Abstract
Rationale
The dorsal raphé nucleus (DRN), the origin for serotonin (5-HT) in forebrain areas, has been implicated in the neural control of escalated aggression. Gamma aminobutyric acid type-A (GABAA) and type-B (GABAB) receptors are expressed in the DRN and modulate 5-HT neuronal activity, and both play a role in the behavioral effect of alcohol.
Objective
The purpose of this study is to examine the interaction between drugs acting on GABA receptors in the DRN and alcohol in their effects on aggressive behaviors.
Method
Male CFW mice, housed with a female, were trained to self-administer ethanol (1.0 g/kg) or water via an operant conditioning panel in their home cage. Immediately after they drank either ethanol or water, the animals were microinfused with a GABAergic drug into the DRN, and their aggressive behaviors were assessed 10 min later. Muscimol (0.006 nmol), a GABAA receptor agonist, escalated alcohol-heightened aggression but had no effect in the absence of ethanol. This effect of muscimol was prominent in the animals that showed alcohol-heightened aggression, but not the animals that reduced or did not change aggressive behavior after ethanol infusion compared to water. On the other hand, the GABAB agonist baclofen (0.06 nmol) increased aggressive behavior similarly in both water and ethanol conditions. Antagonists of the GABAA and GABAB receptors, bicuculline (0.006 nmol) and phaclofen (0.3 nmol) respectively, did not suppress heightened-aggressive behavior induced by ethanol self-administration.
Conclusion
GABAA receptors in the DRN are one of the neurobiological targets of alcohol-heightened aggression. Activation of the GABAB receptors in the DRN also produced escalated aggression, but that is independent of the effect of alcohol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than any other drug, alcohol has been linked to violence and aggression (Miczek et al. 2002, 2004b). Not every individual increases aggressive behavior after alcohol intake, but only a subset of individuals is prone to escalate aggressive behaviors under the influence of alcohol. Preclinical studies using rodents have shown that approximately 30% of individuals escalated aggressive behaviors under the influence of a moderate dose of ethanol (1.0 g/kg) relative to their base level of fighting in the absence of ethanol (Miczek et al. 1992, 1998). This individual difference on alcohol-heightened aggression has been reported in several species including humans, non-human primates, rats, and mice (Fulwiler et al. 2005; Higley et al. 1996; Miczek et al. 1998, 2004b; Virkkunen et al. 1996). The neurobiological basis for the vulnerability for alcohol-heightened aggression has begun to be investigated with a focus on ionotropic receptors.
Most prominently, γ-aminobutyric acid A (GABAA) receptors have been studied extensively as one of the major targets of alcohol (for review, see Kumar et al. 2009). Acute alcohol increases the conductance of Cl− flux through the GABAA receptors (Aguayo 1990; Allan and Harris 1987; Palmer and Hoffer 1990; Suzdak et al. 1986), and chronic exposure to alcohol changes the composition of receptor subunits in the synaptic membrane (Devaud et al. 1995; Liang et al. 2007; Matthews et al. 1998; Papadeas et al. 2001). Prototypical GABAA receptor positive modulators (e.g., benzodiazepines, barbiturates, and neurosteroids) can induce behavioral effects that are similar to those of alcohol including anxiolytic, anticonvulsant, sedative, hypnotic, and pro- and anti-aggressive effects (Barnard et al. 1998; Dar and Wooles 1985; Korpi et al. 2002; Liljequist and Engel 1982; Olsen 1982; Rabow et al. 1995; Sieghart 1995). Low to moderate doses of benzodiazepines and some neurosteroids increase aggressive behaviors in a receptor-selective manner in humans and other species; whereas, higher doses have anti-aggressive effects (Arnone and Dantzer 1980; Bond et al. 1995; Christmas and Maxwell 1970; Cole and Wolf 1970; DiMascio 1973; Ferrari et al. 1997; Fish et al. 2001; Miczek 1974; Olivier et al. 1985; Weerts and Miczek 1996; Weisman et al. 1998). This pattern of effects implicates the GABAA receptor complex as a target for aggression-heightening effects of alcohol. An anatomically discrete analysis is required to identify which brain pathways are regulated by the GABAA receptors to promote alcohol-heightened aggression.
Although there is no clear structural interaction with alcohol, GABAB receptors also appear to play a role in some of the behavioral effects of alcohol. Preclinical and clinical data have shown that the GABAB receptor agonists and positive modulators reduce alcohol withdrawal symptoms (Addolorato et al. 2006; Colombo et al. 2000; File et al. 1991) and also change self-administration of alcohol (Besheer et al. 2004; Colombo et al. 2003; Daoust et al. 1987; Flannery et al. 2004; Maccioni et al. 2008; Moore et al. 2007; Orru et al. 2005; Walker and Koob 2007). Alcohol can modulate the GABAB receptor expression in the rat cerebral cortex (Li et al. 2005), and electrophysiological studies have shown that the GABAB receptor modulates alcohol effects (Ariwodola and Weiner 2004; Wu et al. 2005). GABAB receptor activation can escalate aggressive behaviors when baclofen was systemically administered or microinjected into the dorsal raphé nucleus (DRN) in mice (Takahashi et al. 2010). Thus, it is possible that GABAB receptors are also involved in the alcohol-heightened aggression.
The serotonin (5-HT) system has long been implicated in neurobiological mechanisms of escalated aggression or violence (de Boer and Koolhaas 2005; Miczek et al. 2004a; Olivier 2004), and much evidence suggests the 5-HT system as a major target of alcohol-heightened aggression (Cloninger et al. 1989; Virkkunen et al. 1996; Virkkunen and Linnoila 1993). Gene expression analysis found reduced 5-HT receptor mRNA expressions in several forebrain areas in the male mice that engaged in alcohol-heightened aggression relative to the animals that did not change their aggressive behavior (Chiavegatto et al. 2010). Chronic treatment with an SSRI inhibited the heightened aggression induced by the alcohol without changing the species-typical aggression in mice (Caldwell and Miczek 2008). Therefore, differential activation of the 5-HT system by alcohol may contribute to the individual vulnerability for alcohol-heightened aggression. Forebrain 5-HT is mainly derived from the DRN (Azmitia and Segal 1978; Dahlstrom and Fuxe 1964; Michelsen et al. 2007).In addition to 5-HT cells, a large number of GABA neurons can be found in the DRN, and they modulate the activity of 5-HT neurons (Belin et al. 1983; Gervasoni et al. 2000; Nanopoulos et al. 1982; Wang et al. 1992). Both GABAA and GABAB receptors are expressed on the 5-HT neurons in the DRN and inhibit 5-HT cell firing (Bowery et al. 1987). Due to its role in the behavioral action of alcohol, GABA receptors may modulate the DRN 5-HT system after alcohol consumption and thus, change aggressive behaviors. In this study, we modulated the GABAA or GABAB receptors in the DRN pharmacologically and examined the resulting changes in alcohol-heightened aggression in male mice.
Methods
Subjects
Male CFW mice (Charles River Laboratories, Wilmington, MA), weighed 21–23 g upon arrival. Resident males were housed in pairs with females in a clear polycarbonate cage (28 × 17 × 14 cm) with pine shavings as bedding material. Intruder males were group housed seven to ten per large cage (48 × 26 × 14 cm) with corn cob bedding. All animals were maintained in our vivarium with controlled humidity and temperature (35–40%, 21 ± 1°C) on a reversed 12-h-light/dark cycle (lights off at 7:00 AM). Food (Purina, St. Louis, MO) was freely available, whereas availability of water was limited to 3 h per day. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University. The animals were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 1996).
Ethanol self-administration
After 21 h of water restriction, the female and offspring were removed to a holding cage during the experimental session, and a custom designed aluminum panel (16.5 × 15.9 cm) was inserted into the resident’s home cage (Miczek and de Almeida 2001). The panel has two nose-poke operanda with drinking troughs (3 × 5 cm) on the right and left sides of the panel with cue lights positioned above each operandum and one house light at the center top (Med Associates, Georgia, VT). The fluid receptacle was connected to a syringe containing either water or ethanol (6% w/v), operated by a syringe pump (Med Associates). The panel and syringe pump were controlled by MED-PC software running on a PC (MED-PC for Windows, v. 4.1; Med Associates). During the session, a house light and a cue light over the active operandum (right or left side, counterbalanced across the animals) were illuminated. A nose-poke response was detected by the photobeam sensor in the operanda, and every fifth response into the active operandum was reinforced by the delivery of 50 μl of fluid into the trough (schedule of reinforcement, fixed ratio five). The animals self-administered water or ethanol 5 days per week between 12:00 and 17:00, with ethanol (1.0 g/kg) being available every third experimental session and water on the intervening days.
Resident-intruder test and alcohol-heightened aggressive behavior
After 3 weeks of being housed with a female, the residents were studied for their aggression toward the same intruder male (Miczek and O’Donnell 1978). The female and the pups were removed, and an intruder was introduced into the home cage of the resident male. Their behaviors were recorded for 5 min after the first attack bite or the intruder was removed after 5 min if no attack occurred. This encounter occurred every other day until the animals showed a stable number of attack bites.
Once aggressive behavior had stabilized (< 20% variation), the residents were assessed for alcohol-heightened aggression. Fifteen minutes before the resident-intruder encounter, the residents self-administered water or 1.0 g/kg of ethanol (in an equal volume, 16.9 ml fluid/kg). Aggressive behavior was examined three times per week separated by 48 h, alternatively after consuming either water or ethanol for a total of three times for each condition. These encounters were videotaped and analyzed with the aid of software described later. Animals were categorized as alcohol-heightened aggressors (AHA) if the rate of attack bites after ethanol consumption exceeded that after water consumption by at least two standard deviations. The remaining mice were designated as alcohol non-heightened aggressors (ANA).
Surgery and cannulation
After the stabilization of attack bites, residents were anesthetized by i.p. injection of a mixture of 100 mg/kg of ketamine HCl and 10 mg/kg of xylazine, and stereotaxically implanted with a 26-gauge guide cannula (Plastics One Inc., Roanoke, VA) aimed at the DRN (AP, −4.2 mm; ML, +1.5 mm; DV, −1.9 mm from bregma; angled 26° from vertical) as calculated from a mouse brain atlas (Paxinos and Franklin 2001). A 33-gauge obturator (Plastics One Inc.) that extended 0.5 mm beneath the tip of the guide cannula was inserted after surgery. The obturator was moved daily to prevent blockage and also for habituating the animals to handling. The animals were housed individually for 5 days to recover and then pair-housed with the same female. To prevent gnawing by the female, the obturator and head mount were covered with a quinine polish (Bite it©). One week after the surgery, the residents were assessed for self-administration of ethanol or water again and for fighting before starting microinjection tests.
Microinjection and aggression test
On the test day, the resident mice self-administered water or 1.0 g/kg of ethanol immediately before the microinjection. The obturator was removed and a 33-gauge microinjector (Plastics One Inc, Roanoke, VA) attached to a PE-50 tubing was inserted into the guide cannula. The microinjector extended 2 mm below the end of the guide to reach the DRN. The other end of the tubing was connected to a Hamilton syringe placed into an infusion pump (CMA Microdialysis, North Chelmsford, MA). The drug was infused in a volume of 0.2 μl over 2 min. The microinjector was left in place for 1 min after the infusion to allow the drug to diffuse completely. Ten minutes after the microinjection, an intruder was introduced, which prompted attacks by the resident mouse. The animal’s behaviors were videotaped for detailed behavioral analysis at a later time. An animal received a total of six microinjections, following three drug conditions under both ethanol and water treatments: experiment 1, muscimol (0.006 nmol), bicuculline (0.006 nmol), and saline vehicle; experiment 2, baclofen (0.06 nmol), phaclofen (0.3 nmol), and saline vehicle. All drugs, purchased from Sigma-Aldrich (St. Louis, MO, USA), were dissolved in saline (0.9%). The drug treatments were administered in irregular sequence. The drug doses of muscimol and baclofen were selected based on previous work (Takahashi et al. 2010). The dose of phaclofen was chosen because it inhibited the effect of the 0.06 nmol of baclofen on aggressive behaviors. The dose of bicuculline was chosen on the basis of pilot studies. The selected dose of 0.006 nmol of bicuculline did not have any apparent effects on motor activity; whereas, higher doses of bicuculline (0.01 and 0.06 nmol) produced turning behavior or inhibited motor activity. Previous studies have estimated that the diffusion range of 1 μl muscimol (1 μg/μl) is about 1.7 mm from the injection site (Arikan et al. 2002; Edeline et al. 2002), but another study found that even 0.05 μl of muscimol can diffuse up to 1.4–1.7 mm from the injection site within 15 min (Martin 1991). In the present study, we used low concentrations of drugs with a 0.2 μl-injection volume and slower infusion rate. The animals that received infusions outside of the DRN did not show behavioral effects (Supplementary Table 1). Therefore, the effects of drugs are considered due to its action in the DRN.
Histology
At the end of the experiment, the mice were deeply anesthetized (ketamine and xylazine mixture) and intracardially perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) in phosphate-buffered saline. After post-fixation in the 4% PFA for at least 24 h, the brains were placed into 15% sucrose solution. A microtome was used to slice the brains (60 μm thickness), and the sections were stained with cresyl violet to verify the placement of the cannula. Table 1 summarizes the number of animals used in this study, and Fig. 1 shows the injection site in each animal. Four out of 22 animals in experiment 1 and two out of 16 animals in experiment 2 had infusions, which missed the DRN, and their data were analyzed separately.
Injection sites in the DRN. a Representative mouse coronal brain section (20×) that was stained with cresyl violet. b Schematic representation of injection sites for experiment 1 (GABAA) and experiment 2 (GABAB) in mouse coronal brain section (Paxinos and Franklin 2001). Circles indicate injection sites within the DRN and triangles represent injection sites outside the DRN
Blood ethanol concentration (BEC) measurement
For the analysis of the BECs, we used a new set of animals (n = 7). Because we found an interaction between muscimol and alcohol-heightened aggression, we examined the effect of muscimol on BEC after 1.0 g/kg of ethanol consumption. Once stable self-administration of water and ethanol was established, the animals were subjected to cannulation surgery under anesthesia. After 1 week of recovery, water and ethanol self-administration was reestablished. On the test day, the animals received muscimol (0.006 nmol) or vehicle microinjection into the DRN immediately after they had consumed ethanol or water. Ten minutes later blood samples were collected from their submandibular vein (250 μl) using animal lancets (MEDIpoint, Inc., NY, USA). Blood was collected in 1.5-ml-centrifuge tubes containing 50 μl of heparin (100 U/ml) on ice. Samples were centrifuged to separate plasma, and supernatants were stored at −80°C. Plasma ethanol concentrations were measured by using an Ethanol Assay Kit (BioVision, CA, USA) with high sensitivity (ranging from 0.4 to 40 ppm of ethanol).
Behavior analysis and statistics
Detailed behavioral analysis of the videotape record was performed by an observer whose reliability was established using the Observer software (Observer XT 8.0, Noldus, Wageningen, The Netherlands). The frequency of aggressive behaviors (attack bites, sideway threats, tail rattles, and pursuits) and the duration of non-aggressive behaviors (walking, rearing, grooming, and contact) were quantified as operationally defined and illustrated previously (Grant and Mackintosh 1963; Miczek and O’Donnell 1978). Repeated measures one-way ANOVA was performed to examine the effect of ethanol on aggressive and non-aggressive behaviors relative to water using vehicle microinjection data. The effect of drugs on the alcohol-heightened aggression was examined by using repeated measures of two-way ANOVA. In case of significant F values, Holm–Sidak t-tests were conducted as a post hoc analysis to determine which doses of the drug had significant effects compared to the vehicle (α = 0.05). In this analysis, the effect of the receptor agonist and antagonist were analyzed separately. Three animals in experiment 1 and one animal in experiment 2 could not complete all the injections due to cannula blockage, and those animals were used for analysis of either agonist or antagonist injections which they did complete.
Results
Alcohol-heightened aggression
Table 2 shows the effects of ethanol on aggressive behavior after vehicle microinjection. Self-administration sessions during the microinjection experiments took an average of 3.3 min for water and 3.0 min for 1.0 g/kg of ethanol. Self-administration of ethanol increased attack bites and sideway threats, and significantly reduced tail rattles relative to the measurements after water consumption. Repeated measures ANOVA showed significant main effects of ethanol on attack bites [F(1, 34) = 4.659, p = 0.038], sideway threats [F(1, 34) = 4.457, p = 0.042], and tail rattles [F(1, 34) = 8.544, p = 0.006]. There was no significant difference between the effects of ethanol and water self-administration on non-aggressive behaviors.
Before surgery, we characterized the animals for alcohol-heightened aggression and confirmed that some of the animals showed alcohol-heightened aggression, but not others (Miczek et al. 1998). However, not all animals that met our initial criterion for AHA did so after surgery. Therefore, we analyzed the data from all animals together to examine the interaction between ethanol and the GABA receptors in the DRN. Only when we found an interaction between the drug and ethanol, further analysis was performed in AHA and ANA individuals (see muscimol section).
GABAA receptors in the DRN and alcohol-heightened aggression
Agonist (muscimol, 0.006 nmol)
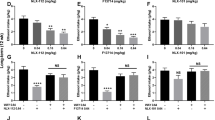
Muscimol significantly increased attack bites after ethanol consumption, but had no effect in the absence of ethanol (Fig. 2a). Two-way ANOVA showed a significant interaction between muscimol and ethanol [F(1, 16) = 4.537, p = 0.049] and the significant main effect of muscimol [F(1, 16) = 6.341, p = 0.023] on attack bites. On the other hand, muscimol slightly reduced walking compared to the vehicle [F(1, 16) = 4.756, p = 0.044] (Table 3). A significant main effect of ethanol was observed only for the measure of rearing [F(1, 16) = 8.901, p = 0.009].
GABAA receptor modulation in the DRN and alcohol-heightened aggression. a The effect of intra-DRN microinjection of GABAA receptor agonist muscimol (Mus) on the frequency of attack bites after water or 1.0 g/kg of alcohol (EtOH) consumption. Asterisk indicates significant difference from the vehicle within the same condition (p<.05). b The effect of intra-DRN microinjection of the GABAA receptor antagonist, bicuculline (Bic), on the frequency of attack bites after water or EtOH consumption. Asterisk indicates significant main effect of alcohol
To examine the interaction between muscimol and ethanol in AHA and ANA individuals, we selected animals that consistently engaged in alcohol-heightened aggression between pre- and post-surgery. Out of 17 animals, four individuals that were categorized as AHA before the surgery also showed increased attack bites (increase > 5) after ethanol consumption when given vehicle (Fig. 3a), and four individuals that were designated as ANA before surgery consistently showed no change or reduced attack bites after ethanol consumption (Fig. 3b). Muscimol increased aggressive behaviors after both water and ethanol self-administration in AHA animals (Fig. 3c). Even though the sample size was very small (AHA, n = 4), repeated measures two-way ANOVA found a significant main effect of the drug [F(1, 3) = 16.963, p = 0.026] on the composed aggression scores (attack bites + sideway threats) within this subset of mice. In contrast, muscimol did not produce any effect in the ANA animals (Fig. 3d).
Interaction between muscimol microinjection into the DRN and alcohol-heightened aggression in AHA and ANA individuals. a AHA individuals (n = 4), left side (Pre), attack bites before the cannula implantation after water or 1.0-g/kg alcohol self-administration. Right side (Post), attack bites after water or 1.0-g/kg alcohol self-administration and vehicle microinjection into the DRN. b ANA individuals (n = 4) that were designated as such before surgery and consistently showed no change or reduced attack bites after alcohol self-administration and intra-DRN vehicle treatment. c The effect of muscimol in AHA individuals on attack bites after either water or alcohol consumption. d The effect of muscimol in ANA individuals on attack bites after water or alcohol consumption. Asterisks indicate significant difference from vehicle within same condition (p < .05)
Antagonist (bicuculline, 0.006 nmol)
There was no significant interaction between bicuculline and ethanol in terms of changes in any behaviors (Fig. 2b, Table 3). Only a significant main effect of the drug was detected on the duration of contacts [F(1, 15) = 4.909, p = 0.043], and bicuculline reduced the duration of contacts relative to the vehicle. A significant effect of ethanol was observed on attack bites [F(1, 15) = 16.547, p = 0.001], sideway threats [F(1, 15) = 6.265, p = 0.0242] and walking [F(1, 15) = 8.373, p = 0.011]. There was no difference in the effect of bicuculline between the AHA and ANA individuals (data not shown).
Blood ethanol concentration
Because there was an interactive effect between ethanol and muscimol, we examined the effect of muscimol on BACs using a separate group of animals (n = 7). After the animals self-administered 1.0 g/kg of ethanol, they received either saline or 0.006 nmol of muscimol into the DRN. Blood samples were collected 10 min after the microinjection, which corresponds to the time when animals were tested for their aggressive behaviors. There was no significant difference between BAC after muscimol microinjection (70.7 ± 4.5 mg/dL) and BAC after saline (73.4 ± 3.9 mg/dL).
GABAB receptors in the DRN and alcohol-heightened aggression
Agonist (baclofen, 0.06 nmol)
Baclofen significantly increased attack bites and sideway threats after both water and ethanol self-administration (Fig. 4a, Table 4). However, there was no significant interaction between the effects of baclofen and ethanol was observed on any behaviors. Repeated measure two-way ANOVA showed a significant main effect of the drug on attack bites [F(1, 14) = 11.591, p = 0.004] and sideway threats [F(1, 14) = 5.094, p = 0.041]. Both ethanol and baclofen reduced the frequency of tail rattles. Significant main effects of the drug [F(1, 14) = 9.674, p = 0.008] and ethanol [F(1, 14) = 5.191, p = 0.039] were observed on tail rattles. There was no significant effect of baclofen on non-aggressive behaviors (Table 4).
GABAB receptor modulation in the DRN and alcohol-heightened aggression. a The effect of intra-DRN microinjection of the GABAB receptor agonist, baclofen (Bac), on the frequency of attack bites after water or 1.0 g/kg of alcohol (EtOH) consumption. b The effect of intra-DRN microinjection of the GABAB receptor antagonist, phaclofen (Pha), on the frequency of attack bites after water or EtOH consumption. Asterisks 2indicate significant difference from the vehicle within the same condition (p < .05)
Antagonist (phaclofen, 0.3 nmol)
There was no significant interaction or main effect of phaclofen on any behaviors (Fig. 4b, Table 4). Only the main effects of ethanol were significant on the frequency of tail rattles [F(1, 15) = 7.642, p = 0.014] and grooming [F(1, 15) = 5.544, p = 0.033].
Discussion
A moderate dose of alcohol can promote heightened aggressive behaviors in a certain proportion of the population, and this phenomenon is observed in several species including humans, monkeys, rats, and mice (Fulwiler et al. 2005; Higley et al. 1996; Miczek et al. 1998, 2004b; Virkkunen et al. 1996; Winslow and Miczek 1985). The present results support a role of GABAA receptors in the DRN in the aggression-heightening effect of alcohol in mice. Previously, Fish et al. (2001) showed that systemic administration of the neurosteroid, allopregnanolone, which acts as a positive allosteric modulator at GABAA receptors, enhanced the pro-aggressive effect of alcohol in mice. A low dose of ethanol (0.6 g/kg) enhanced the pro-aggressive effect of allopregnanolone in the animals who engaged at higher levels of aggressive behavior after ethanol consumption compared to their basal fighting in the absence of ethanol (alcohol-heightened aggressors, AHA), whereas this interactive effect was not observed in alcohol-non-heightened aggressors (ANA). The current data provide evidence that the GABAA receptors in the DRN may be critical for the individual vulnerability to alcohol-heightened aggression. The GABAA agonist, muscimol, when microinjected into the DRN, escalated the frequency of attack bites only after ethanol consumption, but not in the absence of ethanol. Therefore, the strong activation of GABAA receptors in the DRN by ethanol plus muscimol can induce higher vulnerability to alcohol-heightened aggression. Interestingly, when AHA and ANA individuals were separately analyzed, intra-DRN muscimol escalated attack bites only in AHA animals, but not in ANA mice. One implication of these results appears to be that AHA animals may be characterized by higher GABAA receptor activation due to ethanol relative to ANA animals. It is possible that AHA animals have a higher GABAA receptor expression in the DRN compared to ANA. This hypothesis can be addressed in future studies by comparing GABAA receptor expression in the DRN between AHA and ANA animals. In this study, we used previously determined optimally effective doses of ethanol (1.0 g/kg) and muscimol (0.006 nmol). Future studies will address the dose-effect functions for alcohol and muscimol in order to investigate whether the alcohol-muscimol interaction is specific to the AHA individuals or whether ANA individuals can also show heightened aggression with different dose combinations.
In contrast, the GABAA antagonist bicuculline, at a subconvulsant dose, did not inhibit alcohol-heightened aggression. It is possible that bicuculline did not block the site of action for ethanol on GABAA receptors and that 1.0 g/kg of ethanol was sufficiently effective to promote heightened aggression even in the presence of a low dose of bicuculline (0.006 nmol). Interestingly, bicuculline inhibited several behavioral acts in the absence of ethanol (e.g., attack bites and walking), but those effects of bicuculline were rescued by ethanol consumption.
Low doses of alcohol act preferentially via selected GABAA subtype compositions including extrasynaptic GABAA receptors that contain α4β2/3δ and α6β2/3δ subunits (Hanchar et al. 2005; Liang et al. 2007; Sundstrom-Poromaa et al. 2002; Wallner et al. 2006; Wei et al. 2004); however, considerable debate continues about the selectivity of the subunit requirement for specific alcohol actions (Korpi et al. 2007). Also, chronic alcohol treatments reduced the expression of α1 subunits of GABAA receptors and increased or decreased the expression of α4 subunits in the amygdala and cerebral cortex, respectively (Devaud et al. 1995; Matthews et al. 1998; Papadeas et al. 2001). It is possible that GABAA receptor subunit compositions in the DRN differ between AHA and ANA mice, and this difference contributes to the sensitivity to the pro-aggressive property of alcohol. Systemic administration of GABAA-α1 subunit preferring antagonist β-CCt inhibited alcohol-heightened aggression, suggesting the α1 subunit as a functional target (de Almeida et al. 2004). However, β-CCt also reduced species-typical aggression, and the α1 subunit-preferring agonist zolpidem failed to potentiate pro-aggressive effects of ethanol. Further exploration with more selectively acting compounds will be required to identify the subtypes of GABAA receptor that specifically modulate alcohol-heightened aggression.
GABAB receptors have also attracted considerable interest, primarily because agonists and positive modulators of this receptor suppress the intake of alcohol and other drugs (Besheer et al. 2004; Brebner et al. 2002; Colombo et al. 2004; Flannery et al. 2004; Maccioni et al. 2005, 2008; Orru et al. 2005; Walker and Koob 2007). Therefore, we examined the interaction between ethanol and the GABAB receptors in the DRN. We found that the local administration of the GABAB receptor agonist into the DRN did not enhance the aggression-heightening effects of ethanol, but escalated aggressive behavior independent of whether the animal consumed ethanol or water. This finding is consistent with our previous study without any self-administration of ethanol or water (Takahashi et al. 2010). It appears that different neurobiological mechanisms underlie alcohol-heightened aggression (GABAA dependent) and baclofen-escalated aggression (GABAB dependent). Further investigation will be required to address how GABAA and GABAB receptors in the DRN promote different types of aggressive behaviors. GABAB receptors are localized on presynaptic terminals of afferent neurons (e.g., GABAergic and glutamatergic) in addition to postsynaptic 5-HT neurons (Bowery et al. 2002; Cryan and Kaupmann 2005), and we have shown that GABAB receptors on presynaptic terminals may be the critical target for the pro-aggressive effect of baclofen (Takahashi et al. 2010). In contrast, GABAA receptors seem to modulate mainly postsynaptic serotonergic activity in the DRN; microinfusion of the GABAA receptor agonists into the DRN consistently inhibited 5-HT neuronal activity (Colmers and Williams 1988; Gallager and Aghajanian 1976; Innis and Aghajanian 1987; Judge et al. 2004). Thus, GABAA and GABAB receptors may modulate either pre- or post-synaptic neurons or different subsets of 5-HT neurons in the DRN.
References
Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G (2006) Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med 119:276–278
Aguayo LG (1990) Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol 187:127–130
Allan AM, Harris RA (1987) Involvement of neuronal chloride channels in ethanol intoxication, tolerance, and dependence. Recent Dev Alcohol 5:313–325
Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM (2002) A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods 118:51–57
Ariwodola OJ, Weiner JL (2004) Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci 24:10679–10686
Arnone M, Dantzer R (1980) Effects of diazepam on extinction induced aggression in pigs. Pharmacol Biochem Behav 13:27–30
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179:641–668
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291–313
Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF (1983) Immunohistochemical evidence for the presence of γ-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res 275:329–339
Besheer J, Lepoutre V, Hodge CW (2004) GABAB receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6 J mice. Psychopharmacology 174:358–366
Bond AJ, Curran HV, Bruce MS, O’Sullivan G, Shine P (1995) Behavioural aggression in panic disorder after 8 weeks’ treatment with alprazolam. J Affect Disord 35:117–123
Bowery NG, Hudson AL, Price GW (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20:365–383
Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Bonner RM, TI ESJ (2002) International union of pharmacology. XXXIII. Mammalian γ-aminobutyric acidB receptors: structure and function. Pharmacol Rev 54:247–264
Brebner K, Childress AR, Roberts DC (2002) A potential role for GABAB agonists in the treatment of psychostimulant addiction. Alcohol Alcohol 37:478–484
Caldwell EE, Miczek KA (2008) Long-term citalopram maintenance in mice: selective reduction of alcohol-heightened aggression. Psychopharmacology 196:407–416
Chiavegatto S, Quadros IMH, Ambar G, Miczek KA (2010) Individual vulnerability to escalated aggressive behavior by a low dose of alcohol: decreased serotonin receptor mRNA in the prefrontal cortex of male mice. Genes Brain Behav 9:110–119
Christmas AJ, Maxwell DR (1970) A comparison of the effects of some benzodiazepines and other drugs on aggressive and exploratory behaviour in mice and rats. Neuropharmacology 9:17–29
Cloninger CR, Sigvardsson S, Gilligan SB, Von Knorring AL, Reich T, Bohman M (1989) Genetic heterogeneity and the classification of alcoholism. In: Gordis E (ed) Alcohol Research from Bench to Bedside. Haworth Press, Binghampton, NY, pp 3–16
Cole HF, Wolf HH (1970) Laboratory evaluation of aggressive behavior of the grasshopper mouse (Onychomys). J Pharm Sci 59:969–971
Colmers WF, Williams JT (1988) Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett 93:300–306
Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL (2000) Ability of baclofen in reducing alcohol intake and withdrawal severity: I. Preclinical evidence. Alcohol Clin Exp Res 24:58–66
Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL (2003) Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology 167:221–224
Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL (2004) Role of GABAB receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res 6:403–414
Cryan JF, Kaupmann K (2005) Don’t worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43
Dahlstrom A, Fuxe K (1964) Localization of monoamines in the lower brain stem. Experientia 20:398–399
Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F (1987) GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol 4:469–472
Dar MS, Wooles WR (1985) GABA mediation of the central effects of acute and chronic ethanol in mice. Pharmacol Biochem Behav 22:77–84
de Almeida RMM, Rowlett JK, Cook JM, Yin W, Miczek KA (2004) GABAA/α1 receptor agonists and antagonists: effects on species-typical and heightened aggressive behavior after alcohol self-administration in mice. Psychopharmacology 172:255–263
de Boer SF, Koolhaas JM (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526:125–139
Devaud LL, Smith FD, Grayson DR, Morrow AL (1995) Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol 48:861–868
DiMascio A (1973) The effects of benzodiazepines on aggression: reduced or increased? Psychopharmacologia 30:95–102
Edeline JM, Hars B, Hennevin E, Cotillon N (2002) Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem 78:100–124
Ferrari PF, Parmigiani S, Rodgers RJ, Palanza P (1997) Differential effects of chlordiazepoxide on aggressive behavior in male mice: the influence of social factors. Psychopharmacology 134:258–265
File SE, Zharkovsky A, Gulati K (1991) Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology 30:183–190
Fish EW, Faccidomo S, DeBold JF, Miczek KA (2001) Alcohol, allopregnanolone and aggression in mice. Psychopharmacology 153:473–483
Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A (2004) Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res 28:1517–1523
Fulwiler C, Eckstine J, Kalsy S (2005) Impulsive-aggressive traits, serotonin function, and alcohol-enhanced aggression. J Clin Pharmacol 45:94–100
Gallager DW, Aghajanian GK (1976) Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur J Pharmacol 39:357–364
Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH (2000) Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci 20:4217–4225
Grant EC, Mackintosh JH (1963) A comparison of the social postures of some common laboratory rodents. Behaviour 21:246–295
Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M (2005) Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci 8:339–345
Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M (1996) CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40:1067–1082
Innis RB, Aghajanian GK (1987) Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol 143:195–204
Judge SJ, Ingram CD, Gartside SE (2004) GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int 45:1057–1065
Korpi ER, Grunder G, Luddens H (2002) Drug interactions at GABAA receptors. Prog Neurobiol 67:113–159
Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, Luddens H (2007) Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol 41:163–176
Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL (2009) The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 205:529–564
Li SP, Park MS, Jin GZ, Kim JH, Lee HL, Lee YL, Kim JH, Bahk JY, Park TJ, Koh PO, Chung BC, Kim MO (2005) Ethanol modulates GABAB receptor expression in cortex and hippocampus of the adult rat brain. Brain Res 1061:27–35
Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I (2007) Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci 27:12367–12377
Liljequist S, Engel J (1982) Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 78:71–75
Maccioni P, Serra S, Vacca G, Orru A, Pes D, Agabio R, Addolorato G, Carai MA, Gessa GL, Colombo G (2005) Baclofen-induced reduction of alcohol reinforcement in alcohol-preferring rats. Alcohol 36:161–168
Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G (2008) Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783—comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res 32:1558–1564
Martin JH (1991) Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127:160–164
Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL (1998) Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem 70:1160–1166
Michelsen KA, Schmitz C, Steinbusch HW (2007) The dorsal raphe nucleus—from silver stainings to a role in depression. Brain Res Rev 55:329–342
Miczek KA (1974) Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia 39:275–301
Miczek KA, de Almeida RMM (2001) Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology 157:421–429
Miczek KA, O’Donnell JM (1978) Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and l-dopa. Psychopharmacology 57:47–55
Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM (1992) Alcohol and “bursts” of aggressive behavior: Ethological analysis of individual differences in rats. Psychopharmacology 107:551–563
Miczek KA, Barros HM, Sakoda L, Weerts EM (1998) Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res 22:1698–1705
Miczek KA, Fish EW, DeBold JF, de Almeida RMM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology 163:434–458
Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF (2004a) Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci 1036:336–355
Miczek KA, Fish EW, de Almeida RMM, Faccidomo S, DeBold JF (2004b) Role of alcohol consumption in escalation to violence. Ann N Y Acad Sci 1036:278–289
Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL (2007) GABAergic modulation of binge-like ethanol intake in C57BL/6 J mice. Pharmacol Biochem Behav 88:105–113
Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF (1982) Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res 232:375–389
National Research Council (1996) Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington DC
Olivier B (2004) Serotonin and aggression. Ann N Y Acad Sci 1036:382–392
Olivier B, Mos J, Van Oorschot R (1985) Maternal aggression in rats: effects of chlordiazepoxide and fluprazine. Psychopharmacology 86:68–76
Olsen RW (1982) Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol 22:245–277
Orru A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G (2005) Reducing effect of the positive allosteric modulators of the GABAB receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol 525:105–111
Palmer MR, Hoffer BJ (1990) GABAergic mechanisms in the electrophysiological actions of ethanol on cerebellar neurons. Neurochem Res 15:145–151
Papadeas S, Grobin AC, Morrow AL (2001) Chronic ethanol consumption differentially alters GABAA receptor α1 and α4 subunit peptide expression and GABAA receptor-mediated 36Cl− uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res 25:1270–1275
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Rabow LE, Russek SJ, Farb DH (1995) From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse 21:189–274
Sieghart W (1995) Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47:181–234
Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS (2002) Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci 5:721–722
Suzdak PD, Schwartz RD, Skolnick P, Paul SM (1986) Ethanol stimulates γ-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A 83:4071–4075
Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA (2010) GABAB receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci (in press)
Virkkunen M, Linnoila M (1993) Brain serotonin, type II alcoholism and impulsive violence. J Stud Alcohol Suppl 11:163–169
Virkkunen M, Eggert M, Rawlings R, Linnoila M (1996) A prospective follow-up study of alcoholic violent offenders and fire setters. Arch Gen Psychiatry 53:523–529
Walker BM, Koob GF (2007) The γ-aminobutyric acidB receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31:11–18
Wallner M, Hanchar HJ, Olsen RW (2006) Low dose acute alcohol effects on GABAA receptor subtypes. Pharmacol Ther 112:513–528
Wang QP, Ochiai H, Nakai Y (1992) GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull 29:943–948
Weerts EM, Miczek KA (1996) Primate vocalizations during social separation and aggression: effects of alcohol and benzodiazepines. Psychopharmacology 127:255–264
Wei W, Faria LC, Mody I (2004) Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 24:8379–8382
Weisman AM, Berman ME, Taylor SP (1998) Effects of clorazepate, diazepam, and oxazepam on a laboratory measurement of aggression in men. Int Clin Psychopharmacol 13:183–188
Winslow JT, Miczek KA (1985) Social status as determinant of alcohol effects on aggressive behavior in squirrel monkeys (Saimiri sciureus). Psychopharmacology 85:167–172
Wu PH, Poelchen W, Proctor WR (2005) Differential GABAB receptor modulation of ethanol effects on GABAA synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther 312:1082–1089
Acknowledgements
This research was supported by NIAAA grant AA13983.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Frequency of attack bites in animals that received microinfusion outside the DRN target site (DOC 38 kb)
Rights and permissions
About this article
Cite this article
Takahashi, A., Kwa, C., DeBold, J.F. et al. GABAA receptors in the dorsal raphé nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology 211, 467–477 (2010). https://doi.org/10.1007/s00213-010-1920-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1920-x