Abstract
Rationale
Anti-psychotic drugs are widely recognised to produce beneficial effects on impaired cognition in schizophrenia but their mechanism of action is poorly understood. The prefrontal cortex (PFC) and nucleus accumbens (NAC) are key brain loci considered to mediate many of the cognitive deficits associated with schizophrenia and related disorders.
Objectives
To investigate (1) the effects of selective damage to the PFC on visuo-spatial attention and cognition in the rat and (2) the ability of the anti-psychotic drug sulpiride after its intra-NAC administration to ameliorate cognitive and behavioural deficits produced by lesions of the PFC.
Methods
Selective lesions of the medial PFC were made using quinolinic acid in rats previously trained on a five-choice serial reaction time task of sustained visual attention (n = 7). Sham rats received phosphate-buffered saline infusions (n = 7). Following a period of recovery, low doses of sulpiride (0.5ng or 1ng) were infused into the core sub-region of the NAC of sham and lesioned rats immediately prior to testing on the five-choice task.
Results
Lesions of the medial PFC produced a range of impairments on the five-choice task, including decreased attentional accuracy, slower latencies to respond correctly and increased omissions and premature responses, the latter an operational measure of impulsivity. Intra-NAC sulpiride dose-dependently ameliorated the increased impulsivity and attentional impairment present in PFC-lesioned rats.
Conclusions
These findings suggest that attentional and cognitive impairment in schizophrenia may be determined in part by a dysregulation of the subcortical dopamine systems occurring as a consequence of damage to the PFC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the current interest in the possible treatment of cognitive deficits associated with schizophrenia, which have been associated with dysfunction of hippocampal and prefrontal cortical mechanisms, there is controversy as to how anti-psychotic medications themselves impact on cognition. Chronic treatment of experimental animals with a ‘typical’ neuroleptic, the dopamine D2/3 receptor antagonist haloperidol, produces deficits in spatial delayed response (‘working memory’) performance that are remediable by systemic injections of a dopamine D1 receptor agonist (Castner et al. 2000). By contrast, although it is difficult to design definitive studies, much of the literature suggests that typical neuroleptics have an ameliorating influence on cognition in schizophrenia itself, possibly because of their effective anti-psychotic actions (Mortimer 1997).
There are no established disease models of schizophrenia to date, although it is possible to simulate some of the known deficits by brain lesions that disturb cognition (Lipska and Weinberger 2000). The prefrontal cortex (PFC) is central to many of the cognitive deficits in schizophrenia although it is also implicated in other neuropsychiatric disorders such as attention deficit/hyperactivity disorder (ADHD) (Arnsten 2006). These lesion models can thus be used to assess possible therapeutic actions of cognitive-enhancing drugs, as well as the effects of typical and atypical neuroleptics.
In a previous study we employed the five-choice serial reaction time task (5CSRTT) to reveal deficits in visual sustained attention and executive control following excitotoxic lesions of the medial prefrontal cortex (mPFC)—and to assess the impact of dopamine D1 and D2/3 receptor antagonists administered systemically (Passetti et al. 2003). To our surprise, we found that sulpiride, a dopamine D2/3 receptor antagonist, when administered systemically, significantly improved discriminative accuracy on the 5CSRTT. Although a dopamine D1 receptor antagonist, SCH23390, also improved performance, this effect was less marked than that produced by sulpiride. The results were interpreted as consistent with the hypothesis that the mPFC lesion had up-regulated striatal dopamine, consistent with many lines of evidence (e.g. (Braun et al. 1993; Iversen 1971; Jaskiw et al. 1990; Roberts et al. 1994; Wilkinson et al. 1997). Moreover, previous evidence following similar mPFC lesions had shown that there were enhanced cellular and behavioural responses to d-amphetamine in the nucleus accumbens (NAC) (Dalley et al. 1999), suggesting the latter to be the key striatal site mediating the ameliorative effects of sulpiride. Consequently, in this study we directly tested the hypothesis that this amelioration is mediated by dopamine D2/3 receptors in the NAC by infusing sulpiride there in rats with deficits in 5CSRTT performance produced by mPFC lesions.
This experiment also enabled us to examine a further hypothesis relating to top-down inhibitory control of responding by the mPFC. Lesioning the mPFC, especially the infralimbic (IL) region, increases premature (‘impulsive’) responses on the 5CSRTT (Chudasama et al. 2003). This measure of impulsivity has recently been shown to be predictive of escalating rates of self-administration of cocaine on a ‘chronic binge access’ schedule, and correlated with changes in dopamine D2/3 receptors in the NAC (Dalley et al. 2007). Consequently, this study was also able to test the hypothesis that mPFC lesions might increase premature responding which could also be re-mediated via dopamine D2/3 receptor blockade within the NAC.
Materials and methods
Subjects
The subjects were 16 male, hooded Lister rats (Charles River, UK) weighing about 260g at the start of the experiment and housed in pairs under temperature-controlled conditions and alternating 12-h light–12-h dark cycle (lights on at 0700h). They were maintained at 80–85% of their free-feeding weight by restricting access to laboratory chow (Purina, UK) to 18g/day per rat. Water was provided ad libitum. All procedures complied with the requirements of the UK Animals (Scientific Procedures) Act 1986 and in accordance with local institutional guidelines at Cambridge University.
Apparatus
A detailed description of the nine-hole apparatus has been provided previously (Carli et al. 1983; Robbins 2002). Briefly, eight 25 × 25 × 25-cm nine-hole boxes (Paul Fray, UK) were used, each contained within a ventilated and sound-attenuated chamber and illuminated by a 3-W house light. Nine evenly spaced square holes (2.5 × 2.5 × 4cm) containing a 3-W light were set into the curved aluminum wall at the rear of the box, 2cm above the grid floor. A metal cap blocked holes 2, 4, 6 and 8. An infrared beam located at the entrance of each hole enabled detection of nose-poke responses. Every other hole was blocked so that only five of the holes were accessible. A food magazine, into which food pellets (Noyes dustless pellets, 45mg; Sandown Scientific, UK) could be dispensed, was located in the middle of the opposite wall. An infrared beam located horizontally across the entrance to the magazine allowed recording of entries into the magazine. The distance from the centre hole at the rear of the box and the magazine was 25cm. The apparatus and on-line data collection were controlled by means of an Archimedes computer system with software written in ARACHNID (Paul Fray, Cambridge, UK).
Behavioural training
Rats were trained on the 5CSRTT to a stable level of performance as described previously (Granon et al. 2000; Pezze et al. 2007). Briefly, the rats initiated a trial by nose-poking into the magazine. After an inter-trial interval (ITI) of 5s the light at the rear of one of the apertures was presented randomly in one of the five possible locations for a short period of 0.5s. Responses in this aperture within a limited time of illumination of the hole (limited hold period) were recorded as correct responses and were rewarded by the delivery of a food pellet to the magazine. Responses in a non-illuminated hole (incorrect responses), failure to respond within the 5s limited hold period (omission), and responses in one of the apertures during the ITI (premature or impulsive responses) were also recorded and punished by a time-out period of 5s, during which the illumination of the chamber was switched off. The stimulus duration was 30s in the initial training sessions and then progressively reduced to the final duration used during testing (0.5s), depending on the rats’ individual performance. Rats reached the criterion of stable pre-operative performance when they achieved ≥80% accuracy with fewer than 20% omissions. An average of 30 daily sessions, each consisting of approximately 100 trials and lasting 30min was required to reach this criterion. Task performance was assessed by the following behavioural measures:
-
Accuracy: proportion of correct to total correct + incorrect trials, expressed as a percentage.
-
Omissions: proportion of omissions to total trials (correct + incorrect + omission trials), expressed as a percentage.
-
Premature responses: proportion of premature responses to total trials (correct + incorrect + omission trials), expressed as a percentage.
-
Correct response latency: mean duration between the stimulus onset and a nose poke in a correct hole (ms).
-
Magazine latency: mean duration between a nose poke in the correct hole and entry into the food magazine (ms).
-
Perseverative responses: proportion of repeat responses in the response apertures to total trials, expressed as a percentage. Such responses were not punished by a 5s time-out period or cancellation of food reward.
Surgery
After acquisition of the 5CSRTT animals were anaesthetised with Avertin (10g 99% 2,2,2-tribromoethanol (Sigma-Aldrich, Dorset, UK) in 5mg tertiary amyl alcohol and 4.5ml phosphate-buffered saline (PBS), in 40ml ethanol), administered intraperitoneally (1ml/100g), and secured in a stereotaxic frame with the incisor bar set at −3.3mm relative to the interaural line. Eight rats received mPFC lesions induced by infusing 0.09M quinolinic acid (in PBS) according to the following stereotaxic coordinates (mm from bregma or from dura): AP + 3.8; L ± 0.7, DV −1.5 (0.5μl); AP + 3.2; L ± 0.7, DV −3.0 and −1.5 (0.5μl); and AP + 2.6; L ± 0.7, DV −1.5 (0.7μl). As a control, eight rats received sham infusions of PBS at the same coordinates as above. Injections were made using a microsyringe mounted in a Harvard infusion pump and connected to a 30-gauge stainless steel cannula. The quinolinic acid solution was prepared freshly each day. The injection volume was infused over a period of 2min and cannula were left in place for a further 2min. Bilateral cannulae (Plastics One, UK) consisting of a plastic body holding two 21-gauge metal tubes 3.8mm apart and projecting 4mm from the pedestal were implanted 5mm above the injection site in the NAC, at the following coordinates (mm from bregma or from skull): AP + 1.5; L ± 1.9; DV −2.2. (Pezze et al. 2007). Cannulae were secured to the skull with dental acrylic and stainless-steel screws. A wire stylet occluded the guide to maintain patency. One of the sham-operated rats did not recover from surgery leaving seven animals for the control group.
Post-surgical behavioural testing
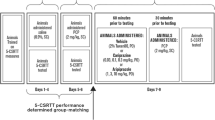
Two weeks after surgery, the rats were tested over nine consecutive daily sessions on the standard schedule of the task in order to establish stable post-operative performance (ITI = 5s, stimulus duration 0.5s). Subsequently, rats were habituated to the infusion procedure during a single session with a single insertion of a non-drug-primed injector for 2min. The infusion study was run over 3-day cycles, each starting with a baseline session. The following day, rats received an infusion of sulpiride or vehicle 5min before testing on the 5CSRTT. On the third day, animals were not tested and remained in their home cage. On each infusion day the stimulus duration was decreased to 0.1s to avoid possible ceiling effects of improved performance under sulpiride. The different drug doses were tested in a counterbalanced order using a Latin-square design.
During each infusion rats were gently restrained while the stylets were removed and the 28-gauge bilateral injector protruding 5mm below the end of the guide cannulae was inserted into the NAC. A volume of 0.5μl of the drug solution or vehicle was infused over a period of 1min into the NAC. The injectors were left in place for a further 1min to allow the diffusion of the drug in the NAC. The injector was then removed and the stylet replaced. Five minutes later rats were tested on the 5CSRTT.
(-)-Sulpiride (Research Biomedicals, Nattick, MA, USA) was freshly prepared each day in acidified 0.9% sodium chloride (0.5ng or 1ng/0.5μl; pH~7). Doses were chosen on the basis of a previous study (Pezze et al. 2007).
Histology
Following completion of the behavioural testing, subjects were given an overdose of sodium pentobarbitone (200mg/ml) and perfused transcardially with 0.01M PBS followed by 4% paraformaldehyde. The brains were removed and post-fixed in 4% paraformaldehyde. Prior to being sectioned the brains were cryo-protected in 20% sucrose over night at 4°C. Coronal sections of 40μm were cut on a freezing microtome, mounted on gelatinised slides and stained with Cresyl violet.
Statistical analysis
Data were subjected to ANOVA (SPSS, 12.0.1, Chicago, IL, USA) with ‘lesion’ as a between-subjects factor and ‘dose’ and ‘day’ as within-subjects factors. Further comparisons were made using Fisher’s PLSD tests where appropriate. All tests of significance were performed at α = 0.05.
Results
Histology
Histological examination revealed that one subject had a unilateral mPFC lesion and hence was discarded from further analysis. The final lesion group size was therefore reduced to seven. Figure 1 shows the extent of the lesion in the mPFC and a reconstruction of the injector tip placements within the NAC. It can be seen that the lesion encompassed the anterior cingulate cortex, prelimbic cortex, and infralimbic cortex and that the injector tips were located in the NAC core sub-region 1.2 and 2.2mm forward of bregma.
Schematic representation of the maximum extent of the mPFC lesion (bold line) and injector tip locations in the nucleus accumbens. Adapted from Paxinos and Watson (1996)
Effects of mPFC lesions on attentional performance
Figure 2 summarises the effects of selective excitotoxic lesions of the mPFC on attentional performance on the 5CSRTT. Data from the final 3 days of post-surgical testing were used to assess the effects of the lesion on performance, which included a significant reduction in attentional accuracy (lesion × time, F 2,24 = 0.35, p = 0.55; lesion, F 1,12 = 8.02, p < 0.02), an increase in omissions (lesion × time, F 2,24 = 0.35, p = 0.71; lesion, F 1,12 = 7.74, p < 0.02) and premature responses (lesion × time, F 2,24 = 0.33, p = 0.72; lesion, F 1,2 = 8.17, p < 0.02) and slower correct response latencies (lesion × time, F 2,24 = 4.03, p < 0.04; lesion, F 1,12 = 14.48, p < 0.003). There was no significant effect of the PFC lesions on total trials completed, magazine latency or collect latencies. Although visual inspection of the data shown in Fig. 2 suggests an increase in perseveration, this effect failed to reach statistical significance (lesion, F 1,12 = 2.71, p = 0.125).
Summary of the main effects of the mPFC lesions on attentional performance on the 5CSRTT. Data show mean behavioural performance of sham control rats (open circles, n = 7) and mPFC-lesioned rats (closed circles, n = 7) averaged over the final 3 days of post-operative training (mean ± SEM). Latencies expressed as centiseconds
Effects of intra-NAC (-)-sulpiride infusions in mPFC-lesioned rats
Low doses of sulpiride infused directly into the NAC significantly improved attentional accuracy and performance in mPFC-lesioned rats compared with sham-operated control rats (see Fig. 3). This improvement was significant relative to saline for the 0.5ng dose (saline versus 0.5ng dose, p < 0.02). By contrast, sulpiride had no significant effect on accuracy in the sham control group. Sulpiride did however increase omissions (dose, F 2,24 = 4.66, p < 0.02, an effect that was restricted to sham animals (dose, F 2,24 = 7.44, p < 0.008; saline vs 1ng, p < 0.003). Of note, sulpiride also significantly decreased the number of premature responses in mPFC-lesioned rats (lesion × dose, F 2,24 = 3.48, p < 0.05)]. Post-hoc tests revealed this effect to be present following both doses of sulpiride (saline versus 0.5ng and saline versus 1ng, both p < 0.03). However, sulpiride had no significant effect on correct response latencies, which were significantly slower in mPFC-lesioned rats (F 1,12 = 10.84, p < 0.007). Finally, there were no significant effects of sulpiride in PFC-lesioned rats on total trials completed, collect latency or perseveration.
Discussion
The main finding in this experiment was that the intra-accumbens administration of the relatively selective dopamine D2/3 receptor antagonist sulpiride dose-dependently ameliorated several of the behavioural deficits produced by lesions of the mPFC on the 5CSRTT. This is a striking result, replicating the amelioration also found by Passetti and colleagues (2003) but now highlighting the NAC as a likely site of action, based on the findings of Dalley et al (1999) concerning prefrontal cortex–nucleus accumbens interactions. There are several implications of these findings, perhaps the most important being that modulation of dopamine D2/3 receptors at the level of the NAC significantly blocked the ‘impulsive’ responding induced by the mPFC lesion. This is consistent with the notions that (1) ‘spontaneous’ impulsivity in the 5CSRTT is associated with altered dopamine D2/3 receptor function and (2) that this may arise in part from impaired top-down inhibitory control exerted by the mPFC on accumbens outflow. The findings are also relevant to understanding the factors that may lead to cognitive impairment in schizophrenia, as sulpiride, an ‘atypical’ anti-psychotic drug improved some aspects of attentional function and executive control resulting from prefrontal cortical impairment. This suggests that some, though probably not all, of the cognitive spectrum of deficits found in schizophrenia may result from a dysregulation of subcortical dopamine systems by prefrontal damage—just as hypothesised for the psychotic symptoms themselves (Weinberger 1987) and consistent with recent evidence in unmedicated schizophrenic subjects (Meyer-Lindenberg et al. 2002).
Intra-accumbens sulpiride tended to remove the impairment in discriminative accuracy as well as reducing the enhanced impulsivity in the mPFC-lesioned rats, suggesting effects on both attention and response control. In sham-operated rats, intra-accumbens sulpiride tended dose-dependently (though non-significantly) to reduce accuracy, as found in a previous study in intact rats (Pezze et al. 2007). Thus, although only the low dose of sulpiride significantly improved percent accuracy (from about 65% to 74%) in the mPFC-lesioned rats, the higher dose also greatly reduced the large performance differential between the lesion and sham-operated groups (see Fig. 3). The ameliorative effects of sulpiride were likely not secondary to simple motivational, sedative or tranquilising effects, as (1) the main motivational variable of latency to collect reward was unaffected by the drug (nor indeed by the lesion itself), (2) the accuracy measure is a choice variable which equates motor requirements and (3) because impulsivity was not reduced by the low dose of sulpiride in sham-operated rats. Moreover, impulsivity is not generally associated with impaired discriminative accuracy (Chudasama et al. 2003) and was halved in the mPFC-lesioned rats at the low dose of sulpiride, which had no effect on the sham controls. These latter findings are consistent with work showing that (1) intra-accumbens neuroleptics reduce impulsivity in rats, when produced for example by d-amphetamine (Cole and Robbins 1989; Pattij et al. 2007), (2) that d-amphetamine-induced impulsivity is blocked by lesions of the NAC shell region (Murphy et al. 2008) and (3) that dopamine D2/3 receptors in the NAC are altered in spontaneously hyper-impulsive rats (Dalley et al. 2007).
It is not possible at this time to define the precise anatomical sub-region of the NAC contributing to these effects, even though the majority of the cannulae placements successfully targeted the core sub-region. This is because of the likely diffusion of sulpiride within the ventral striatum, including the NAC shell. We do not consider that the small volume administered, and the rate of infusion employed, in the present study (0.5μl delivered over 1min) led to significant diffusion to neighbouring brain regions, as the estimated spread would be approximately only 1mm (Myers et al. 1971). However, we cannot completely exclude some possible dorsal striatal mediation of these effects. Nor can we be certain about the precise sub-region of PFC involved as the lesion encompassed the entire medial PFC although previous research indicates dissociable roles of the anterior cingulate cortex and infralimbic cortex in discriminative accuracy and response control, respectively (Chudasama et al. 2003; Murphy et al. 2005)
The precise mechanism by which sulpiride achieves its therapeutic effects in the mPFC-lesioned rats is also open to debate. However, it seems likely that the impairments in performance are partly caused by the sequelae of prefrontal damage operating on the striatal control of behavioural output via cortico-striatal ‘loops’ (Voorn et al. 2004). For example, the drug may ameliorate the gross distractibility and behavioural disorganisation produced by mPFC lesions, potentially via neuromodulatory effects on the cortical cholinergic system mediated by D2-like receptors in the NAC (Brooks et al. 2007). It is of course entirely possible that completely different approaches to remediation, for example, through boosting noradrenergic function (see (Chudasama et al. 2005; Newman et al. 2008) might also be effective in mPFC-lesioned rats. The present findings are also entirely compatible with the parallel findings of Ridley and colleagues (Ridley et al. 1993) in marmoset monkeys that perseverative choice in a visual discrimination task produced by prefrontal damage was ameliorated by the typical neuroleptic haloperidol, although we found no significant effects of mPFC lesions on the very different index of perseverative behaviour employed in this task.
Overall, the results reported in this study show that it is important to consider the possibility that brain damage can lead to deficits produced in part as sequelae to cascading changes in function of distal brain regions. Thus some, though not all, of the attentional and executive impairments resulting from prefrontal cortical dysfunction can be remediated by reducing the impact of dopamine at D2/3 receptors within the NAC. This finding serves to remind us that approaches to cognitive enhancement in complex neuropsychiatric disorders such as schizophrenia and ADHD will likely have to depend on several, somewhat independent, but hopefully compatible, therapeutic approaches.
References
Arnsten AF (2006) Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 67(Suppl 8):7–12
Braun AR, Jaskiw GE, Vladar K, Sexton RH, Kolachana BS, Weinberger DR (1993) Effects of ibotenic acid lesion of the medial prefrontal cortex on dopamine agonist-related behaviors in the rat. Pharmacol Biochem Behav 46:51–60
Brooks JM, Sarter M, Bruno JP (2007) D2-like receptors in nucleus accumbens negatively modulate acetylcholine release in prefrontal cortex. Neuropharmacology 53:455–463
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Castner SA, Williams GV, Goldman-Rakic PS (2000) Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 287:2020–2022
Chudasama Y, Nathwani F, Robbins TW (2005) D-Amphetamine remediates attentional performance in rats with dorsal prefrontal lesions. Behav Brain Res 158:97–107
Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Cole BJ, Robbins TW (1989) Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res 33:165–179
Dalley JW, Thomas KL, Howes SR, Tsai TH, Aparicio-Legarza MI, Reynolds GP, Everitt BJ, Robbins TW (1999) Effects of excitotoxic lesions of the rat prefrontal cortex on CREB regulation and presynaptic markers of dopamine and amino acid function in the nucleus accumbens. Eur J Neurosci 11:1265–1274
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270
Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000) Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20:1208–1215
Iversen SD (1971) The effect of surgical lesions to frontal cortex and substantia nigra on amphetamine responses in rats. Brain Res 31:295–311
Jaskiw GE, Karoum FK, Weinberger DR (1990) Persistent elevations in dopamine and its metabolites in the nucleus accumbens after mild subchronic stress in rats with ibotenic acid lesions of the medial prefrontal cortex. Brain Res 534:321–323
Lipska BK, Weinberger DR (2000) To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 23:223–239
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF (2002) Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 5:267–271
Mortimer AM (1997) Cognitive function in schizophrenia—do neuroleptics make a difference? Pharmacol Biochem Behav 56:789–795
Murphy ER, Dalley JW, Robbins TW (2005) Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 179:99–107
Murphy ER, Robinson ES, Theobald DE, Dalley JW, Robbins TW (2008) Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. Eur J Neurosci 28:353–363
Myers RD, Tytell M, Kawa A, Rudy T (1971) Micro-injection of 3H-acetylcholine, 14C-serotonin and 3H-norepinephrine into the hypothalamus of the rat: diffusion into tissue and ventricles. Physiol Behav 7:743–751
Newman LA, Darling J, McGaughy J (2008) Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 200:39–50
Passetti F, Levita L, Robbins TW (2003) Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav Brain Res 138:59–69
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 191:587–598
Paxinos G, Watson C (1996) The rat brain in stereotaxic co-ordinates. 3rd edition. Academic Press, San Diego
Pezze MA, Dalley JW, Robbins TW (2007) Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 32:273–283
Ridley RM, Clark BA, Durnford LJ, Baker HF (1993) Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience 52:595–604
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW (1994) 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci 14:2531–2544
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27:468–474
Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669
Wilkinson LS, Dias R, Thomas KL, Augood SJ, Everitt BJ, Robbins TW, Roberts AC (1997) Contrasting effects of excitotoxic lesions of the prefrontal cortex on the behavioural response to D-amphetamine and presynaptic and postsynaptic measures of striatal dopamine function in monkeys. Neuroscience 80:717–730
Acknowledgments
This study was conducted within the Cambridge University Behavioural and Clinical Neuroscience Institute and was funded by a Wellcome Trust Programme grant (076274/z/04/z) and a consortium joint award from the MRC and Wellcome Trust (G0001354). MAP was supported by a Marie Curie Fellowship and is currently supported by a Medical Research Scotland Jean-Baxter Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pezze, M.A., Dalley, J.W. & Robbins, T.W. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology 202, 307–313 (2009). https://doi.org/10.1007/s00213-008-1384-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1384-4