Abstract
Background
Selective serotonin reuptake inhibitors (SSRIs) alleviate many affective disturbances in human clinical populations and are used in animal models to study the influence of serotonin (5-HT) on aggressive behavior and impulsivity.
Objective
We hypothesized that long-term SSRI treatment may reduce aggressive behavior escalated by alcohol consumption in mice. Therefore, aggression was tested in male CFW mice to determine whether repeated citalopram (CIT) administration reduces alcohol-heightened aggression.
Materials and methods
Resident male mice self-administered alcohol by performing an operant response on a panel placed in their home cage that delivered a 6% alcohol solution. Mice repeatedly confronted an intruder 15 min after self-administration of either 1 g/kg alcohol (EtOH) or water (H2O). Aggressive behaviors were higher in most mice when tests occurred after EtOH intake relative to H2O. Once baseline aggression was established, animals were injected (i.p.) twice daily with 10 mg/kg CIT or saline (SAL) for 32 days. Every 4 days throughout the CIT treatment period, aggressive encounters occurred 6 h after CIT injections, with testing conditions alternating between EtOH and H2O intake.
Results
Aggression was only modestly affected by CIT in the first 2 weeks of treatment. However, by day 17 of CIT treatment, alcohol-heightened aggressive behavior was abolished, while baseline aggression remained stable. These data lend support for the role of the 5-HT transporter in the control of alcohol-related aggressive behavior, and the time course of effects suggests that a change in density of 5HT1A autoreceptors is necessary before antidepressant drugs produce beneficial outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Alcohol can increase aggression in some individuals (Bushman and Cooper 1990) especially in individuals with a propensity for impulsive aggressive behavior. According to the 2000 World Report on Violence and Health, more than half of all violent crimes, including assaults, homicides, and rapes, are committed while under the influence of alcohol (Krug et al. 2002), and injuries due to interpersonal violence tend to occur in close proximity to places where alcohol is sold (Gruenewald et al. 2006). Alcohol heightens aggressive behavior in the laboratory for both human and non-human subjects (Parker and Auerhahn 1998; Miczek et al. 2002; see Miczek et al. 2004b). However, most of the time, alcohol consumption does not result in violence, but rather is considered a pleasurable activity that facilitates social interactions, while alcohol-related violence occurs in a small minority of the population (Brady et al. 1998; Cloninger 1987; Linnoila et al. 1983). Preclinical research shows a similar population trend, with a small proportion of rodents (between 15 and 30%) consistently exhibiting escalated attack behavior after alcohol intake (Fish et al. 2001; Miczek et al. 1992, 1998; van Erp and Miczek 1997). It has been hypothesized that a preexisting genetic vulnerability predisposes an individual to drink and to engage in violence as a function of certain environmental influences (Higley and Bennett 1999; Higley et al. 1996; Meyer-Lindenberg et al. 2006).
Several studies link disorders of the serotonergic system to aggression (Olivier et al. 1995; Popova 2006; Summers et al. 2005; van der Vegt et al. 2003a, b; Linnoila and Virkkunen 1992), and heightened alcohol responsivity (Badawy et al. 1995). Historically, a negative correlation between the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid and impulsive aggressive behavior and risk-taking behavior has been reported in human (Brown et al. 1979, 1982; Linnoila et al. 1983) and non-human primate populations (Fairbanks et al. 2001; Higley et al. 1992; Mehlman et al. 1994). Further evidence identifies regulatory polymorphisms in tryptophan hydroxylase, 5-HT transporter (5-HTT), and monoamine oxidase A promoters in humans (Beitchman et al. 2006; Brunner et al. 1993; Caspi et al. 2002; Haberstick et al. 2006; Hennig et al. 2005; Manuck et al. 2000; Verona et al. 2006) and primates (Barr et al. 2004; Wendland et al. 2006), which are related to violent and impulsive behaviors and, in some cases, also to alcohol consumption (see Wrase et al. 2006).
Serotonergic tone in frontal cortex is thought to be important, specifically for the inhibition of impulsive aggressive behavior, and many treatments aimed at alleviating aggressive behavior in psychiatric patients have targeted serotonergic neurons, especially the transporter molecules (Barkan et al. 2006; Blader 2006; see Bond 2005; Miczek et al. 2002; Reist et al. 2003). Aggressive behavior is reduced after acute systemic administration of direct and indirect serotonergic agonists, particularly the full and partial 5HT1B receptor agonists, CP94,253, anpirtoline, zolmitriptan, and eltoprazine (de Boer et al. 1999; Fish et al. 1999; De Almeida et al. 2001, 2006; see Miczek et al. 2004a; Bannai et al. 2007). Treatment with SSRIs has been shown to reduce aggression in patients with borderline personality disorder (New et al. 2004). Citalopram, one of the most selective of the SSRIs, reduced impulsive aggression in clinical and preclinical studies (Reist et al. 2003; Peremans et al. 2005).
Although SSRIs have been found to reduce aggressive behavior in rodents (Delville et al. 1996; Ferris et al. 1997; Pinna et al. 2003), results have been variable. SSRIs differentially affect aggressive behavior in juvenile compared to adult hamsters (Taravosh-Lahn et al. 2006), and the specific aggression-reducing effects of CIT and escitalopram in isolated mice has been less promising (Sanchez et al. 2003). The present experiment is designed to address whether long-term SSRI maintenance, mimicking the clinical regimen, may reduce alcohol-heightened aggressive behavior in mice. To accomplish this objective, chronic daily CIT injections were implemented to increase serotonergic tone in experienced resident male mice. Aggressive behavior was examined after oral self-administration of alcohol during periodic confrontations with intruder males.
Materials and methods
Subjects
Adult male CFW mice (Charles River Laboratories, Wilmington, MA, USA), approximately 60 days old and weighing between 20 and 25 g upon arrival, were used as residents (n = 31) in the present experiment. All resident mice were housed in clear polycarbonate cages (29 × 19 × 13 cm) with female conspecifics throughout the experiment. In addition, stimulus mice serving as intruder animals consisted of CFW males, housed six to eight mice per cage in large polycarbonate cages (48 × 27 × 17 cm). Mice were housed in a temperature- and humidity-controlled vivarium, maintained at 21–23°C and 30–40% humidity, on a 12:12-h reversed light photocycle (lights off 0600 hours/lights on 1800 hours). All procedures were conducted following strict adherence to the Guide for the Care and Use of Laboratory Animals (National Research Council 1996), and all experimental protocols were approved by the Animal Care and Use Committee at Tufts University.
Drugs
Citalopram hydrobromide (1-[3(Dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile hydrobromide) was obtained from Forest Laboratories and dissolved in 0.9% SAL to equal a 1mg/ml solution. Injection volumes were 1 ml/100 g body weight. EtOH solutions (6%w/v) consisted of 95% EtOH diluted with tap H2O.
Experimental procedures
Alcohol self-administration procedure
To ensure reliable EtOH self-administration in resident male mice, all animals were restricted to 3 h of access to H2O each day. After 21 h of H2O restriction, an aluminum panel was inserted into the home cage, which contained two nose-poke holes with fluid receptacles on the left and right sides of the panel, one of which was designated active and one inactive, with a light illuminated over the active hole and a houselight located on the center of the panel (Miczek and de Almeida 2001). Upon emitting an operant nose poke response (FR 5 schedule) into the active hole (right or left side, counterbalanced), a pump delivered 0.05 ml of fluid into the receptacle located on the panel. Each day, animals self-administered EtOH in increasing concentrations using a modified sucrose-fading technique (Samson 1986) wherein animals initially drank a 10% sucrose-sweetened fluid, after which, EtOH was gradually added to the fluid in 1% increments while sucrose was gradually faded out until mice self-administered 6% EtOH in H2O. Once sucrose fading was completed, resident mice self-administered 1 g/kg EtOH in their home cages on a daily basis (3–4 days per week), usually within 2–3 min per trial (2.65 ± 0.20, mean±SEM).
Resident–intruder confrontations
Baseline aggressive behavior
After 3 weeks of cohabitation with a female, male resident mice were screened for aggressive behavior during confrontations with an intruder (Miczek and O’Donnell 1978). The screening procedure entailed removal of the female and any pups from the home cage, after which, an intruder male was placed into the home cage. Fighting behavior was observed for 5 min after the first biting attack, or the interaction was terminated after 5 min if no attack occurred. Aggression testing procedures were repeated using the same intruder for each resident every other day, 3 days per week, until aggressive behavior reached stable levels, defined by three consecutive interactions in which attack bite counts were within 15% of the animals’ individual average. Stable aggressive behavior was evident by most residents after seven to ten confrontations, and mice demonstrated an average of 22 ± 8 bites per session.
Alcohol-heightened aggressive behavior
Once stable aggressive behavior was reached, animals were tested for EtOH-heightened aggressive behavior during resident–intruder confrontations, which took place exactly 15 min after each animal reached completion of 1 g/kg EtOH in the self-administration session. Twice per week, animals were examined for aggressive behavior, with test conditions alternating between EtOH and H2O drinking (in an equal volume, 1.69 ml fluid/100 g body weight). Three determinations of each resident’s attack bites directed toward intruders, under both EtOH and H2O conditions, were obtained, and alcohol-heightened aggressive behavior was calculated from these measurements.
Chronic treatment with citalopram
After baseline aggressive behavior was determined under both EtOH and H2O drinking conditions, animals were treated twice daily with 10 mg/kg (s.c.) CIT (n = 14) or SAL (n = 17) in a volume of 1 ml/100 g body weight. This dose was chosen based on reports in mice of anti-immobility effects in the forced swim and tail-suspension tests, which revealed 10 mg/kg as a consistently effective dose (Cryan and Mombereau 2004; Dziedzicka-Wasylewska et al. 2006; Perrault et al. 1992), and based on the knowledge that 10 mg/kg CIT is able to achieve steady-state plasma levels in rodents that correspond to clinically effective doses in humans (Kugelberg et al. 2003). Daily injections were administered between the hours of 0800 and 0930 hours and between 1700 and 1850 hours. Twice daily CIT treatment was repeated for 32 days. As with baseline aggression testing, tests for aggressive behavior were conducted twice a week after drinking either EtOH or H2O. Every test for aggressive behavior was conducted 5–7 h after that morning’s injection. This post-injection interval was chosen to obtain behavioral measurements at the drug’s average elimination half-life (5 h) and to avoid testing during peak plasma levels of CIT (i.e., 30 min, as observed at comparable doses in the rat; Cremers et al. 2000).
Aggressive behavior after an acute citalopram injection
After 1 month of chronic CIT or SAL treatment, animals were tested once for alcohol-heightened aggression 30 min after an injection of 10 mg/kg CIT and once 30 min after a SAL injection. These tests were conducted on the last 2 days of repeated CIT (or SAL) treatment to be used as a comparison between aggression tests conducted 4–6 h post-injection to those conducted 30 min post-injection in chronically treated animals. The rationale for these last two tests is to determine whether aggressive behavior is differentially affected at a post-injection time point which represents peak plasma levels of the drug in comparison to behavior observed during chronic treatment at the longer post-injection intervals used in the present experiment.
Blood alcohol content determination
On the last day of the experiment, 15 min after self-administration of 1 g/kg EtOH, blood was collected from mice via intraorbital sinus puncture under isoflurane anesthesia. Blood samples were prepared using an ethanol assay, NAD-ADH reagent (Diagnostic Chemicals, Canada), and determined via UV/VIS spectrophotometer.
Data analyses
Aggressive and non-aggressive behaviors were measured by a trained experimenter during observational analyses of videotaped interactions. The experimenter was trained and practiced analysis techniques until inter-rater and self-reliability scores for all behaviors reached 90% reliability (average inter- and intra-rater reliability scores were 93 and 94%, respectively). Interactions were scored using the Noldus Observer software package. The Observer automatically scored counts, durations, and latencies of every behavior. As previously defined and illustrated (Miczek and O’Donnell 1978), aggressive behaviors included bites, threats, pursuits, and tail rattles. Bites were operationally defined as any contact with the teeth directed by the resident toward the intruder. A threat was defined when the resident mouse directed a sideways posture toward the intruder, regardless of whether the threat resulted in a bite or not. Pursuits were defined when a resident chased an intruder with the intruder actively fleeing from the resident.
Non-aggressive behaviors included locomotion, rearing, grooming, and social contact with the intruder. Locomotion was measured as any movement of the resident mouse not otherwise specified (included walking, digging, or jumping and flipping against the cage top). Rearing was defined when the two front paws were elevated off the floor. Grooming was defined when the resident engaged in autogrooming, cleaning any part of its body with the forepaws, or scratching the body with the hindpaws. Social contact was measured when the resident made nasal contact with the body of the intruder.
Data were analyzed using SPSS software for the behavioral sciences. One-way analysis of variance (ANOVA) was used to examine baseline differences in aggressive and non-aggressive behaviors for testing that occurred after either EtOH or H2O drinking. A mixed design, repeated measures ANOVA was performed to analyze the effect of chronic CIT treatment on aggressive behavior under both H2O and EtOH conditions from pre-experimental baseline through the last day of CIT treatment. The within-subjects factor, week, consisted of five levels, baseline, and 4 weeks of drug treatment. The between subjects factor, drug, compared SAL and CIT treated subjects. Levine’s test for equality of variance and Mauchly’s test of sphericity were used, and, where appropriate, corrections were applied. Mice demonstrating substantially augmented attack behavior after drinking EtOH, in excess of two standard deviations of baseline, were designated alcohol-heightened aggressors (AHA). This determination represents a statistical outlier criterion (Barnett and Lewis 1984) previously shown to distinguish animals that reliably demonstrate alcohol-heightened aggressive behavior from those that do not (Miczek et al. 1998). Those that did not evidence the same degree of heightened aggression due to EtOH were designated alcohol non-heightened aggressors (ANA).
Results
Alcohol drinking
All mice quickly consumed (within 2.27 ± 0.33 min) 1 mg/kg EtOH 15 min before aggression testing. BAC was determined for a sample (n = 18) of mice which was measured 15 min after consumption of 1 g/kg EtOH. Mean BAC for the sample was 71.7 ± 1.9 mg/dl, and no differences were found between CIT and SAL conditions for BAC determination.
Alcohol-heightened aggressive behavior
Resident mice were screened for aggressive behavior repeatedly until stable baseline levels were reached, defined as ≤15% variability between three consecutive agonistic encounters. Once fighting experience was established, each resident male directed an average of 16.3 attacks (±1.3) toward an intruder. Fighting conditions alternated between EtOH and water self-administration. Three determinations were conducted assessing aggression after both EtOH and H2O consumption, and EtOH drinking heightened attack behavior by 36%. The difference in fighting behavior after EtOH vs H2O consumption was statistically significant [t(30) = 7.871, p < 0.001]. Fourteen mice (45%) demonstrated alcohol-heightened aggressive behavior (mean attacks = 26.28 ± 2.27) in excess of two standard deviations above baseline (mean attacks = 12.94 ± 1.59) and were therefore designated AHAs. Preliminary analyses showed that the 14 mice which met the statistical criterion for AHA were not different from ANA mice in their response to CIT. Thus, AHA and ANA mice were analyzed together as one sample.
Effect of CIT on alcohol-heightened aggressive behavior
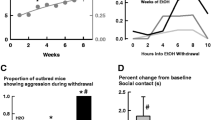
Repeated daily treatment with CIT significantly reduced baseline aggressive behavior, and after 2 weeks of chronic treatment, CIT completely eliminated alcohol-heightened aggressive behavior. A mixed design repeated measures ANOVA revealed a main effect of week [F(3,89) = 7.149, p < 0.001], a main effect of drug [F(1,29) = 8.175, p < 0.01], and a significant interaction of week × drug [F(3,89) = 2.822, p < 0.05] on total aggression (defined as the sum frequency of bites, threats, pursuits, and tail rattles) after drinking EtOH. The Holm–Sidak method for pairwise multiple comparisons confirmed that CIT significantly decreased aggressive behavior during weeks 3, 4, and 5 compared to baseline (all p < 0.05), while the SAL group demonstrated no significant change over the 5 weeks of treatment. Importantly, aggression was significantly different between CIT vs SAL groups for EtOH-related aggression week 3 [t(29) = 3.167, p < 0.01] and week 4 [t(22) = 2.971, p < 0.01], but not weeks 1 and 2 of chronic drug treatment, which demonstrates that the effect of CIT on alcohol-related aggression requires at least 14 days of repeated daily injections (see Fig. 1).
Effects of repeated, twice daily (10 mg/kg) CIT injections on aggressive behavior in mice after drinking either EtOH or H2O. Frequency of aggressive acts (defined as sum of attack bites, threats, pursuits, and tail rattles)±SEM. Plus symbols indicate statistical significance of behavior relative to baseline (++p < 0.01; +++p < 0.001). Asterisks indicate a significant effect of CIT compared to SAL (**p < 0.01; ***p < 0.001). n = 14 and 17
A second ANOVA revealed a main effect of week [F(4,116) = 6.062, p < 0.001] and a main effect of drug [F(1,29) = 5.127, p < 0.05] on total aggression after drinking H2O. The interaction between drug × week on total aggression was not significant. Figure 1 demonstrates that the effect of CIT on baseline (H2O) aggression emerged early and remained stable throughout the remaining 3 weeks.
Analyses of attack behaviors alone revealed that CIT significantly reduced the number of attack bites. A main effect of drug [F(1,29) = 5.347, p < 0.05] and a main effect of week [F(4,116) = 11.401, p < 0.001] was found for attack frequency after drinking EtOH. Although the interaction between CIT × week was not significant, the overall effect of CIT on attacks was clearly more pronounced during the last 2 weeks of treatment (see Fig. 2). In fact, analyses of alcohol-heightened aggressive behaviors for CIT and SAL groups during each week of treatment showed that the heightening effect of EtOH on aggressive behavior was maintained throughout the treatment period for SAL animals (all p < 0.01), but disappeared in CIT-treated animals after the second week of treatment. Attack bite frequency measured after drinking H2O did not differ between CIT and SAL mice.
Frequency of attack bites at baseline and during chronic CIT treatment. A significant effect of drug was found (p < 0.05). The aggression reducing effect of CIT was more pronounced during weeks 3 and 4 where attacks after drinking EtOH were significantly (p < 0.01) lower in CIT (filled circles) than SAL-treated animals (filled diamonds). Alcohol-heightened attack behavior was eliminated after 2 weeks of CIT treatment, yet remained elevated in SAL-treated mice. Asterisks indicate a significant difference between aggression after EtOH relative to H2O (p < 0.001). n = 14 and 17
Effect of CIT on non-aggressive behaviors
A mixed design, repeated measures ANOVA on each non-aggressive behavior measured during testing (both EtOH and H2O drinking conditions) revealed no significant differences based on drug or week for all behaviors including social contact, grooming, and rearing. A main effect of week emerged for locomotor behavior after drinking EtOH [F(3,116) = 5.692, p < 0.01], but had neither a main effect of drug nor interaction of drug × week. As illustrated in the Table 1, both CIT and SAL groups showed an equally substantial decline in locomotion during the last testing session after drinking EtOH.
Effect of an acute CIT injection on alcohol-related aggression
A subgroup of animals (n = 26) was tested once for alcohol-induced aggression 30 min after an injection of 10 mg/kg CIT and, counterbalanced on a separate day, 30 min after a SAL injection. For animals maintained on repeated daily SAL injections, one injection of CIT resulted in significantly lower levels of attack bites compared to a single injection of SAL. An ANOVA was performed comparing the between-subjects factor treatment group, which refers to chronically maintained CIT vs SAL mice, and the within-subjects factor drug, either 0 (SAL) or 10 mg/kg CIT. A main effect of drug was detected [F(1,24) = 20.107, p < 0.001], and an interaction between treatment group × drug was found on attack bites [F(1,24) = 8.018, p < 0.01]. Holm–Sidak post hoc comparisons confirmed that CIT resulted in significantly lower attack bites compared to SAL injections [t(25) = 5.898, p < 0.001] and showed that animals chronically maintained on SAL showed a significant reduction in aggressive behavior 30 min after CIT injections, but animals with a history of chronic CIT treatment did not evidence the same reduction in aggressive behavior (Fig. 3). Similarly, CIT injections reduced individual measurements of threats and tail rattles (all p < 0.01). Mean threat frequency (±SEM) for SAL and CIT was 13.8 ± 1.4 and 8.3 ± 0.9, respectively. Mean tail rattle frequency (±SEM) for SAL and CIT was 12.3 ± 1.4 and 4.7 ± 0.8, respectively. A main effect was found for locomotor behavior 30 min post-injection. Interestingly, an acute CIT injection resulted in significantly higher rates of locomotor behavior than a SAL injection [t(25) = 2.953, p < 0.001] for both treatment groups, with no significant interaction between treatment group × drug.
Aggressive behavior 30 min after an acute injection of CIT, 15 min after drinking alcohol. Only SAL-maintained animals show a pronounced reduction in aggressive behavior after an acute injection of CIT. Compare to day 1 of chronic treatment (inset) wherein no differences emerged between CIT and SAL when interactions occurred 6 h post-injection. Therefore, a single injection of CIT reduces alcohol-related aggression, but this reduction is only apparent during elevated plasma levels of the drug. n = 10 and 16
Discussion
The present experiments represent the first investigation of long-term antidepressant treatment on alcohol-heightened aggression in rodents. The purpose of conducting tests of aggressive behavior at a time divorced from initial rapid drug effects is to mimic human treatment strategies in an effort to find enduring, rather than transient, changes in behavior. Until now, most behavioral studies have examined acute actions of antidepressants (Crowley et al. 2005; de Boer et al. 1999, 2000; Sanchez et al. 2003). Those examining long-term antidepressant treatment have utilized depression- and anxiety-like behavioral protocols such as forced swim, tail suspension, open field, novelty-suppressed feeding, and ultrasonic distress vocalizations (Connor et al. 2000; Santarelli et al. 2003; Fish et al. 2004), research which consistently shows that antidepressants dose-dependently reduce anxiety- and depression-like behaviors. Some research on rodent aggression has shown that acute administration of SSRIs lengthens attack latency (Sanchez et al. 2003) and has been found to dose-dependently reduce aggressive behaviors (de Boer et al. 1999, 2000; Taravosh-Lahn et al. 2006), but antidepressants have also produced variable effects on attack frequency in rodents (Rilke et al. 2001). A series of studies reported that long-term SSRI treatment affects establishment of dominance hierarchies and enhances agonistic behavior and social competence in colony housed rodents (Mitchell 2005; Mitchell and Redfern 1997; Mitchell et al. 1991), yet none have examined changes in alcohol-related aggressive, social, and locomotor behavior after repeated daily SSRI treatment. The present results represent an encouraging development in the search for therapeutic strategies aimed at alleviating chronic alcohol-related violence in humans.
Chronic treatment with SSRI, but not other types of antidepressants, modulate the uptake process of 5-HT, reduces SERT binding in the CA3 region of the hippocampus (Benmansour et al. 1999), and desensitizes the 5HTT (Piñeyro et al. 1994). Of all the SSRI antidepressants, CIT and ESC are the most selective for blocking 5HTT. These results therefore demonstrate that the 5HTT is important for the regulation of aggressive behavior and suggest that blockade of the transporter is a useful strategy for reducing strong aggressive tendencies in individuals.
Because alcohol-related violence in humans is thought to be an enduring and maladaptive trait, it is important not only to distinguish between alcohol-heightened (or escalated) and species-typical forms of aggressive behavior but also to distinguish between both short- and long-term consequences of altered monoamine levels. Citalopram increases 5-HT levels immediately, reaching peak plasma concentration in approximately 30–40 min, and cortical increases in extracellular 5-HT levels are maximally increased 40–60 min post-injection (Cremers et al. 2000). The rationale for conducting a single probe of aggressive behavior 30 min after an acute injection of CIT was to demonstrate that changes in aggressive behavior occur differentially based on whether animals are chronically vs acutely treated with CIT and whether behavior is altered during peak plasma levels of the drug compared to a 6-h post-injection interval. Indeed, CIT reduced aggressive behavior acutely in SAL-maintained animals, suggesting that even transient increases in extracellular 5-HT are effective. Compared to the first day of chronic treatment, when aggression testing occurred 6 h post-injection, the difference between SAL and CIT is far more substantial. Thus, the aggression-reducing effect of acute CIT administration is likely transient in nature, while the more dramatic long-term changes in the reduction of escalated aggression occur after repeated administration.
Chronic citalopram treatment affected baseline (water condition) aggression, an effect which emerged by the second week and persisted throughout the treatment period. However, the reduction in alcohol-heightened aggressive behavior was most impressive. Alcohol-related aggression was reduced by CIT treatment, and although this effect began to emerge during the second week of treatment, the main effect of alcohol on aggressive behavior was completely abolished by the third week of treatment, a time course which is very similar to the temporal effects of antidepressants on depressive symptoms in humans. This also corresponds to the time when autoreceptors are believed to have become desensitized (see Blier and de Montigny 1998; Ceglia et al. 2004). At the same time, these effects correspond to a time when hippocampal neurogenesis is known to be enhanced (Santarelli et al. 2003) and effects are also congruent with the return of suppressed 5-HT firing activity observed in the hippocampus after chronic CIT treatment in rats (Mansari et al. 2005).
Acute treatment with CIT or ESC maximally increases extracellular 5-HT (Ceglia et al. 2004), and prolonged treatment with CIT may not result in significantly higher dialysate 5-HT during a CIT challenge compared to SAL-maintained animals (Auerbach and Hjorth 1995; Hjorth and Auerbach 1999). However, multiple studies do report functional changes in the 5-HT levels and changes in brain serotonin transporter (SERT density and mRNA expression in the raphe) after chronic SSRI antidepressant treatment (Benmansour et al. 1999).
Projections terminating in the prefrontal cortex are thought to be important for the regulation of impulsive aggressive behavior as well as behavioral responses to alcohol (Badawy et al. 1995; Fahlke and Hansen 1999; Hinkers et al. 2006; see Wrase et al. 2006). Somatodendritic 5HT1A autoreceptors located on cell bodies within the raphe are thought to be important for the latency of SSRI effects (Ceglia et al. 2004; De Vry et al. 2004; Santarelli et al. 2003). Serotonin levels depend on raphe neuronal activity, and although antidepressants enhance terminal 5-HT levels immediately, antidepressant efficacy is delayed likely due to the time required for autoreceptor desensitization (Hughes et al. 2007; also see Kalsner 2000).
Whether autoreceptor desensitization accounts for some of the behavioral effects seen here could be determined by incorporating daily administration of 5HT1A receptor antagonists (e.g., WAY-100635) in addition to SSRI treatment wherein SSRI effects would be predicted to emerge earlier. de Boer et al. (2000) found that WAY-100635 combined with S-15535 resulted in additive aggression-reducing effects. In addition, WAY-100635 augments the increased extracellular 5-HT in the dialysate due to CIT (Cremers et al. 2000; Hjorth et al. 1997), supporting the supposition that 5-HT1A autoreceptors play a regulatory role in the onset of SSRI treatment effects. Therefore, this strategy could currently be implemented to reduce alcohol-heightened aggression more rapidly.
The clinical translation of the present findings suggests that long-term SSRI treatment may be a useful therapeutic strategy for reducing escalated aggressive behavior due to alcohol consumption.
References
Auerbach SB, Hjorth S (1995) Effect of chronic administration of the selective serotonin (5-HT) uptake inhibitor citalopram on extracellular 5-HT and apparent autoreceptor sensitivity in rat forebrain in vivo. Naunyn-Schmiedeberg’s Arch Pharmacol 352:597–606
Badawy AAB, Morgan CJ, Lovett JWT, Bradley DM, Thomas R (1995) Decrease in circulating tryptophan availability to the brain after acute ethanol consumption by normal volunteers: implications for alcohol-induced aggressive behaviour and depression. Pharmacopsychiatry 28:93–97
Bannai M, Fish EW, Faccidomo S, Miczek KA (2007) Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology 193:295–304
Barkan T, Peled A, Modai I, Barak P, Weizman A, Rehavi M (2006) Serotonin transporter characteristics in lymphocytes and platelets of male aggressive schizophrenia patients compared to non-aggressive schizophrenia patients. Eur Neuropsychopharmacol 16:572–579
Barnett V, Lewis T (1984) Outliers in statistical data. Wiley, Chichester
Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD (2004) Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry 61:1146–1152
Beitchman JH, Baldassarra L, Mik H, De Luca V, King N, Bender D, Ehtesham S, Kennedy JL (2006) Serotonin transporter polymorphisms and persistent pervasive childhood aggression. Am J Psychiatry 163:1103–1105
Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A (1999) Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci 19:10494–10501
Blader JC (2006) Pharmacotherapy and postdischarge outcomes of child inpatients admitted for aggressive behavior. J Clin Psychopharmacology 26:419–425
Blier P, de Montigny C (1998) Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry 44:313–323
Bond AJ (2005) Antidepressant treatments and human aggression. Eur J Pharmacol 526:218–225
Brady KT, Myrick H, McElroy S (1998) The relationship between substance use disorders, impulse control disorders, and pathological aggression. Am J Addict 7:221–230
Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF (1979) Aggression in humans correlates with cerebrospinal-fluid amine metabolites. Psychiatry Res 1:131–139
Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK (1982) Aggression, suicide, and serotonin—relationships to CSF amine metabolites. Am J Psychiatry 139:741–746
Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA (1993) Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–580
Bushman, Cooper (1990) Effects of alcohol on human aggression: an integrative research review. Psychol Bull 107:341–354
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R (2002) Role of genotype in the cycle of violence in maltreated children. Science 297:851–854
Ceglia I, Acconcia S, Racasso C, Colovic M, Caccia S, Invernizzi RW (2004) Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol 142:469–478
Cloninger CR (1987) Neurogenetic adaptive-mechanisms in alcoholism. Science 236:410–416
Connor TJ, Kelliher P, Shen Y, Harkin A, Kelly JP, Leonard BE (2000) Effect of subchronic antidepressant treatments on behavioral, neurochemical, and endocrine changes in the forced-swim test. Pharmacol Biochem Behav 65:591–597
Cremers TIFH, de Boer P, Liao Y, Bosker FJ, den Boer JA, Westerink BHC, Wikström HV (2000) Augmentation with a 5-HT1A, but not a 5-HT1B receptor antagonist critically depends on the dose of citalopram. Eur J Pharmacol 397:63–74
Crowley JJ, Blendy JA, Lucki I (2005) Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology 183:257–264
Cryan JF, Mombereau C (2004) In search of a depressed mouse: utility of models for studying behavior in genetically modified mice. Mol Psychiatry 9:326–357
de Almeida RMM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA (2001) Zolmitriptan—a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology 157:131–141
de Almeida RMM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA (2006) 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology 185:441–450
de Boer SF, Lesourd M, Mocaer E, Koolhaas JM (1999) Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-HT1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J Pharmacol Exp Ther 288:1125–1133
de Boer SF, Lesourd M, Mocaer E, Koolhaas JM (2000) Somatodendritic 5-HT1A autoreceptors mediate the anti-aggressive actions of 5-HT1A receptor agonists in rats: an ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology 23:20–33
Delville Y, Mansour KM, Ferris CF (1996) Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav 59:813–816
De Vry J, Schreiber R, Melon C, Dalmus M, Jentzsch KR (2004) 5-HT1A receptors are differentially involved in the anxiolytic- and antidepressant-like effects of 8-OH-DPAT and fluoxetine in the rat. Eur Neuropsychopharmacol 14:487–495
Dziedzicka-Wasylewska M, Faron-Gorecka A, Kusmider M, Drozdowska E, Rogoz Z, Siwanowicz J, Caron MG, Bonisch H (2006) Effect of antidepressant drugs in mice lacking the norepinephrine transporter. Neuropsychopharmacology 31:2424–2432
Fahlke C, Hansen S (1999) Alcohol responsiveness, hyperreactivity, and motor restlessness in an animal model for attention-deficit hyperactivity disorder. Psychopharmacology 146:1–9
Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT (2001) Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology 24:370–378
Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y (1997) Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci 17:4331–4340
Fish EW, Faccidomo S, Miczek KA (1999) Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology 146:391–399
Fish EW, Faccidomo S, DeBold JF, Miczek KA (2001) Alcohol, allopregnanolone and aggression in mice. Psychopharmacology 153:473–483
Fish EW, Faccidomo S, Gupta S, Miczek KA (2004) Anxiolytic-like effects of escitalopram, citalopram, and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther 308:474–480
Gruenewald PJ, Freisthler B, Remer L, LaScala EA, Treno A (2006) Ecological models of alcohol outlets and violent assaults: crime potentials and geospatial analysis. Addiction 101:666–677
Haberstick BC, Smolen A, Hewitt JK (2006) Family-based association test of the 5HTTLPR and aggressive behavior in a general population sample of children. Biol Psychiatry 59:836–843
Hennig J, Reuter M, Netter P, Burk C (2005) Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behav Neurosci 119:16–25
Higley JD, Bennett AJ (1999) Central nervous system serotonin and personality as variables contributing to excessive alcohol consumption in non-human primates. Alcohol Alcohol 34:402–418
Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Linnoila M, Vickers JH (1992) Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry 49:436–441
Higley JD, Suomi SJ, Linnoila M (1996) A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res 20:629–642
Hinkers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A (2006) Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry 60:282–287
Hjorth S, Auerbach SB (1999) Autoreceptors remain functional after prolonged treatment with a serotonin reuptake inhibitor. Brain Res 835:224–228
Hjorth S, Westlin D, Bengtsson HF (1997) WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology 36:461–465
Hughes ZA, Starr KR, Scott CM, Newson MJ, Sharp T, Watson JM, Hagan JJ, Dawson LA (2007) Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649915-B. Psychopharmacology 192:121–133
Kalsner S (2000) The question of feedback at the somadendritic region and antidepressant drug action. Brain Res Bull 52:467–473
Krug EG, Mercy JA, Dahlberg LL, Zwi AB, Lozano R (eds) (2002) World Report on Violence and Health. World Health Organization, Geneva, Switzerland
Kugelberg FC, Carlsson B, Ahlner J, Bengtsson F (2003) Stereoselective single-dose kinetics of Citalopram and its metabolites in rats. Chirality 15:622–629
Linnoila M, Virkkunen M (1992) Aggression, suicidality, and serotonin. J Clin Psychiatry 53:46–51
Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK (1983) Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 33:2609–2614
Mansari ME, Sanchez C, Chouvet G, Renaud B, Haddjeri N (2005) Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology 30:1269–1277
Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF (2000) A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res 95:9–23
Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M (1994) Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman-primates. Am J Psychiatry 151:1485–1491
Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR (2006) Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci 103:6269–6274
Miczek KA, de Almeida (2001) Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology 157:421–429
Miczek KA, O’Donnell JM (1978) Intruder-evoked aggression in isolated and non-isolated mice—effects of psychomotor stimulants and l-dopa. Psychopharmacology 57:47–55
Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM (1992) Alcohol and “bursts” of aggressive behavior: ethological analysis of individual differences in rats. Psychopharmacology 107:551–563
Miczek KA, Barros HM, Sakoda L, Weerts EM (1998) Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res 22:1698–1705
Miczek KA, Fish EW, deBold JF, de Almeida RMM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology 163:434–458
Miczek KA, Faccidomo S, De Almeida RMM, Bannai M, Fish EW, DeBold JF (2004a) Escalated aggressive behavior—new pharmacotherapeutic approaches and opportunities Youth violence: scientific approaches to prevention. Ann NY Acad Sci 1036:336–355
Miczek KA, Fish EW, De Almeida RMM, Faccidomo S, DeBold JF (2004b) Role of alcohol consumption in escalation to violence. Youth violence: scientific approaches to prevention. Ann NY Acad Sci 1036:278–289
Mitchell PJ (2005) Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol 526:147–162
Mitchell PJ, Redfern PH (1997) Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav Pharmacol 8:585–606
Mitchell PJ, Fletcher A, Redfern PH (1991) Is antidepressant efficacy revealed by drug-induced changes in rat behaviour exhibited during social interaction? Neurosci Biobehav Rev 15:539–544
New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, O’Flynn K, Siever LJ (2004) Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology 176:451–458
Olivier B, Mos J, van Oorschot R, Hen R (1995) Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 28:80–90
Parker RN, Auerhahn K (1998) Alcohol, drugs, and violence. Annu Rev Sociology 24:291–311
Peremans K, Audenaert K, Hoybergs Y, Otte A, Goethals I, Gielen I, Blankaert P, Vervaet M, van Heeringen C, Dierckx R (2005) The effect of citalopram hydrobromide on 5-HT2A receptors in the impulsive-aggressive dog, as measured with I-123-5-I-R91150 SPECT. Eur J Nucl Med Mol Imaging 32:708–716
Perrault G, Morel E, Zivkovic B, Sanger DJ (1992) Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav 42:45–47
Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A (2003) In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci 100:2035–2040
Pińeyro G, Blier P, Dennis T, de Montigny C (1994) Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci 14:3036–3047
Popova NK (2006) From genes to aggressive behavior: the role of serotonergic system. BioEssays 28:495–503
Reist C, Nakamura K, Sagart E, Sokolski KN, Fujimoto KA (2003) Impulsive aggressive behavior: open-label treatment with citalopram. J Clin Psychiatry 64:81–85
Rilke O, Iwill K, Jahkel M, Oehler J (2001) Behavioral and neurochemical effects of anpirtoline and citalopram in isolated and group housed mice. Prog Neuro-Psychopharmacol Biol Psychiatry 25:1125–1144
Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food-sated and water-sated rats. Alcohol Clin Exp Res 10:436–442
Sanchez C, Bergqvist PBF, Brennum LT, Gupta S, Hogg S, Larsen A, Wilborg O (2003) Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology 167:353–362
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809
Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Overli O, Hoglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N (2005) Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool 78:679–694
Taravosh-Lahn K, Bastida C, Delville Y (2006) Differential responsiveness to fluoxetine during puberty. Behav Neurosci 120:1084–1092
van der Vegt BJ, van de Wall EHEM, Moya-Albiol L, Martinez-Sanchis S, Kato K, de Boer SF, Koolhaas JM (2003a) Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci 117:667–674
van der Vegt BJ, Lieuwes N, Cremers TIFH, de Boer SF, Koolhaas JM (2003b) Cerebrospinal fluid monoamine and metabolite concentrations and aggression in rats. Horm Behav 44:199–208
van Erp AMM, Miczek KA (1997) Increased aggression after ethanol self administration in male resident rats. Psychopharmacology 131:287–295
Verona E, Joiner TE, Johnson F, Bender TW (2006) Gender specific gene-environment interactions on laboratory-assessed aggression. Biol Psychol 71:33–41
Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ (2006) Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet 36:163–172
Wrase J, Reimold M, Puls I, Kienast T, Heinz A (2006) Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci 6:53–61
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (USPHS Award AA013983 and Fellowship Award F32AA015013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caldwell, E.E., Miczek, K.A. Long-term citalopram maintenance in mice: selective reduction of alcohol-heightened aggression. Psychopharmacology 196, 407–416 (2008). https://doi.org/10.1007/s00213-007-0972-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0972-z