mGlu2/3 receptors are presynaptic and enriched in brain areas associated with cognition, e.g., the hippocampus (HIP), neocortex, amygdala, and striatum (Cartmell and Schoepp 2000). mGlu2/3 agonists, such as LY354740, LY379268, and LY404039, have been reported to be more potent inhibitors of PCP-induced than amphetamine-induced locomotor activity (Moghaddam and Adams 1998; Cartmell et al. 1999; Swanson and Schoepp 2002; Woolley et al. 2008). A recent phase IIA clinical trial showed that LY2140023, a prodrug that is converted into the mGlu2/3 agonist LY404039, improved positive and negative symptoms in patients with schizophrenia (Patil et al. 2007). A second phase II study with LY2140023 failed to show clinical improvement in schizophrenic patients, but it was a failed, and, therefore, inconclusive study since the positive control, olanzapine, also did not differentiate from placebo (Kinon 2009).
LY354740 has been reported to reverse PCP-induced working memory deficits (Moghaddam and Adams 1998) and deficits of social novelty discrimination secondary to neonatal PCP administration in rats (Harich et al. 2007). LY354740 was also reported to attenuate ketamine-induced working memory deficits in humans (Krystal et al. 2005). However, Schlumberger et al. (2009) reported that LY354740 did not modify PCP-induced working memory deficits in a spontaneous alternation task or in the passive avoidance test. Another mGlu2/3 agonist, LY487379, had no effect on cognitive impairment in active allothetic place avoidance induced by MK-801 (Vales et al. 2010). LY354740 has been reported to induce memory impairments in rats (Aultman and Moghaddam 2001) whereas LY379268 exacerbated PCP-induced disruption of attentional performance in the five-choice serial reaction time task (Amitai and Markou 2010). Possible synergistic effects between mGlu2/3 agonists and typical or atypical APDs have not yet been investigated. Uslaner et al. (2009) reported that the effects of LY379268 and M100907, a 5-HT2A inverse agonist, on amphetamine-induced and MK-801-induced psychotomor activity, were significantly greater when administered together than when administered separately.
This study was designed to test whether the mGlu2/3 agonist, LY379268, by itself, or in combination with a sub-effective dose of atypical APDs which have high affinity for 5-HT2A receptors (clozapine and lurasidone), a typical APD (haloperidol), or the 5-HT2A inverse agonist, pimavanserin, are able to reverse the PCP-induced NOR deficit in rats.
Material and methods
Animals
Thirty-four female Long–Evans (LE) rats (8 or 9 weeks old; Harlan Sprague Dawley, Inc, Indianapolis, IN, USA) were used as subjects for NOR experiments 1–3; 43 rats were used as subjects for experiments 4–6. LE rats were housed in groups of three or four on a 12-h light/dark cycle (lights on at 7:00 a.m.). All experiments were conducted during the light phase. Food and water were available ad libitum. All experiments were conducted in accordance with the Vanderbilt animal committee regulations.
Drugs
Lurasidone was provided from Dainippon Sumitomo Pharma (Osaka, Japan). Pimavanserin was provided by Acadia Pharmaceuticals (Torrence, CA). Clozapine was obtained from Novartis (Basel, Switzerland). Haloperidol and PCP were obtained from Sigma–Aldrich (St. Louis, MO, USA). LY379268 and LY341495 were purchased from Tocris Bioscience (Ellisville, MO, USA). PCP, haloperidol, and pimavanserin were dissolved in distilled water. Lurasidone was dissolved 0.5% methylcellulose, 0.2% Tween80. Clozapine was dissolved in a small amount of 0.1 M phosphoric acid, and the pH was adjusted to 6 to 7 with 0.1 N NaOH. LY379268 was dissolved in saline. LY341495 was dissolved in a small amount of 0.1 M sodium hydroxide and then diluted with saline. All drugs or vehicle administered intraperitoneally (i.p.) in a volume of 1 ml/kg body weight.
Drug treatment
LE rats were randomly assigned to two treatment groups: nine were treated with vehicle (saline, i.p.), and the remainder were treated with PCP (2 mg/kg, i.p.) twice daily for 7 days. Subsequently, animals were given a 7-day washout period prior to NOR testing, and each rat was tested three times in the NOR paradigm, as in previous studies (Grayson et al. 2007; Snigdha et al. 2010). To reduce carryover effects, a 7-day washout period was given between each of the test sessions. The criterion for continuing to test rats was exploration times in the acquisition and retention phases to either of two objects ≥5 s. If a rat did not explore at least that amount in either of these two phases, its data were excluded from analysis. This rarely occurred and did not affect the ability to complete the analysis using the data from the remaining animals of that group. All experiments consisted of six to nine rats.
Novel object recognition test
Testing was carried out according to a previously validated method (Grayson et al. 2007; Snigdha et al. 2010). The object recognition test was performed in an open field comprising a square box made of Plexiglas (52 × 52 × 31 cm). The floor of the box was white with black gridlines forming nine identical squares on it; all other walls were black. All rats were habituated to the test environment and NOR arena for three consecutive days prior to the first NOR test. Habituation consisted of placing the subjects in the empty NOR arena for 1 h. Rats were given a further 3-min habituation on the day of testing. After the 3-min habituation period, the rats were given two 3-min trials (an acquisition trial and a retention trial), separated by a 1-min inter-trial return to their home cage during which the objects were removed, and the arena was cleaned. During the acquisition trial, the animals were allowed to explore two identical objects (A1 and A2) for 3 min. During the retention trial, the animals explored a familiar object (A) from the acquisition trial and a novel object (B) for 3 min. The familiar object presented during the retention trial was a duplicate of the object presented in acquisition trial to avoid any olfactory trails. Behavior in all trials was recorded on video for subsequent blind scoring for the object exploration. Object exploration is defined by animal’s licking, sniffing, or touching the object with the forepaws while sniffing, but not leaning against, turning around, standing, or sitting on the object. The exploration time (seconds) of each object in each trial was recorded manually by the use of two stopwatches. The discrimination index (DI) [(time spent exploring the novel object − time spent exploring the familiar object) / total exploration time] was then calculated for retention trial.
Data analysis
All data are expressed as mean ± SEM (n = 6–9 per group). Exploration data were analyzed by a repeated-measures two-way ANOVA followed by the pairwise comparison when a significant effect was detected by the ANOVA. DI data were analyzed by one-way ANOVA followed by the Bonferroni test when a significant effect was detected by the ANOVA.
Results
Effect of lurasidone in subchronic PCP-treated rats (experiments 1 and 2)
In the acquisition phase, there was no significant difference in time spent exploring the two identical objects in any group (Figs. 1a and 2a). In the retention phase, vehicle-treated animals explored the novel object significantly longer than the familiar objects (p < 0.05 and p < 0.01 each, Figs. 1b and 2b). The ability to discriminate novel and familiar objects was abolished by subchronic PCP-treatment (Figs. 1b and 2b). Lurasidone (0.01 and 0.03 mg/kg) failed to reverse the PCP-induced NOR deficit (Fig. 1b). However, higher doses of lurasidone (0.1 and 0.5 mg/kg) significantly attenuated the deficit (p < 0.01, each; Fig. 2b). The DI was significantly reduced following subchronic PCP treatment (p < 0.01); 0.1 and 0.5 mg/kg lurasidone significantly (p < 0.05 and p < 0.01, respectively) reversed the PCP-induced reduction in DI (Figs. 1c and 2c).
Effect of acute administation of lurasidone (0.01, 0.03 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of lurasidone (0.01, 0.03 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 7 per group). b Effect of lurasidone (0.01, 0.03 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 7 per group). *p < 0.05, significant difference in time spent exploring the novel compared with the familiar object. c Effect of lurasidone (0.01, 0.03 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 7 per group). **p < 0.01, significant decrease in DI compared with the vehicle group
Effect of acute administation of lurasidone (0.1, 0.5 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of lurasidone (0.1, 0.5 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 7 to 8 per group). b Effect of lurasidone (0.1, 0.5 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 7 to 8 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. c Effect of lurasidone (0.1, 0.5 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 7 to 8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. # p < 0.05, ## p < 0.01, significant reversal in DI compared with PCP group
Effect of LY379268 in subchronic PCP-treated rats (experiment 3)
In the acquisition trial, statistical analysis showed no significant difference in time spent exploring the identical two objects in any group (Fig. 3a). In the retention trial, vehicle-treated animals explored the novel object significantly more than the familiar object (p < 0.05). In subchronic PCP-treated rats, there was no significant difference between the time spent exploring the novel and the familiar object and LY379268 at doses of 1 and 3 mg/kg did not attenuate the PCP-induced deficit (Fig. 3b). Statistical analysis showed subchronic PCP treatment significantly reduced the DI (p < 0.05). LY379268 (1 and 3 mg/kg) failed to improve the reduction of DI induced by subchronic PCP treatment (Fig. 3c).
Effect of acute administation of LY379268 (1, 3 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LY379268 (1, 3 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 7 to 8 per group). b Effect of LY379268 (1, 3 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 7 to 8 per group). *p < 0.05, significant difference in time spent exploring the novel compared with the familiar object. c Effect of LY379268 (1, 3 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 7 to 8 per group). *p < 0.05, significant decrease in DI compared with the vehicle group
Effect of clozapine and LY341495 in subchronic PCP-treated rats (experiment 4)
In the acquisition trial, statistical analysis showed no significant difference in time spent exploring the identical two objects in any group (Fig. 4a). In the retention trial, vehicle-treated rats showed exploratory preference for the novel object and subchronic PCP treatment abolished the preference (p < 0.01). 0.3 mg/kg clozapine, but not 0.1 mg/kg, significantly reversed the PCP-induced NOR deficit (p < 0.01). Co-administration of the mGlu2/3 antagonist, LY341495, with clozapine (0.3 mg/kg) blocked the effect of clozapine to restore the NOR deficit in PCP-treated rats (Fig. 4b). The DI was significantly reduced following subchronic PCP treatment (p < 0.01); treatment with 0.3 mg/kg clozapine, but not 0.1 mg/kg, significantly improved the NOR deficit induced by PCP (p < 0.05). Clozapine (0.3 mg/kg) did not reverse the PCP-induced NOR deficit when LY341495 (1 mg/kg) was co-administered (Fig. 4c).
Effect of acute administation of clozapine (0.1, 0.3 mg/kg) and clozapine (0.3 mg/kg) plus LY341495 (1 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of clozapine (0.1, 0.3 mg/kg, i.p.) and clozapine (0.3 mg/kg, i.p.) plus LY341495 (1 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 6–8 per group). b Effect of clozapine (0.1, 0.3 mg/kg, i.p.) and clozapine (0.3 mg/kg, i.p.) plus LY341495 (1 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 6–8 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. c Effect of clozapine (0.1, 0.3 mg/kg, i.p.) and clozapine (0.3 mg/kg, i.p.) plus LY341495 (1 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 6–8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. #p < 0.05, significant reversal in DI compared with PCP group
Effect of LY379268 plus atypical APDs, haloperidol, or pimavanserin in subchronic PCP-treated rats (experiment 5 and 6)
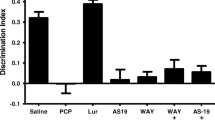
In the acquisition trial, statistical analysis showed no significant difference in time spent exploring the identical two objects in any group (Figs. 5a and 6a). In the retention trial, vehicle-treated animals spent significantly longer exploring the novel object compared with familiar objects (p < 0.05, p < 0.01, each; Figs. 5b and 6b). In subchronic PCP-treated rats, there was no significant difference between the time spent exploring the novel and the familiar object (Fig. 5b). Haloperidol (0.1 mg/kg) plus LY379268 (1 mg/kg) or pimavanserin (3 mg/kg) plus LY379268 (1 mg/kg) did not affect the exploration times of the PCP-treated rats in the retention trial (Figs. 5b and 6b). Statistical analysis showed that the combination of LY379268 (1 mg/kg) and sub-effective dose of lurasidone (0.03 mg/kg) or clozapine (0.1 mg/kg) successfully reversed the PCP-induced deficit in NOR (p < 0.05, p < 0.01, respectively; Figs. 5b and 6b). Subchronic PCP treatment significantly reduced the DI compared with control rats (p < 0.01, p < 0.05, each; Figs. 5c and 6c). Haloperidol (0.1 mg/kg) or pimavanserin (3 mg/kg) in combination with LY379268 (1 mg/kg) did not affect the PCP-induced reduction in DI (Figs. 5c and 6c). A sub-effective dose of lurasidone (0.03 mg/kg) in combination with LY379268 (1 mg/kg) significantly improved the PCP-induced reduction in DI (Fig. 5c). There was a trend for sub-effective dose of clozapine (0.1 mg/kg) plus LY379268 (1 mg/kg) to restore the DI in PCP-treated rats (p = 0.08; Fig. 6c).
Effect of acute administation of LY379268 (1 mg/kg) plus haloperidol (0.1 mg/kg) and LY379268 (1 mg/kg) plus lurasidone (0.03 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LY379268 (1 mg/kg, i.p.) plus haloperidol (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus lurasidone (0.03 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 6–8 per group). b Effect of LY379268 (1 mg/kg, i.p.) plus haloperidol (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus lurasidone (0.03 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 6–8 per group). *p < 0.05, significant difference in time spent exploring the novel compared with the familiar object. c Effect of LY379268 (1 mg/kg, i.p.) plus haloperidol (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus lurasidone (0.03 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 6–8 per group). **p < 0.01, significant decrease in DI compared with the vehicle group. ## p < 0.01, significant reversal in DI compared with PCP group
Effect of acute administation of LY379268 (1 mg/kg) plus clozapine (0.1 mg/kg) and LY379268 (1 mg/kg) plus pimavanserin (3 mg/kg) on PCP-induced cognitive impairment in NOR test. a Effect of LY379268 (1 mg/kg, i.p.) plus clozapine (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus pimavanserin (3 mg/kg, i.p.) on exploration of two identical objects in the acquisition trial in NOR test. Data are shown as mean ± SEM (n = 8 to 9 per group). b Effect of LY379268 (1 mg/kg, i.p.) plus clozapine (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus pimavanserin (3 mg/kg, i.p.) on exploration of a novel and a familiar object in the retention trial in NOR test. Data are shown as mean ± SEM (n = 8 to 9 per group). **p < 0.01, significant difference in time spent exploring the novel compared with the familiar object. c Effect of LY379268 (1 mg/kg, i.p.) plus clozapine (0.1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) plus pimavanserin (3 mg/kg, i.p.) on the DI. Data are shown as mean ± SEM (n = 8 to 9 per group). *p < 0.05, significant decrease in DI compared with the vehicle group
Discussion
The mGlu2/3 agonist, LY379268, alone, did not attenuate the PCP-induced NOR deficit at a dose which has previously been reported to block the locomotor effects of PCP and, to a lesser extent, amphetamine, in rodents (Moghaddam and Adams 1998; Cartmell et al. 1999; Swanson and Schoepp 2002; Woolley et al. 2008). Lurasidone, a novel atypical APD, like clozapine, produced a dose-dependent reversal of PCP-induced deficit in NOR. Co-administration of LY379268, with a sub-effective dose of clozapine or lurasidone, but not haloperidol or pimavanserin, also significantly reversed the PCP-induced NOR deficit. Moreover, the ameliorating effect of clozapine on NOR in PCP-treated rats was blocked by pretreatment with the mGlu2/3 antagonist, LY341495. Whereas the combined effect of submaximal doses of LY379268 and the 5-HT2A inverse agonist, M100907 block acutely administered PCP- and amphetamine-induced locomotor activity in rats (Uslaner et al. 2009), the ability of LY379268 to reverse the effects of subchronic PCP on NOR, was not potentiated by the 5-HT2A inverse agonist, pimavanserin.
Our results confirm that subchronic PCP treatment (2 mg/kg, i.p., twice a day for 7 days) induces a robust, persistent impairment in NOR and that atypical APDs, but not typical APDs, reverse this deficit (Grayson et al. 2007; Snigdha et al. 2010). The procedure of using the same rats in up to three studies, separated by a 7-day washout period, was validated by conducting the same experiment as the first in one group of animals and the last in another group of animals, and obtaining the same results (Snigdha et al. 2010).
LY379268 alone (1 and 3 mg/kg) did not improve the NOR deficit, despite evidence that these doses of LY379268 attenuate PCP-induced hyperlocomotion (Cartmell et al. 1999; Swanson and Schoepp 2002). Our results are in accord with some previous studies showing that mGlu2/3 agonists did not improve cognitive impairment induced by non-competitive NMDA receptor antagonists in a spontaneous alternation task, a passive avoidance test (Schlumberger et al. 2009), and in active allothetic place avoidance (Vales et al. 2010). Moreover, the mGlu2/3 agonist, LY354740, failed to improve the deficit in pre-pulse inhibition induced by acute PCP (Schreiber et al. 2000) or ketamine (Imre et al. 2006) administration, and LY379268 did not improve the impairment in conditioned emotional response in isolation reared rats (Jones et al. 2010). On the other hand, mGlu2/3 agonists have been reported to improve working memory and social novelty discrimination disrupted by non-competitive NMDA receptor antagonists (Krystal et al. 2005; Moghaddam and Adams 1998; Harich et al. 2007). LY379268 also reversed post-weaning social isolation-induced locomotor hyperactivity, deficits in NOR, and deficits in acoustic startle response in the pre-pulse inhibition paradigm (Jones et al. 2010). This suggests that mGlu2/3 agonists attenuate some domains of cognitive impairments but not others, as has been reported for atypical APDs in schizophrenia (Meltzer and McGurk 1999). Our results suggest that the combination of a sub-effective dose of an atypical APD with an mGlu2/3 agonist might produce a broader improvement in cognition in patients with schizophrenia than either drug alone.
Lurasidone has more potent 5-HT2A than D2 receptor blocking properties (Ishiyama et al. 2009), as do the five other atypical antipsychotic drugs which have been shown to reverse the effects of PCP in this model (Grayson et al. 2007; Snigdha et al. 2010). This is consistent with other evidence that the reversal of the PCP-induced deficit in NOR by atypical APDs is facilitated by extensive blockade of 5-HT2A receptors (Snigdha et al. 2010). There is significant clinical evidence that 5-HT2A receptor blockade can diminish psychopathology, including psychotic symptoms in patients with schizophrenia or Parkinson’s disease, some of which we have summarized elsewhere (Meltzer et al. 2010). This includes a placebo-controlled trial in acutely psychotic patients treated with the 5-HT2A inverse agonist, SR43469B (Meltzer et al. 2004). Further, a recent PET study which measured 5-HT2A receptor occupancy with [18F] altanserin PET in 15 first-episode antipsychotic-naïve schizophrenia patients before and after 6 months of quetiapine treatment reported that a 5-HT2A receptor occupancy level between 60% and 70% appeared to exert the optimal 5-HT2A receptor-related treatment effect on positive symptoms (Rasmussen et al. 2010).
It is noteworthy that co-administration of LY379268 and sub-effective doses of the atypical APDs, clozapine and lurasidone, but not haloperidol or pimavanserin, ameliorated the subchronic PCP-induced NOR deficit. These negative results suggest that an interaction of mGlu2/3 agonism with either D2 or 5-HT2A receptors alone is insufficient to reverse the effects of subchronic PCP, but do not exclude the possibility that both D2 and 5-HT2A receptor blockade, in combination with other neurochemical effects of the atypical APDs, contribute to the synergism of mGlu2/3 agonists with atypical APDs. The dose of haloperidol used here was based on previous studies of its ability to weakly enhance cortical DA efflux (Ichikawa and Meltzer 1991; Kuroki et al. 1999). The dose of pimavanserin has been shown to achieve essentially 100% 5-HT2A receptor occupancy (Vanover et al. 2006). mGlu2/3 and 5-HT2A receptors co-localize in cortical pyramidal neurons and form a heterodimer complex (González-Maeso et al. 2008). mGlu2/3 agonists, like 5-HT2A inverse agonists, decrease 5-HT2A agonist-induced head twitch (Gewirtz and Marek 2000) and excitatory postsynapitc potentials (Marek et al. 2000). Recent studies showed that LY379268 (1 mg/kg) and the 5-HT2A inverse agonist, M100907 (0.2 mg/kg), modestly decreased amphetamine-induced hyperlocomotion when given alone, whereas the combination markedly attenuated amphetamine- and MK-801-induced hyperlocomotion (Uslaner et al. 2009). These considerations led Pehrson and Moghaddam (2010) to suggest that mGlu2/3 agonists may be working, in part, through a serotonergic signaling mechanism to attenuate the ability of amphetamine to increase DA efflux. LY379268 may induce functional changes in these heterodimer complexes and 5-HT2A receptor signal transduction, thereby producing the synergistic effects of LY379268 and sub-effective doses of the atypical APDs, clozapine and lurasidone. In this study, LY379268 3 mg/kg tended to increase total exploration time in both trials. A similar effect has been reported with pimavanserin (Snigdha et al. 2010). Additional studies are necessary to clarify the effect of mGlu2/3 agonists and 5-HT2A inverse agonists on exploration time in PCP-treated rodents.
The ameliorative effect of clozapine on NOR was blocked by the mGlu2/3 antagonist, LY341495, whereas LY341495 was reported not to inhibit the effect of clozapine on PCP-induced hyperlocomotion (Cartmell et al. 1999). Although it was reported that LY341495 improved acquisition of spatial learning in rodents (Higgins et al. 2004), we found that LY341495 by itself did not attenuate the PCP-induced NOR deficit (data not shown). These data suggest that mGlu2/3 agonism is a necessary component of the neural circuit activated by clozapine, and probably other atypical APDs related to clozapine, to reverse the effect of subchronic PCP treatment on NOR, but that mGlu2/3 agonism is insufficient by itself to reverse the effect of PCP. mGlu2/3 receptors are negatively coupled to adenylyl cyclase (Prézeau et al. 1994) as are DA D2 receptors (Senogles et al. 1988) and 5-HT1A receptors (Devivo and Maayani 1985), while 5-HT7 receptors are positively coupled to adenylyl cyclase (Lovenberg et al. 1993). Atypical APDs have complex effects as partial agonists and inverse agonists at both 5-HT1A and 5-HT7 receptors (Newman-Tancredi et al. 2005: Rauly-Lestienne et al. 2007). As clozapine and lurasidone, as well as other atypical APDs, are likely to be acting at D2, 5-HT1A and 5-HT7 receptors at clinically effective doses, all of which have been shown to have a role in cognition, further study is warranted to determine if the effect of mGlu2/3 agonists and antagonists may be mediated, in part, by actions on cyclic AMP-dependent signaling mechanisms.
mGlu2 receptors, which are considered to mediate the antipsychotic effect of the mGlu2/3 agonists (Woolley et al. 2008), are expressed in perirhinal cortical neurons, although the expression profile, functional roles, and intracellular signaling of the various neurotransmitter receptors in this region are not well known (Harris et al. 2004). There is evidence for involvement of the perirhinal cortex in human recognition memory (Yassa and Stark 2008), as well as NOR performance in rodents. Thus, lesions of perirhinal cortex impair performance in rodent NOR (Winters et al. 2004). Patients with schizophrenia, compared to normals, have been reported to have reduced volumes, relative to cranial size, in left and right perirhinal cortex, which was associated with decreased olfactory threshold sensitivity but not impaired memory performance (Turetsky et al. 2003). The mGlu2 in perirhinal cortex might contribute to the ability of atypical APDs to ameliorate the PCP-induced NOR deficit. The results reported suggest the need for clinical trials of the combination of atypical APDs with mGlu2/3 agonists to improve some domains of cognition in schizophrenia especially declarative memory.
Atypical APDs, e.g., clozapine, preferentially enhance DA efflux in the rat mPFC and HIP (Kuroki et al. 1999; Chung et al. 2004). We have suggested this may contribute to their ability to improve cognitive function in patients with schizophrenia (Meltzer and McGurk 1999). LY379268, 1 mg/kg, s.c. by itself, did not increase DA efflux in the mPFC or HIP, but the combination of this dose and a sub-effective dose of lurasidone significantly increased mPFC and HIP DA efflux in awake freely moving rats (Huang et al. submitted). It has been reported that prefrontal cortical DA utilization is reduced by subchronic PCP treatment in rats (Jentsch et al. 1998). This has been suggested to be due to the loss of dendritic spine synapses (Hajszan et al. 2006). Clozapine, but not haloperidol, have been shown to increase the formation of dendritic spines (Critchlow et al. 2006). Chronic administration of the atypical APD, olanzapine, but not haloperidol, has been shown to reverse 6-hydroxydopamine-induced abnormalities in pyramidal neuron dendrites (Wang and Deutch 2008). Whether the ability of clozapine and lurasidone alone, or in combination with LY379268, is based on changes in dendritic spines, which can occur very rapidly (Li et al. 2011), requires further study.
In conclusion, these results indicate that mGlu2/3 agonism is relevant to the ability of clozapine and lurasidone to ameliorate the effect of subchronic PCP treatment on NOR, a putative model for the cognitive dysfunction of schizophrenia. The results reported here suggest that combined administration of mGlu2/3 agonists, with at least some atypical APDs, may be a means to augment their ability to improve cognition in schizophrenia. Co-administration of a mGlu2/3 agonist with an atypical APD may also facilitate the antipsychotic efficacy of both agents, thereby permitting lower doses of each, with a diminution of side effect burden of either agent.
References
Amitai N, Markou A (2010) Effects of metabotropic glutamate receptor 2/3 agonism and antagonism on schizophrenia-like cognitive deficits induced by phencyclidine in rats. Eur J Pharmacol 639(1–3):67–80
Aultman JM, Moghaddam B (2001) Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology Berl 153(3):353–364
Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA (2002) Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 159(6):1018–1028
Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75(3):889–907
Cartmell J, Monn JA, Schoepp DD (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291(1):161–170
Chung YC, Li Z, Dai J, Meltzer HY, Ichikawa J (2004) Clozapine increases both acetylcholine and dopamine release in rat ventral hippocampus: role of 5-HT1A receptor agonism. Brain Res 1023:54–63
Coyle JT (2006) Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 26(4–6):365–384
Critchlow HM, Maycox PR, Skepper JN, Krylova O (2006) Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Mol Cell Neurosci 32(4):356–365
Devivo M, Maayani S (1985) Inhibition of forskolin-stimulated adenylate cyclase activity by 5-HT receptor agonists. Eur J Pharmacol 119(3):231–234
Enomoto T, Ishibashi T, Tokuda K, Ishiyama T, Toma S, Ito A (2008) Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav Brain Res 186(2):197–207
Gewirtz JC, Marek GJ (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23(5):569–576
Goldman-Rakic PS, Selemon LD (1997) Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 23(3):437–458
González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452(7183):93–97
Gray JA, Roth BL (2007) Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull 33(5):1100–1119
Grayson B, Idris NF, Neill JC (2007) Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 184(1):31–38
Gunduz-Bruce H (2009) The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60(2):279–286
Hagger C, Buckley P, Kenny JT, Friedman L, Ubogy D, Meltzer HY (1993) Improvement in cognitive functions and psychiatric symptoms in treatment-refractory schizophrenic patients receiving clozapine. Biol Psychiatry 34:02–712
Hagiwara H, Fujita Y, Ishima T, Kunitachi S, Shirayama Y, Iyo M, Hashimoto K (2008) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antipsychotic drug perospirone: role of serotonin 5-HT1A receptors. Eur Neuropsychopharmacol 18(6):448–454
Hajszan T, Leranth C, Roth RH (2006) Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry 60:639–644
Harich S, Gross G, Bespalov A (2007) Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology Berl 192(4):511–519
Harris SL, Cho K, Bashir ZI, Molnar E (2004) Metabotropic glutamate receptor signalling in perirhinal cortical neurons. Mol Cell Neurosci 25(2):275–287
Hashimoto K, Fujita Y, Shimizu E, Iyo M (2005) Phencyclidine-induced cognitive deficits in mice are improved by subsequent sub-chronic administration of clozapine, but not haloperidol. Eur J Pharmacol 519:114–117
Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, Woltering T, Nakanishi S, Mutel V (2004) Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology 46(7):907–917
Ichikawa J, Meltzer HY (1991) Differential effects of repeated treatment with haloperidol and clozapine on dopamine release and metabolism in the striatum and the nucleus accumbens. J Pharmacol Exp Ther 256(1):348–357
Ichikawa J, Dai J, Meltzer HY (2002) Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5HT1A receptor agonism. Brain Res 956:349–357
Imre G, Fokkema DS, Ter Horst GJ (2006) Subchronic administration of LY354740 does not modify ketamine-evoked behavior and neuronal activity in rats. Eur J Pharmacol 544(1–3):77–81
Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y (2007) Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol 572(2–3):160–170
Ishiyama T, Loebel A, Cucciaro J, Horisawa T, Tokuda K, Ishibashi T, Stahl S (2009) Receptor binding profile of lurasidone: a novel psychotropic agent under development for schizophrenia and bipolar disorder. ACNP 48th Annual Meeting Poster Abstr 75
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148(10):1301–1308
Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, Roth RH (1998) Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neurosci Lett 258(3):175–178
Jones CA, Brown AM, Auer DP, Fone KC (2010) The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology Berl. doi:10.1007/s00213-010-1931-7
Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA, CATIE Investigators and the Neurocognitive Working Group (2007) Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry 64:633–647
Kinon BJ (2009) LY2140023 monohydrate: an agonist at the mGlu2/3 receptor for the treatment of schizophrenia. International Congress on Schizophrenia Research, San Diego
Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S (1999) NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry 7(3):125–143
Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A (2005) Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology Berl 179(1):303–309
Kuroki T, Meltzer HY, Ichikawa J (1999) Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther 288:774–781
LaPorte DJ, Lahti AC, Koffel B, Tamminga CA (1996) Absence of ketamine effects on memory and other cognitive functions in schizophrenia patients. J Psychiatr Res 30(5):321–330
Li Q, Deng Z, Zhang Y, Zhou X, Nagerl V, Wong S (2011) A global spatial similarity optimization scheme to track large numbers of dendritic spines in time-lapse confocal microscopy. IEEE Trans Med Imaging 30(3):632–641
Liegeois JF, Ichikawa J, Meltzer HY (2002) 5-HT(2A) receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Res 947:157–165
Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11(3):449–458
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000) Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292(1):76–87
McKibben CE, Jenkins TA, Adams HN, Harte MK, Reynolds GP (2010) Effect of pretreatment with risperidone on phencyclidine-induced disruptions in object recognition memory and prefrontal cortex parvalbumin immunoreactivity in the rat. Behav Brain Res 208(1):132–136
Meltzer HY, Huang M (2008) In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res 172:177–197
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255
Meltzer HY, Matsubara S, Lee JC (1989) The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull 25:390–392
Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study Group (2004) Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 161(6):975–984
Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH (2010) Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson's disease psychosis. Neuropsychopharmacology 35(4):881–892
Meyer JM, Loebel AD, Schweizer E (2009) Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs 18(11):1715–1726
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281(5381):1349–1352
Moghaddam B, Bunney BS (1990) Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem 54(5):1755–1760
Morgan CJ, Huddy V, Lipton M, Curran HV, Joyce EM (2009) Is persistent ketamine use a valid model of the cognitive and oculomotor deficits in schizophrenia? Biol Psychiatry 65(12):1099–1102
Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T (2009) Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology Berl 202(1–3):315–328
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128(3):419–432
Newman-Tancredi A, Assié MB, Leduc N, Ormière AM, Danty N, Cosi C (2005) Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int J Neuropsychopharmacol 8(3):341–356
Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV (1997) Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology 11(3):437–446
Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13(9):1102–1107
Pehrson AL, Moghaddam B (2010) Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology Berl 211(4):443–455
Prézeau L, Carrette J, Helpap B, Curry K, Pin JP, Bockaert J (1994) Pharmacological characterization of metabotropic glutamate receptors in several types of brain cells in primary cultures. Mol Pharmacol 45(4):570–577
Rasmussen H, Ebdrup BH, Erritzoe D, Aggernaes B, Oranjem B, Kalbitzer J, Pinborg LH, Baaré WFC, Svarer C, Lublin H, Knudsen GM, Glenthoj B (2010) Serotonin2A receptor blockade and clinical effect in first-episode schizophrenia patients treated with quetiapine. Psychopharmacology Berl. doi:10.1007/s00213-010-1941-5
Rauly-Lestienne I, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Cussac D (2007) Differential profile of typical, atypical and third generation antipsychotics at human 5-HT7a receptors coupled to adenylyl cyclase: detection of agonist and inverse agonist properties. Naunyn Schmiedebergs Arch Pharmacol 376(1–2):93–105
Schlumberger C, Schäfer D, Barberi C, Morè L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W (2009) Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav Pharmacol 20(1):56–66
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loor K, Leysen JE (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology Berl 124:57–73
Schreiber R, Lowe D, Voerste A, De Vry J (2000) LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. Eur J Pharmacol 388(2):R3–R4
Senogles SE, Amlaiky N, Berger JG, Caron MG (1988) Biochemical properties of D1 and D2 dopamine receptors. Adv Exp Med Biol 235:33–41
Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY (2010) Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 332(2):622–631
Stefanovic A, Brandner B, Klaassen E, Cregg R, Nagaratnam M, Bromley LM, Das RK, Rossell SL, Morgan CJ, Curran HV (2009) Acute and chronic effects of ketamine on semantic priming: modeling schizophrenia? J Clin Psychopharmacol 29(2):124–133
Swanson CJ, Schoepp DD (2002) The group II metabotropic glutamate receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate (LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J Pharmacol Exp Ther 303(3):919–927
Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE (2003) Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry 60(12):1193–1200
Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, Hutson PH (2009) Combined administration of an mGlu2/3 receptor agonist and a 5-HT 2A receptor antagonist markedly attenuate the psychomotor-activating and neurochemical effects of psychostimulants. Psychopharmacology Berl 206(4):641–651
Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A (2010) The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. Eur J Pharmacol 639(1–3):91–98
Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, Del Tredici AL, Piu F, Schiffer HH, Ott TR, Burstein ES, Uldam AK, Thygesen MB, Schlienger N, Andersson CM, Son TY, Harvey SC, Powell SB, Geyer MA, Tolf BR, Brann MR, Davis RE (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317(2):910–918
Wang HD, Deutch AY (2008) Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology 33:1276–1286
Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ (2004) Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24(26):5901–5908
Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8(3):457–472
Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN (2008) The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology Berl 196(3):431–440
Yassa MA, Stark CE (2008) Multiple signals of recognition memory in the medial temporal lobe. Hippocampus 18(9):945–954
Acknowledgements
This research was supported, in part, by a grant from Dainippon Sumitomo Pharma Co., Ltd. HYM is supported, in part, by contributions from the Ritter and Weisman Family Foundations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horiguchi, M., Huang, M. & Meltzer, H.Y. Interaction of mGlu2/3 agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology 217, 13–24 (2011). https://doi.org/10.1007/s00213-011-2251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2251-2