Abstract
Rationale
Blockade of N-methyl-d-aspartic acid (NMDA) receptors in the rat medial prefrontal cortex (mPFC) impairs performance in the five-choice serial reaction time task (5-CSRTT) and increases glutamate (GLU) release. Recent research suggests that excessive GLU release may be critical for attention deficits.
Objectives
We tested this hypothesis by investigating the effects of the atypical antipsychotics sertindole and clozapine on 3-(R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP)-induced performance deficits in the 5-CSRTT and on the CPP-induced GLU release in the mPFC.
Methods
The 5-CSRTT, a test of divided and sustained visual attention providing indices of attentional functioning (accuracy of visual discrimination), response control (anticipatory and perseverative responses) and intracortical microdialysis in conscious rats were used to investigate the effects of sertindole and clozapine.
Results
Low doses of sertindole (0.02–0.32 mg/kg) prevented CPP-induced accuracy deficits, anticipatory over-responding and the rise in GLU release. In contrast, doses ranging from 0.6 to 2.5 mg/kg had no effect or even enhanced the effect of CPP on anticipatory responding. Similarly, 2.5 mg/kg sertindole was unable to reverse CPP-induced rise in GLU release. Clozapine (2.5 mg/kg) prevented accuracy deficits and the increase in anticipatory responding and abolished the rise in GLU release induced by CPP.
Conclusions
These findings show that the ameliorating effects of sertindole and clozapine on NMDA receptor dependent attention deficit is associated with suppression in GLU release in the mPFC. This supports the proposal that suppression in GLU release might be a target for the development of novel drugs aimed at counteracting some aspects of cognitive deficits of schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysfunctional glutamate (GLU) transmission in the medial prefrontal cortex (mPFC) has been implicated in aspects of cognitive deficits of schizophrenia, including attention disorders and deficits in executive functions (Braff 1993; Frith 1987; Javitt and Zukin 1991). Hypofunction of N-methyl-d-aspartic acid (NMDA) receptors in the pathophysiology of schizophrenia stems from clinical observation that non-competitive NMDA receptor antagonists such as phencyclidine (PCP) and ketamine cause schizophrenia-like symptoms in normal people and exacerbates psychotic and negative symptoms in schizophrenic patients and cognitive deficits associated with the disease (Javitt and Zukin 1991; Krystal et al. 1994; Lahti et al. 1995; Luby et al. 1962; Malhotra et al. 1997). Based on these findings, administration of single or repeated doses of NMDA receptor antagonists has become a widely used pharmacological model of schizophrenia in rodents. Findings that drugs that abolished cognitive deficits induced by PCP and ketamine also prevented increased extracellular GLU in the mPFC (Moghaddam et al. 1997; Moghaddam and Adams 1998) suggested that excessive GLU on non-NMDA receptors in the mPFC might cause cognitive impairment. Accordingly, selective blockade of mPFC NMDA receptors with the competitive NMDA receptor antagonist 3-(R)-2-carboxypiperazin-4-propyl-1 phosphonic acid (CPP) caused attention deficit (decreased accuracy of visual discrimination) and loss of inhibitory response control (increase in anticipatory and perseverative responses) in a task of divided and sustained attention such as the five-choice serial reaction time task (5-CSRTT) (Mirjana et al. 2004). Furthermore, CPP increased extracellular GLU in the mPFC (Ceglia et al. 2004). These effects were prevented by the blockade of 5-HT2A receptors (Ceglia et al. 2004; Mirjana et al. 2004) or stimulation of 5-HT1A and 5-HT2C receptors (Calcagno et al. 2009; Carli et al. 2006). In addition, antipsychotic drugs such as haloperidol and clozapine also prevented CPP-induced deficits in rats’ performance in the 5-CSRTT although, differences were noted in the ability of these drugs to control various aspects of performance such as anticipatory and perseverative responding and accuracy (Baviera et al. 2008). Antipsychotic drugs show a complex pharmacology involving various monoaminergic receptors (Arnt and Skarsfeldt 1998). Although the greater efficacy of newer antipsychotics compared to the first-generation antipsychotics in controlling cognitive disturbances in schizophrenic patients is controversial, there is some indication that drugs such as clozapine (a prototype of atypical antipsychotics), characterized by high affinity and antagonistic effect at 5-HT2 receptors are somewhat more effective than conventional antipsychotics in improving some aspects of cognitive deficits (Harvey and Keefe 2001; Keefe et al. 1999; Meltzer and McGurk 1999). However, it has been argued that cognitive improvement with clozapine might be due to practice effects with the testing instruments (Goldberg et al. 2007).
Sertindole is a novel antipsychotic drug showing efficacy against positive and negative symptoms of schizophrenia and low propensity to cause extrapyramidal side effects (Kane and Tamminga 1997; Zimbroff et al. 1997). It has also been shown to improve some aspects of cognitive functions of schizophrenic patients (Gallhofer et al. 2007) and reverse cognitive deficits induced by acute or subchronic PCP in rats such as Morris’ water maze test, and in tests of reversal learning, object recognition and attentional set shifting (Didriksen et al. 2007; Idris et al. 2010; Rodefer et al. 2008).
The present study investigated the ability of sertindole to improve CPP-induced performance impairment in the 5-CSRTT and changes in GLU release. The behavioural and neurochemical effects of sertindole were compared to that of clozapine.
Materials and methods
Animals
Male Lister–Hooded rats (Charles River, UK) weighed between 300 and 350 g before surgery and throughout the experiments were used in behavioural studies, whereas rats used in microdialysis studies were male CD rats (Charles River, Calco, Italy), which weighed approximately 250–300 g. They were housed at a constant room temperature (21 ± 1°C) and relative humidity 50 ± 5%, with a 0700–1900 h day/night cycle. Food was freely available for CD rats while Lister–Hooded rats had limited access to food (about 15 g of Altromin pellets for rats) at the end of each day's testing to keep the animals at 85–90% of their initial free-feeding weight. Water was freely available for all rats. Lister Hooded rats were preferred for behavioural studies as they reach high levels of performance after training on the 5CSRT task. On the other hand, CD rats were preferred for microdialysis as previous studies were done on this strain (Calcagno et al. 2009; Calcagno et al. 2006; Ceglia et al. 2004) and no gross differences in the response to intracortical CPP were noted between Lister Hooded and CD rats (Carli et al. 2010).
All experiments were conducted in conformity with the institutional guidelines that are in compliance with national (D.L. n. 116, G.U., suppl. 40, 18 Febbraio 1992, Circolare No. 8, G.U., 14 Luglio 1994) and international laws and policies (EEC Council Directive 86/609, OJ L 358,1, Dec.12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Behavioural studies
Five-choice serial reaction time task
The apparatus consisted of four specially designed boxes (Campden Ins. UK) controlled online by Whisker software (Cambridge University Technical Services, Ltd. UK). The apparatus and training procedures are described elsewhere (Carli et al. 1983).
Briefly, rats were trained to wait for a fixed time (5 s) before a brief visual stimulus (0.5 s) was presented in one of the five holes. While the light was on and for a short period afterwards (limited hold), response in the hole that was illuminated (correct response) resulted in the delivery of a food pellet. Responses in the holes that had not been illuminated (incorrect responses) or failure to respond within the limited hold (omissions) caused the house light to be turned off for a short period (time out). Throughout the study, each rat had only one session consisting of 100 trials per day.
The following performance measures were recorded: choice accuracy (the proportion of correct responses in the total number of correct plus incorrect responses), omissions (the proportion of omissions in the total number of correct + incorrect + omissions), anticipatory responses (responses made in the holes during the waiting period before presentation of the target), perseverative responses (responses repeated in the holes after a correct response but before collecting the food pellet). Correct response latency (the time from the stimulus onset to a correct response) and magazine latency (the time from the correct response to the collection of food from the magazine) were also recorded. Rats were considered to have acquired the task when their accuracy was about 80% correct responses with no more than 20% omissions.
Surgery, drug administration and microinjection procedure
Rats previously trained to a stable level of performance were anesthetized by an intraperitoneal (IP) injection (2 ml/kg) of 40 mg/ml ketamine and 5 mg/ml xylazine. All animals received 0.1 mg/kg atropine sulfate IP. The rats were secured in a stereotaxic frame (model 900, David Kopf Instruments, USA) with the incisor bar set at −3.3 mm relative to the inter-aural line. Bilateral 23-gauge, stainless steel guide cannulae (Cooper’s Needles, U.K.) were implanted in the mPFC using standard stereotaxic techniques. The coordinates used were: AP +3.7 mm from bregma, L ±0.7 mm from midline and DV −2.8 from dura (Paxinos and Watson 1986). Thirty-gauge stainless steel stylets were inserted flush with the end of the guide cannulae.
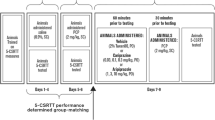
On each test day, rats were given vehicle (2 ml/kg) or sertindole (0.02, 0.08, 0.32, 0.625, 1.25 and 2.5 mg/kg) orally, whereas clozapine (2.5 mg/kg) or 2 ml/kg vehicle were given intraperitoneally. Four hours later (30 min for clozapine), while the rat was held, the stylets were removed and two injection units terminating 2 mm below the tip of the guides were inserted. One microliter per hemisphere of CPP (50 ng/μl) or vehicle was delivered into the mPFC, at a rate of 0.5 μl/min, with a 10-μl syringe mounted in a CMA/100 infusion pump (CMA Microdialysis, Sweden), connected by PE10 tubing to the injection units, which were left in place for 1 min to allow for diffusion.
Microdialysis and analytical procedures
Behaviourally naive rats were anesthetized with 3 ml/kg Equithesin and placed on a stereotaxic apparatus (model 900, David Kopf Instruments, USA). A hole was drilled in the skull and a small incision made in the dura with a bent needle tip. The probe, while being perfused (1 μl/min) with artificial cerebrospinal fluid (aCSF), was lowered slowly into the rat mPFC and fixed vertically to the skull using two to three stainless steel anchorage screws and acrylic cement. Stereotaxic coordinates (in mm) for the probe tip were: AP=+3.7, L = ±0.7, V=−4.8 from bregma and dura surface according to the stereotaxic atlas (Paxinos and Watson 1986).
Vertical dialysis probes were prepared essentially as described elsewhere (Robinson and Whishaw 1988) with a dialysis membrane made of Cuprophan (Sorin Biomedica, Italy; 216 μm outer diameter; 3,000 Da cutoff). The exposed membrane was 4 mm long. Rats were allowed to recover from anesthesia one per cage with free access to food and water. About 20 h after surgery, the rat was placed in a cage and the inlet cannula of the probes connected by polyethylene tubing to a 2.5-ml plastic syringe containing artificial cerebrospinal fluid (aCSF; composition (in mM): 145 NaCl, 3 KCl, 1.26 CaCl2⋅2 H2O, 1 MgCl2⋅6 H2O, 7.2 glucose in distilled water and buffered at pH 7.4 with 2 mM sodium phosphate buffer). Probes were perfused at a constant flow rate of 1 μl/min with a CMA/100 microinfusion pump (CMA/Microdialysis, Stockholm, Sweden). After 30–60 min washout, consecutive 20-min samples of perfusate were collected in minivials with a refrigerated fraction collector (Microsampler 820, TSE, Germany). At least four samples were collected before drug administration (basal).
Concentrations of GLU and 5-HT in the dialysate were measured in the same sample by high performance liquid chromatography coupled to electrochemical (5-HT) or fluorometric (GLU) detection as previously described (Ceglia et al. 2004).
Histology
At the end of behavioural and neurochemical experiments, rats were deeply anesthetized with chloral hydrate (400 mg/kg, IP) and killed by decapitation. The brain was removed and frozen on dry ice. Correct probe and cannula placement was checked by visual inspection of the tracks on 30-μm coronal sections. Only rats with correct cannula and probe placements were included in the results.
Drugs
CPP (Tocris, USA) was dissolved in saline (behavioural experiments) or in aCSF (microdialysis). Sertindole (H. Lundbeck A/S, Denmark) was dissolved in water with the addition of few drops of lactic acid, buffered with NaOH to pH 6–7 and administered orally 4 h before administering CPP. This long pre-treatment time is due to a half-life of 13–15 h (Didriksen et al. 2007). Clozapine (Tocris) was dissolved in water with the addition of few drops of lactic acid, buffered with NaOH to pH 6–7 and was injected intraperitoneally 30 min before CPP.
Statistics
Behavioural studies
The effects of sertindole on CPP-induced performance deficits were tested as follows: a group of 11 rats was employed to test the effects of 0.625 and 1.25 mg/kg, whereas 2.5 mg/kg sertindole was tested in 15 rats. Doses of 0.02, 0.08 and 0.32 mg/kg sertindole were tested in 12 rats. Another group of 11 rats was used to test the effects of clozapine.
The effects of CPP in combination with various doses of sertindole or clozapine on: (a) the percentage of correct responses, (b) the percentage of omissions, (c) mean correct response latency, (d) the number of anticipatory responses and (e) the number of perseverative responses were analyzed by repeated-measures two-way analysis of variance (ANOVA) with factors sertindole or clozapine and CPP. The means of the individual treatment combinations were compared by Tukey’s test.
Microdialysis
Extracellular levels of GLU were expressed as percentages of basal values and analyzed by ANOVA for repeated measures with treatments (CPP, sertindole or clozapine) as the between-subject factors and time as the within-subject factor. The analysis was applied to the part of the curve corresponding to the duration of CPP infusion (60 min) (from 240 to 300 min after sertindole or from 20 to 80 min after clozapine). The effect of sertindole in rats given vehicle or CPP was analyzed separately and the corresponding data are presented in separate panels. Post-hoc comparisons were done with Tukey’s test.
Results
Behavioural studies
Effects of high doses of sertindole
Table 1a shows that 0.6 and 1.25 mg/kg sertindole had no effects on CPP-induced reduction in accuracy (% correct responses) as indicated by the lack of significant interaction sertindole × CPP (F 2,50 = 0.17, P > 0.05) no effect of sertindole (F 2,50 = 0.2, P > 0.05) but a significant effect of CPP (F 1,50 = 24.4, P < 0.0001). Post-hoc Tukey’s test indicated that CPP reduced accuracy (V + CPP versus V + V; Tukey’s test, P < 0.05) and that 0.625 and 1.25 mg/kg sertindole did not prevent this effect (S0.6 + CPP and S1.25 + CPP versus V + CPP; Tukey’s tests, P > 0.05). On its own, sertindole had no effect on accuracy (S0.6 + V or S1.2 + V versus V + V, Tukey’s test, P > 0.05).
The effects of 0.625 and 1.25 mg/kg sertindole on CPP-induced increase in the number of anticipatory responses (Table 1a) (S × CPP, F 2,50 = 4.98, P = 0.01; S, F 2,50 = 6.7, P = 0.002; CPP, F 1,50 = 49.6, P < 0.0001) was bell shaped. CPP significantly increased the number of anticipatory responses (V + V versus V + CPP, Tukey’s test, P < 0.05) and 0.625 mg/kg sertindole significantly enhanced this effect (S0.6 + CPP versus V + CPP; Tukey’s test, P < 0.05). However, 1.25 mg/kg had no effect on the anticipatory over-responding (S1.25 + CPP versus V + CPP, Tukey’s test, P > 0.05).
Perseverative over-responding induced by CPP was not affected by sertindole (S × CPP, F 2,50 = 0.7, P > 0.05; S, F 2,50 = 0.13, P > 0.05; CPP, F 1,50 = 43.5, P ≤ 0.0001) (Table 1a). Comparison between treatment means found a significant effect of CPP (V + V versus V + CPP; Tukey’s test, P < 0.05) but no effect of sertindole (S0.6 + CPP or S1.2 + CPP versus V + V; Tukey’s test, P > 0.05).
Sertindole did not affect the CPP-induced increases in the percentage of omissions (S × CPP, F2,50 = 0.7, P > 0.05; S, F2,50 = 1.4, P > 0.05; CPP, F1,50 = 53.4, P < 0.0001) or the latency to make a correct response (S × CPP, F2,50 = 0.1, P > 0.05; S, F2,50 = 0.2, P > 0.05; CPP, F1,50 = 4.4, P = 0.04). There was a significant increase in omissions and the correct response latency due to CPP (for both measures, V + CPP versus V + V, Tukey’s test, P < 0.05), but no effect of sertindole (for both measures, S0.6 + CPP or S1.25 + CPP versus V + CPP, both Tukey’s tests, P > 0.05). By itself sertindole had no effect on these performance measures (S0.6 + V or S1.2 + V versus V + V, both Tukey’s tests, P > 0.05).
In Table 1b, we report the effects of 2.5 mg/kg sertindole, CPP and their combination on various parameters of the 5-CSRTT. Two-way repeated-measures ANOVA indicated a significant effects of CPP on all measures (% correct, F 1,42 = 17.6, P < 0.0001; anticipatory, F 1,42 = 12.4, P < 0.001; perseverative, F 1,42 = 34.6, P < 0.0001; % omissions, F 1,42 = 78.5, P < 0.0001; correct response latencies, F 1,42 = 13.4, P < 0.0005). The F values of interaction between sertindole and CPP or of the effect of sertindole on the various measures were not statistically significant and are not reported.
Effects of low doses of sertindole
As shown in Fig. 1a, low doses of sertindole (S) prevented CPP-induced reduction in accuracy. Two-way repeated-measures ANOVA showed a significant interaction sertindole × CPP (F 3,77 = 6.3, P = 0.0007) and significant effects of sertindole (F 3,77 = 5.8, P = 0.001) and CPP (F 1,77 = 58.8, P < 0.0001). Post-hoc comparisons indicated that CPP reduced accuracy (V + CPP versus V + V; Tukey’s test, P < 0.05) and that 0.02, 0.08 and 0.32 mg/kg sertindole prevented this effect (S0.02 + CPP, S0.08 + CPP and S0.32 + CPP versus V + CPP; Tukey’s tests, P < 0.05). On its own sertindole had no effect on accuracy (S0.02 + V or S0.08 + V or S0.32 + V versus V + V, Tukey’s test, P > 0.05).
Histograms are mean ± SEM of 12 rats. vehicle (0) or sertindole 0.02, 0.08 and 0.32 mg/kg were administered orally 4 h before bilateral injections of vehicle (+VEHICLE) or 50 ng/μl CPP (+CPP) into the mPFC. Ten minutes later, the rats started the test session. Rats were treated according to a Latin square design. Only one test session per week was performed
In the experiment on the effect of low doses of sertindole (Fig. 1a), CPP seems somewhat more effective in reducing the number of correct response than in other experiments. It could be argued that with a lower baseline level of performance sertindole might produce a proportionally greater increase compared for instance to the baseline group in experiment of Fig. 2a. Although this possibility cannot be ruled out, observation of individual data showed that low doses sertindole improved rats performance to a similar extent regardless of the magnitude of CPP’s effect.
Histograms are mean ± SEM of 11 rats. Vehicle (0) or clozapine 1.25 and 2.5 mg/kg were injected intraperitoneally 30 min before bilateral injections of vehicle (+VEHICLE) or 50 ng/μl CPP (+CPP) into the mPFC. Ten minutes later, the rats started the test session. Rats were treated according to a Latin square design with at least 72 h of washout period between test sessions
The effects of low doses of sertindole on CPP-induced increase in the number of anticipatory responses are shown in Fig. 1b. Repeated-measures two-way ANOVA indicated no significant interaction between sertindole and CPP (F 3,77 = 1.9, P > 0.05) but significant main effects of CPP (F 1,77 = 93.3, P < 0.0001) and sertindole (F 3,77 = 2.7, P = 0.05). Comparison between means showed that anticipatory responses were increased after CPP (V + V versus V + CPP, Tukey’s test, P < 0.05) and that only the intermediate dose of 0.08 mg/kg (S0.08 + CPP versus V + CPP; Tukey’s test, P < 0.05) reduced this increase (S0.02 + CPP or S0.32 + CPP versus V + CPP, Tukey’s test, P > 0.05).
Perseverative over-responding induced by CPP was not affected by sertindole (S × CPP, F 3,77 = 1.1, P > 0.05; S, F 3,77 = 1.9, P > 0.05; CPP, F 1,77 = 82.8, P < 0.0001) (Fig. 1c). Comparison between treatment means found a significant effect of CPP (V + V versus V + CPP; Tukey’s test, P < 0.05) but no effect of sertindole in CPP injected rats (S0.02 + CPP or S0.08 + CPP or S0.32 + CPP versus V + CPP; Tukey’s test, P > 0.05) or in rats given vehicle (S0.02 + V or S0.08 + V or S0.32 + V versus V + V; Tukey’s test, P > 0.05).
As shown in Table 2a, the CPP-induced increases in the percentage of omissions (S × CPP, F3,77 = 0.2, P > 0.05; S, F3,77 = 0.6, P > 0.05; CPP, F1,77 = 85.4, P < 0.0001) was not affected by any dose of sertindole (S0.02 + CPP or S0.08 + CPP or S0.32 + CPP versus V + CPP, all Tukey’s tests, P > 0.05). On its own, these low doses of sertindole had no effect on omissions (S0.02 + V or S0.08 + V or S0.32 + V versus V + V, all Tukey’s tests, P > 0.05). The correct response latency was increased by CPP and this effect was prevented by sertindole (S × CPP, F3,77 = 2.9, P = 0.04; S, F3,77 = 5.2, P = 0.002; CPP, F1,77 = 121.5, P < 0.0001). Post-hoc comparisons of various treatments means showed that 0.08 (S0.08 + CPP versus V + CPP; P < 0.05) and 0.32 mg/kg (S0.32 + CPP versus V + CPP; P < 0.05) but not 0.02 mg/kg (S0.02 + CPP versus V + CPP; P > 0.05) sertindole prevented the increase in correct response latencies induced by CPP (V + CPP and V + V; P < 0.05).
Effects of clozapine
Figure 2a shows that both 1.25 and 2.5 mg/kg doses of clozapine (C) prevented the CPP-induced reduction in accuracy. Two-way repeated-measures ANOVA showed a significant interaction between clozapine and CPP (C × CPP, F 2,50 = 5.3, P < 0.01) and significant effects of CPP (CPP, F 1,50 = 5.5, P < 0.02) but not clozapine (C, F 2,50 = 0.8, P > 0.05). This interaction reflected the fact that CPP reduced % correct responses (V + CPP versus V + V, Tukey’s test, P < 0.05) and that clozapine prevented this effect (C1.25 + CPP or C2.5 + CPP versus V + CPP, Tukey’s test, P < 0.05). No dose of clozapine by itself had any effect on accuracy (C1.25 + V or C2.5 + V versus V + V, Tukey’s test, P > 0.05).
The CPP-induced increase in anticipatory responding (Fig. 2b) was dose-dependently reduced by clozapine (C × CPP, F 2,50 = 3.3, P < 0.05; C, F 2,50 = 5.6, P < 0.005; CPP, F 1,50 = 20.6, P < 0.0001) while perseverative over-responding was not affected (C × CPP, F 2,50 = 0.7, P > 0.05; C, F 2,50 = 2.3, P > 0.05; CPP, F 1,50 = 15.7, P < 0.0002) (Fig. 2c). Post-hoc comparisons of treatment means showed that rats injected with CPP made significantly more anticipatory (V + CPP versus V + V, Tukey’s test, P < 0.05) and perseverative responses (V + CPP versus V + V, Tukey’s test, P < 0.05) than vehicle controls and that pre-treatment with 1.25 and 2.5 mg/kg clozapine dose-dependently prevented the effects of CPP (C1.25 + CPP or C2.5 + CPP versus V + CPP) on anticipatory (both Tukey’s test, P < 0.05) but not perseverative responding (both Tukey’s test, P > 0.05). On its own, clozapine (C1.25 + V or C2.5 + V versus V + V) had no effect on the number of anticipatory or perseverative responses (both, Tukey’s test, P > 0.05).
Table 2b shows that the increase in the percentage of omissions (C × CPP, F2,50 =0.8, P > 0.05; C, F2,50 = 2.8, P = 0.06; CPP, F1,50 = 31.9, P < 0.0001) and latency to make a correct response (C × CPP, F2,50 = 4.7, P = 0.01; C, F2,50 = 1.4, P > 0.05; CPP, F1,50 = 11.3, P < 0.001) induced by CPP was not affected by clozapine. Comparison of treatment means indicated no effect of clozapine on CPP-induced increase in omissions or the latency to make a correct response (for both measures C1.25 + CPP or C2.5 + CPP and V + CPP; Tukey’s test, P > 0.05). However, 2.5 mg/kg clozapine increased the proportion of omissions and the latency to make a correct response in vehicle injected rats (for both measures C2.5 + V versus V + V; Tukey’s test, P < 0.05).
Mean basal levels of GLU
Mean basal levels of GLU (±SEM) in pmol/20 μl for each treatment groups were as follows: Figure 3a: V + aCSF, 16.8 ± 1.0 (n = 7); V + CPP, 14.6 ± 2.2 (n = 6); S0.02 + CPP, 15.1 ± 3.5 (n = 5), S0.32 + CPP, 14.8 ± 1.6 (n = 5); S2.5 + CPP, 16.9 ± 1.9 (n = 6). Figure 3b: S0.02 + aCSF, 14.5 ± 2.6 (n = 5), S0.32 + aCSF, 14.6 ± 1.9 (n = 5); S2.5 + aCSF, 13.7 ± 3.5 (n = 5). Figure 4: V + aCSF, 13.8 ± 2.1 (n = 5); V + CPP, 15.0 ± 1.9 (n = 5); C + CPP, 17.1 ± 1.3 (n = 5), C + aCSF, 16.2 ± 1.1 (n = 5).
Effects of sertindole (S) on CPP-induced rise of extracellular GLU in the mPFC. Drug or vehicle (V) were given orally (arrows) 4 h before the infusion of 100 μM CPP (a) or aCSF (b) through the probe. Horizontal bar indicates the duration of CPP infusion. Experimental groups in (a) were as follows: V + aCSF (n = 7), V + CPP (n = 6), S0.02 mg/kg + CPP (n = 5), S0.32 mg/kg + CPP (n = 5), S2.5 mg/kg + CPP (n = 6). (b) V + aCSF the same as in a (dotted line), S0.02 mg/kg + aCSF (n = 5), S0.32 mg/kg + aCSF (n = 5) and S 2.5 mg/kg + aCSF (n = 5). Data are expressed as mean percentages of basal values ± SEM. For the sake of clarity, data from 20 to 220 min were omitted from (a). The whole curves are shown in Fig. S1. *P < 0.05 versus basal values (Tukey’s test)
Effects of clozapine on CPP-induced rise of extracellular GLU in the mPFC. Clozapine (CLOZ) or vehicle (V) were given orally (arrows) 20 min before the infusion of 100 μM CPP or aCSF through the probe. Horizontal bar indicates the duration of CPP or aCSF infusion. Experimental groups were as follows: V + V, V + CPP, CLOZ + CPP, CLOZ + aCSF. Data are expressed as percentages of basal values and are the mean ± SEM of five rats per group. *P < 0.05 versus basal values (Tukey’s test)
No significant differences in basal GLU were found across groups (F 11,52 = 0.33, P > 0.05; one-way ANOVA). Pooled data yield mean basal GLU levels of 15.3 ± 0.6 pmol/20 μl (n = 63).
Effects of sertindole and clozapine on GLU efflux in the mPFC
The effect of sertindole on basal and CPP-induced rise of extracellular GLU is shown in Fig. 3. The infusion of 100 μM CPP through the probe increased extracellular GLU in the rat mPFC, reaching 305% of basal values at 60 min. The effect of CPP was prevented by sertindole (Fig. 3a). ANOVA indicated a significant effect of sertindole (F3,18 = 14.3, P < 0.0001), CPP (F3,54 = 7.5, P = 0.0003) and sertindole × CPP interaction (F9,54 = 5.4, P < 0.0001). Post-hoc analysis showed that in rats given 0.02 and 0.32 mg/kg sertindole CPP had no significant effects while a significant increase of GLU was found in those given 2.5 mg/kg sertindole. The increase of extracellular GLU in rats pretreated with vehicle or 2.5 mg/kg sertindole was not significantly different.
Figure 4 shows the effect of 2.5 mg/kg clozapine on basal and CPP-induced rise of extracellular GLU (Fig. 4a). In rats pre-treated with vehicle, extracellular GLU reached 298% of basal values 60 min after CPP infusion. ANOVA shows that CPP had no significant effect in rats given 2.5 mg/kg clozapine (clozapine, F1,8 = 14.4, P = 0.005; CPP, F3,24 = 5.8, P = 0.004; clozapine × CPP, F3,24 = 5.4, P = 0.006).
Individually, sertindole and clozapine had no significant effect on extracellular GLU (Figs. 3b and 4b).
Discussion
The data show that sertindole and clozapine, at doses preventing the CPP-induced attention deficit in the 5-CSRTT, abolished CPP’s effects on cortical GLU release. Thus, suppression of GLU release may play a major role in the effects of these drugs on accuracy deficit (a measure of attentional functioning) in the 5-CSRTT and further extend our previous observations that 5-HT receptor agents preventing accuracy deficits, abolish cortical GLU release induced by CPP or MK-801 (Calcagno et al. 2009; Calcagno et al. 2006; Ceglia et al. 2004; Lopez-Gil et al. 2009). In addition, the mGlu 2/3 receptor agonist LY379268 reversed CPP-induced impairment in attentional functioning and the effect on cortical GLU release (Pozzi et al., submitted). Thus, these findings strongly favour the hypothesis that excessive cortical GLU release is deleterious for cognitive functions dependent on the mPFC (Moghaddam and Adams 1998).
Possible limitation to this interpretation is the disparity in the CPP administration between behavioural and microdialysis experiments. As in behavioural experiments CPP was injected into the mPFC for 1 min while continuous infusion over 1 h was used in microdialysis experiments, it may be argued that the two conditions are not comparable. However, we found that the intracortical injection of 50 ng/μl CPP, the same dose and route used in the 5-CSRTT, yielded a rapid increase of extracellular GLU which was similar in magnitude to that observed after its infusion through the probe (Calcagno et al. 2009). It should be noted that CPP’s effects on GLU was measured in behaviourally naïve rats and thus, it could not be assumed that CPP would have had similar effect on GLU in rats performing the 5-CSRTT.
As summarized in Table 3, no consistent relationship can be established between the CPP-induced impulsivity (as measured by anticipatory responses), or perseverative responding and changes in prefrontocortical GLU release (Calcagno et al. 2006; Carli et al. 2006).
Impulsivity in the 5-CSRTT has been consistently associated with changes in endogenous 5-HT stores and release (Carli and Samanin 2000; Harrison et al. 1997; Winstanley et al. 2004a). However, no association between the increase in 5-HT release in the PFC and CPP-induced impulsivity, impairment in accuracy or perseverative over-responding (Table 3) was reported (Calcagno et al. 2006; Carli et al. 2006). Thus, the ability of sertindole and clozapine to reverse CPP-induced rise in 5-HT release (Figs. S1 and S2) is unlikely to have contributed to their ability to control accuracy or other aspects of performance deficits.
The association between cognitive deficits and enhanced GLU release is in contrast with the hypothesized hypofunction of NMDA receptors in schizophrenia. However, an unbalance between excitatory and inhibitory drive due to the loss of cortical GABA interneurons (Benes and Berretta 2001; Lewis and Moghaddam 2006) has been suggested to underlie the schizophrenia pathophysiology (Gordon 2010). Enhanced GLU release in response to blockade of NMDA receptors probably reflects the reduced drive of cortical inhibitory interneurons (Homayoun and Moghaddam 2007; Jackson et al. 2004). Consistently, NMDA receptor antagonists including CPP, reduced extracellular GABA in the rat mPFC (Calcagno et al. 2009; Yonezawa et al. 1998). In addition, blockade of NMDA receptors caused cortical activation in rodents (Gozzi et al. 2008; Homayoun and Moghaddam 2007; Jackson et al. 2004; Suzuki et al. 2002) and these effects were prevented by the blockade of 5-HT2A receptors or stimulation of mGlu 2/3 receptors (Gozzi et al. 2010; Homayoun et al. 2005), which also prevented the release of GLU induced by NMDA receptor blockade (Ceglia et al. 2004; Moghaddam and Adams 1998). Cortical activation in response to the NMDA receptor antagonist ketamine was also observed in human volunteers (Breier et al. 1997; Vollenweider et al. 1997), suggesting that NMDA receptors of the prefrontal cortex may be involved in mediating the cognitive impairment caused by this drug both in humans and rodents. Although we found a consistent relationship between the ability of drugs to improve CPP-induced attention deficits and suppress cortical GLU release (summarized in Table 3), it cannot be ruled out that other mechanisms, such as an increase in cortical DA release, may have contributed (see Robbins 2002).
One of the features of the 5-CSRTT is that different aspects of attention control, such as attentional capacity, as indexed by accuracy of correctly reporting the location of a brief visual stimulus, and the inhibitory response control related to executive attention processes and assessed by anticipatory and perseverative responses, are under control of distinct neural substrates and neurotransmitter mechanisms (Chudasama and Robbins 2006; Passetti et al. 2002; Robbins 2002). These measures of executive attention processes may also be dissociated at the level of receptor mechanisms (Besson et al. 2010; Carli et al. 2006; Granon et al. 2000; Winstanley et al. 2003; Winstanley et al. 2004b). Specifically, accuracy has been shown to depend on prefronto-cortical dopamine D1 (Granon et al. 2000) as well as serotonin 5-HT1A and 5-HT2A receptors (Carli et al. 2006; Winstanley et al. 2003) whereas the role of D2 receptors (Baviera et al. 2008; Granon et al. 2000) is controversial as D2 antagonists have been reported to have no effect but also to prevent the accuracy deficit (Passetti et al. 2003; Pezze et al. 2009). In addition, serotonin 5-HT2A and 5-HT1A and dopamine D2 receptors exert differential control over anticipatory and perseverative responding (Baviera et al. 2008; Carli et al. 2006).
Although the mechanisms accounting for the observed findings were not addressed in the present study, comparing the effects of sertindole and clozapine with those of selective antagonists at neurotransmitter receptors may help suggest possible mechanisms by which these drugs might improve different aspects of cognitive abilities.
Sertindole and clozapine resembles the 5-HT2A receptor antagonist, M100907, in preventing CPP-induced GLU release and deficits in accuracy and impulsivity but not perseverative responding (Ceglia et al. 2004; Higgins et al. 2003; Mirjana et al. 2004). The lowest doses of sertindole preventing these effects preferentially occupy 5-HT2A receptor in vivo (ED50, 0.09–0.13 mg/kg) (Idris et al. 2010; Schotte et al. 1996) and are close to the ED50 values for the antagonism of 5-HT2A receptor agonists-induced head twitches (0.015 mg/kg) and of DOI-induced discriminative stimulus (0.034 mg/kg) (Sanchez and Arnt 2000).
Sertindole is a 5-HT2C, 5-HT6, D2 and α1-adrenoceptor antagonist (Arnt 1992; Schotte et al. 1996). Blockade of some of these receptors either alone or in combination might contribute or limit sertindole’s effects on attention and GLU release. Specifically, the α1-adrenoceptor antagonist prazosin prevented GLU release induced by the non-competitive NMDA receptor antagonist MK-801 (Lopez-Gil et al. 2009). By contrast, blockade of 5-HT2C receptors is unlikely to contribute to sertindole’s effects on attention and GLU release as the selective antagonist SB242084 had opposite or no effects (Calcagno et al. 2009; Higgins et al. 2003).
D2 receptor antagonism may contribute to the effect of sertindole on impulsivity, an effect shared by haloperidol, but hardly explains the efficacy of this drug on correct responses (Baviera et al. 2008). The picture might be even more complex with clozapine, which shares the affinity for some 5-HT and DA receptor subtypes with sertindole, but in addition interacts with muscarinic, histaminergic H1, and α2-adrenergic receptors (Arnt and Skarsfeldt 1998; Schotte et al. 1996).
The effects of sertindole on CPP-induced attention deficits and cortical GLU release are biphasic as low doses (0.02–0.32 mg/kg) prevented CPP’s effects while at 0.6–2.5 mg/kg it had no effect or even enhanced anticipatory responding, an effect shared by the 5-HT2C receptor antagonist SB242084 (Higgins et al. 2003). This suggests that high doses of sertindole may act on mechanisms, such as 5-HT2C receptors, that mask the ability of sertindole to prevent CPP effects. Consistently, at doses of 0.5 mg/kg or above, sertindole reversed 5-HT2C receptor-mediated discriminative stimulus of MK-212, a preferential 5-HT2C receptor agonist (Sanchez and Arnt 2000) and blockade of these receptors by SB242084 prevented the effect of M100907 on CPP-induced GLU release in the rat mPFC (Calcagno et al. 2009). By contrast, the stimulation of 5-HT2C receptors with Ro60-0175 mimicked the effects of M100907 on GLU and CPP-induced deficits in accuracy and impulsivity in the 5-CSRTT (Calcagno et al. 2009). However, it cannot be excluded that other mechanisms may account for the inverted U-shaped dose-dependent effects of sertindole on attention and GLU release.
A non-significant, transient increase of basal GLU release was observed in rats given 2.5 mg/kg sertindole (present study) and previous studies showed that 2.5 and 10 mg/kg sertindole significantly increased basal extracellular GLU in the rat mPFC (Mork et al. 2007, 2009). This effect is shared by the selective 5-HT6 receptor antagonist SB271046 suggesting a possible contribution of these receptors to sertindole action on GLU. Interestingly, 1.3 and 2.5 mg/kg sertindole and the selective 5-HT6 receptor antagonist SB271046 abolished the deficit caused by subchronic phencyclidine in an attentional set shifting task. Clozapine, olanzapine, haloperidol and M100907 were ineffective in this task while they consistently reduced the effects of NMDA receptor antagonists in the 5-CSRTT (Baviera et al. 2008; Higgins et al. 2003; Rodefer et al. 2008).
Four hours after oral administration of 2.5 mg/kg sertindole, the plasma concentration in food deprived Lister Hooded rats was 290 ± 43 ng/ml (mean ± SEM), which is higher than those reported in previous studies (Didriksen et al. 2006; Olsen et al. 2008; Rodefer et al. 2008) suggesting that the lack of effect of high doses of sertindole cannot be accounted for by insufficient drug exposure. The discrepancies between the effective doses of sertindole in the 5-CSRTT and attentional set shifting might depend on differences in underlying neurochemical changes induced by acute and subchronic blockade of NMDA receptors. Acute and subchronic NMDA receptor antagonists had opposite effects on extracellular GLU in the mPFC (Fattorini et al. 2008). However, differences in cognitive processes taxed by the tasks employed in different studies could not be disregarded since olanzapine and clozapine, which had no effects in attentional set shifting (Rodefer et al. 2008) significantly attenuated the effect of subchronic PCP in a reversal learning paradigm (Abdul-Monim et al. 2006).
The present study shows that sertindole ameliorates NMDA receptor-dependent deficit in attentional functioning and suppresses GLU release in the mPFC most likely by blocking 5-HT2A receptors. These findings support the proposal that excessive GLU release impairs cognitive functions and clinical observation that neurocognitive measures reflecting patients’ capacity to maintain vigilance in the Continuous Performance Test, the human analogous of the rat 5-CSRTT, improve the most in schizophrenic patients receiving atypical antipsychotics that are potent 5-HT2A receptor antagonists (Keefe et al. 2004; Keefe et al. 1999; Meltzer and McGurk 1999). However, this conclusion is somewhat limited by the results of clinical trials addressing the beneficial effect of antipsychotic drugs in ameliorating cognitive deficit of schizophrenia, which indicated their limited efficacy and substantial similarity between novel and older drugs (Green et al. 2002; Keefe et al. 2007).
In conclusion, suppression of GLU release might be a target for the development of novel therapeutic strategies aimed at counteracting some aspects of cognitive deficits of schizophrenia.
References
Abdul-Monim Z, Reynolds GP, Neill JC (2006) The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res 169:263–73
Arnt J (1992) Sertindole and several antipsychotic drugs differentially inhibit the discriminative stimulus effects of amphetamine, LSD and St 587 in rats. Behav Pharmacol 3:11–18
Arnt J, Skarsfeldt T (1998) Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18:63–101
Baviera M, Invernizzi RW, Carli M (2008) Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 196:269–80
Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27
Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW (2010) Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology 35:560–569
Braff DL (1993) Information processing and attention dysfunctions in schizophrenia. Schizophr Bull 19:233–259
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D (1997) Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 154:805–811
Calcagno E, Carli M, Baviera M, Invernizzi RW (2009) Endogenous serotonin and serotonin2C receptors are involved in the ability of M100907 to suppress cortical glutamate release induced by NMDA receptor blockade. J Neurochem 108:521–532
Calcagno E, Carli M, Invernizzi RW (2006) The 5-HT(1A) receptor agonist 8-OH-DPAT prevents prefrontocortical glutamate and serotonin release in response to blockade of cortical NMDA receptors. J Neurochem 96:853–560
Carli M, Baviera M, Invernizzi RW, Balducci C (2006) Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology 31:757–767
Carli M, Calcagno E, Mainolfi P, Mainini E, Invernizzi RW (2010) Effects of aripiprazole, olanzapine and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex. Psychopharmacology. doi:10.1007/s00213-010-2065-7
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Carli M, Samanin R (2000) The 5-HT1A receptor agonist 8-OH-DPAT reduces rats' accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT1A receptors. Psychopharmacology 149:259–268
Ceglia I, Carli M, Baviera M, Renoldi G, Calcagno E, Invernizzi RW (2004) The 5-HT receptor antagonist M100, 907 prevents extracellular glutamate rising in response to NMDA receptor blockade in the mPFC. J Neurochem 91:189–199
Chudasama Y, Robbins TW (2006) Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73:19–38
Didriksen M, Kreilgaard M, Arnt J (2006) Sertindole, in contrast to clozapine and olanzapine, does not disrupt water maze performance after acute or chronic treatment. Eur J Pharmacol 542:108–115
Didriksen M, Skarsfeldt T, Arnt J (2007) Reversal of PCP-induced learning and memory deficits in the Morris' water maze by sertindole and other antipsychotics. Psychopharmacology (Berl) 193:225–233
Fattorini G, Melone M, Bragina L, Candiracci C, Cozzi A, Pellegrini Giampietro DE, Torres-Ramos M, Perez-Samartin A, Matute C, Conti F (2008) GLT-1 expression and Glu uptake in rat cerebral cortex are increased by phencyclidine. Glia 56:1320–1327
Frith CD (1987) The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 17:631–648
Gallhofer B, Jaanson P, Mittoux A, Tanghoj P, Lis S, Krieger S (2007) Course of recovery of cognitive impairment in patients with schizophrenia: a randomised double-blind study comparing sertindole and haloperidol. Pharmacopsychiatry 40:275–286
Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG (2007) Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry 64:1115–1122
Gordon JA (2010) Testing the glutamate hypothesis of schizophrenia. Nat Neurosci 13:2–4
Gozzi A, Crestan V, Turrini G, Clemens M, Bifone A (2010) Antagonism at serotonin 5-HT(2A) receptors modulates functional activity of frontohippocampal circuit. Psychopharmacology (Berl) 209:37–50
Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A (2008) Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology 33:1690–1703
Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000) Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20:1208–1215
Green MF, Marder SR, Glynn SM, McGurk SR, Wirshing WC, Wirshing DA, Liberman RP, Mintz J (2002) The neurocognitive effects of low-dose haloperidol: a two-year comparison with risperidone. Biol Psychiatry 51:972–978
Harrison AA, Everitt BJ, Robbins TW (1997) Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133:329–342
Harvey PD, Keefe RS (2001) Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 158:176–184
Higgins GA, Enderlin M, Haman M, Fletcher PJ (2003) The 5-HT(2A) receptor antagonist M100, 907 attenuates motor and 'impulsive-type' behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 170:309–319
Homayoun H, Jackson ME, Moghaddam B (2005) Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol 93:1989–2001
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Idris N, Neill J, Grayson B, Bang-Andersen B, Witten LM, Brennum LT, Arnt J (2010) Sertindole improves sub-chronic PCP-induced reversal learning and episodic memory deficits in rodents: involvement of 5-HT(6) and 5-HT (2A) receptor mechanisms. Psychopharmacology (Berl) 208:23–36
Jackson ME, Homayoun H, Moghaddam B (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A 101:8467–8472
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308
Kane JM, Tamminga CA (1997) Sertindole (Serdolect): preclinical and clinical findings of a new atypical antipsychotic. Expert Opin Investig Drugs 6:1729–1741
Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA (2007) Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry 64:633–647
Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA (2004) Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry 161:985–995
Keefe RS, Silva SG, Perkins DO, Lieberman JA (1999) The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 25:201–222
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995) Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13:9–19
Lewis DA, Moghaddam B (2006) Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol 63:1372–1376
Lopez-Gil X, Artigas F, Adell A (2009) Role of different monoamine receptors controlling MK-801-induced release of serotonin and glutamate in the medial prefrontal cortex: relevance for antipsychotic action. Int J Neuropsychopharmacol 12:487–499
Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF (1962) Model psychoses and schizophrenia. Am J Psychiatry 119:61–67
Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A (1997) Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17:141–150
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255
Mirjana C, Baviera M, Invernizzi RW, Balducci C (2004) The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology 29:1637–1647
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352
Mork A, Witten LM, Arnt J (2007) Differentiating effects of sertindole and risperidone on extracellular levels of neurotransmitters in the frontal cortex of conscious rats. In: Neuroscience So (ed), XXXVII Annual Meeting of the Society of Neuroscience, San Diego, CA, pp 500.13
Mork A, Witten LM, Arnt J (2009) Effect of sertindole on extracellular dopamine, acetylcholine, and glutamate in the medial prefrontal cortex of conscious rats: a comparison with risperidone and exploration of mechanisms involved. Psychopharmacology (Berl) 206:39–49
Olsen CK, Brennum LT, Kreilgaard M (2008) Using pharmacokinetic-pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur J Pharmacol 584:318–327
Passetti F, Chudasama Y, Robbins TW (2002) The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 12:1254–1268
Passetti F, Levita L, Robbins TW (2003) Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav Brain Res 138:59–69
Paxinos G, Watson C (1986) The Rat Brain in Stereotaxic Coordinates. Academic, San Diego, CA
Pezze MA, Dalley JW, Robbins TW (2009) Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl) 202:307–313
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Robinson TE, Whishaw IQ (1988) Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res 450:209–224
Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J (2008) Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology 33:2657–2666
Sanchez C, Arnt J (2000) In-vivo assessment of 5-HT2A and 5-HT2C antagonistic properties of newer antipsychotics. Behav Pharmacol 11:291–298
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124:57–73
Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y (2002) Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience 114:769–779
Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J (1997) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7:25–38
Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167:304–314
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004a) Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29:1331–1343
Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004b) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176:376–385
Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H (1998) Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol 341:45–56
Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, Sebree TB, Wallin BA, Kashkin KB (1997) Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group Am J Psychiatry 154:782–791
Acknowledgements
This work was supported by a research grant from H. Lundbeck A/S, Denmark.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Effects of sertindole on CPP-induced rise of extracellular 5-HT in the mPFC. Sertindole (S) or vehicle (V) were given orally (arrows) 4 h before the infusion of 100 μM CPP (a) or aCSF (b) through the probe. Horizontal bar indicates the duration of CPP infusion. Experimental groups in a were as follows: V + aCSF (n = 6), V + CPP (n = 6), S0.02 mg/kg + CPP (n = 5), S0.32 mg/kg + CPP (n = 5), S2.5 mg/kg + CPP (n = 6). b V + aCSF the same as in a (dotted line), S0.02 mg/kg + aCSF (n = 5), S0.32 mg/kg + aCSF (n = 5) and S 2.5 mg/kg + aCSF (n = 5). Data are expressed as mean percentages of basal values ± SEM. For the sake of clarity, data from 20 to 220 min were omitted from a. The whole curves are shown in Fig. S1. *P < 0.05 versus basal values (Tukey’s test) (DOC 183 kb)
Fig. S2
Effects of clozapine on CPP-induced rise of extracellular 5-HT in the mPFC. Clozapine (CLOZ) or vehicle (V) were given orally (arrows) 20 min before the infusion of 100 μM CPP or aCSF through the probe. Horizontal bar indicates the duration of CPP or aCSF infusion. Experimental groups were as follows: V + V, V + CPP, CLOZ + CPP, CLOZ + aCSF. Data are expressed as percentages of basal values and are the mean ± SEM of five rats per group. *P < 0.05 versus basal values (Tukey’s test) (DOC 81 kb)
Rights and permissions
About this article
Cite this article
Carli, M., Calcagno, E., Mainini, E. et al. Sertindole restores attentional performance and suppresses glutamate release induced by the NMDA receptor antagonist CPP. Psychopharmacology 214, 625–637 (2011). https://doi.org/10.1007/s00213-010-2066-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2066-6