Abstract
One of the most serious man-made concerns today is the ever-increasing amount of plastic waste overwhelming the planet. The worldwide interest in using polymers consistently expanded over the years. Because of the plastic wastes thrown into the environment, outrageously the plastic pollution is increasing. In the present study, degradation of PVC and polyethylene-derived synthetic polymers has been carried out. The fungi and bacteria were isolated from the soil of the plastic waste environment and were used for the biodegradation of plastic films. Successful bacterial candidates for biodegradation were identified after screening. The bacterial strain Sb1 was identified as Bacillus licheniformis and Sb2 as Achromobacter xylosoxidans. The fungal strains Sf.1 and Sf.2 were identified as Aspergillus niger and Aspergillus glaucus, respectively. The degraded polymeric films were critically assessed by following the characterization methods like weight loss, FTIR and SEM. The results indicate that the polymers of polyethylene sample showed 32.2% degradation using bacterial strains and 40% using fungal strains in a time duration of just 4 weeks. PVC samples degraded 17 and 32% by fungal strains after 4 weeks. The changes in surface topography was confirmed by scanning electron microscopy and the changes in functional groups intensity was observed using the FTIR. Different parameters, varying temperature, pH, and inoculum concentration, were also evaluated, which implied that plastic waste treated by fungal and bacterial strains gives significant (p < 0.05) result in polymer degradation. As a result, the current research gave a scientific justification that bacteria and fungus could be further developed as promising candidates for plastic bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term "plastic" refers to a wide spectrum of high molecular weight polymers, the commercial plastics used in society, such as food and drink containers, textile fibers, and building materials, are limited to a few varieties. Every year, about 320 million tons of plastic are manufactured, which is expected to threefold by 2034 (Ritchie 2018; European Parliament 2020). As a result, around eight million metric tons of plastic garbage are deposited each year into either landfills or the ocean, causing environmental destruction. (Alvarez-Barragan et al. 2016). Apart from direct exposure, poor plastic waste management has been linked to substantial health consequences and increased mortality in low- and middle-income nations because of open burning and obstruction of rivers, resulting in higher rates of respiratory and waterborne infections, respectively (Williams et al. 2019).
Synthetic plastics account for about 80 percent of overall plastic use worldwide. Polyethylene, polypropylene, polystyrene, polyester polyurethane (PU), polyvinylchloride (PVC), polyethylene terephthalate (PET), polytetrafluoroethylene (PTFE), nylon and polycarbonate are some of the most commonly used synthetic plastics (Alvarez-Barragan et al. 2016). In many respects, plastic has helped the human being and with the population outburst and economic prosperity, it is the use increased. These plastics are used in the aeronautics, building and construction industries, packaging, electronics, transport and refrigerator insulation (Khan et al. 2017). Ethylene long-chain polymers such as low-density polyethylene and high-density polyethylene have been used commercially in packaging. In the packaging industry, these long-chain polymers are seeking attention because of their low weight (Begum et al. 2015). Despite that, Plastics have many benefits, but they also pose significant environmental threats (Awasthi et al. 2017).
Pakistan is a developing country with a long history of dealing with plastic trash issues. Plastic waste mostly found in the form of plastic bags, which are commonly utilized for everyday activities. According to the Environmental Protection Agency, Pakistan consumes about 55 billion disposable plastic bags per year. This plastic waste has built up in water reservoirs and canals, obstructing wastewater treatment systems (Mansoor 2019). The huge plastic waste needs to be reduced by the locally isolated bacteria and fungi in a cost-effective and environment-friendly manner. The microbial degradation of plastic waste is gaining more attention in current scenario. Biodegradation is the natural and cost-effective process by which microorganisms transform the complex polymers into the simple monomers by releasing the intracellular or extracellular enzymes (Chaudhary and Vijayakumar 2019). Microorganisms were used to evaluate the biodegradability of polymers, which included both natural and synthetic polymers (Tamnou et al. 2021). In many studies, it was reported that bacteria and fungi like Penicillium sp. Vibrio sp. Bacillus sp. and Aspergillus sp. Streptomyces sp. and Pseudomonas sp. could degrade plastic waste (Odusanya et al. 2013; Sarkhel et al. 2020). The fungus species Aspergillus flavus, Aspergillus versicolor, and Fusarium solani were reported as potential candidates in biodegradation of plastics (Nanda et al. 2010; Sowmya et al. 2014; Das et al. 2018).
The goal of this research was to isolate and identify bacteria and fungi that can degrade PVC and polyethylene. Weight loss, FTIR, and findings of SEM analysis were used as proof of biodegradation, confirming that bacteria are capable of degradation.
Material and methods
Isolation and primary screening
The soil samples were collected from the plastic waste dumping sites of Multan, Pakistan and the depth was approximately 5 cm. Each sample was labeled and taken to the laboratory under sterile conditions. Then all the information including the sampling date, location, and site was recorded. The collected samples were processed directly to the isolate the plastic-degrading bacteria and fungi. Isolation of fungal and bacterial strains was obtained using the serial dilution methods. Each sample was spread onto nutrient agar (Sigma Aldrich, USA) plates and potato dextrose agar (Sigma Aldrich, USA) supplemented with the different types of fine polymeric powders PVC (Poly vinyl chloride) and Polyethylene (PE) (Sigma Aldrich, USA). Plates were incubated at 37 °C for one week. The streak plate method was used to further purify the fungal and bacterial strains that showed halo zones of activity. The primary screening of microbial isolates was based on the clearance zone; these isolates were selected to further check their biodegradation potential (Sarkhel et al. 2020).
Molecular characterization of microbial isolates by ribotyping
The bacterial and fungal isolates, which showed maximum biodegradation properties, were subjected to 16S and 18S ribosomal RNA sequencing. The obtained sequences were subjected to a BLAST search for retrieving the best homologous sequences. Finally, the phylogenetic tree was constructed using the aligned sequences of the 16S rRNA gene using Neighbor-joining method on MEGA7.0 software.
In vitro study of degradation potential of microorganisms
Plastic deterioration in the liquid medium
To study the biodegradation potential of fungal and bacterial isolates 50 ml of Minimal salt media (MSM) (Sigma Aldrich, USA) was used just to provide the carbon-free medium. The polymer films/strips used were purchased from commercial manufacturers and plastic waste dumping sites. The isolates of bacteria and fungi in minimal media (Sigma Aldrich, USA) supplemented with Polymer films/strips were incubated in an incubator shaker at 150 rpm for one month. The initial weight of all polymeric films (2 × 2 cm) were recorded. These PVC films and Polyethylene strips were surfaces sterilized using the 70% ethyl alcohol and then oven dry for 24 h. The control comprising of minimal salt medium and polymeric films/strips without microbial isolates were used (Sarkhel et al. 2020).
Plastic deterioration test in soil
The PVC and Polyethylene films were buried in autoclaved soil pots with bacterial and fungal inoculum then incubated for two months in dark settings. The control comprising of autoclaved soil pots and polymeric films/strips without microbial inoculum was used.
After two months of incubation films/strips of plastics (PVC and Polyethylene) washed with 70% ethyl alcohol, followed by oven dry and then weigh for the weight loss measurement. Scanning electron microscopy carried out the morphological analysis of these polymer samples (Jain et al. 2021).
Biodegradation end products analysis
Determination of degradation by weight loss method
The rate of biodegradation was determined by the reduction in weight, recorded after one month of incubation using the method of weight loss. The plastic films/strips were washed in 70% ethyl alcohol, oven-dried, and weighed then the weight loss percentage computed using the formula (Sarkhel et al. 2020).
The formula for weight loss percentage is:
Scanning electron microscopy
After 1-month incubation of the bacterial and fungal cultures, the plastic samples were washed with 70% ethanol and then with distilled water to clean their surface. The morphological analysis of these polymeric samples was carried out by scanning electron microscope (VEGA3 – TESCAN Czech Republic). The Scanning electron microscopy reveals the structural changes in control and treated films with the microbial isolates. A sample was adhered to SEM holder stub using carbon tape and gold sputtering was carried out before the analysis (Chaudhary and Vijayakumar 2019).
Fourier-transform infrared (FTIR) analysis
The polymer films of PVC and polyethylene were incubated with the Microbial isolates for 30 days in MSM broth. The plastic sheets were removed from1 the medium after 30 days washed with the distilled water and 70% Ethyl alcohol. The polymer sheets were then oven-dried and analyzed by FTIR (Bruker, Germany) to confirm degradation (Kumari et al. 2014).
Impact of different parameters on plastic degradation
The degradation of polymeric films were performed at different pH and temperature levels.
Effect of temperature
The polymer films of both PVC and polyethylene with the bacterial and fungal inoculum were kept under an incubation period of 4 weeks at varying temperatures: 25, 37 and 45 ℃.The various temperatures ranges were used to analyze the best-optimized temperature condition suitable for the growth of these microorganisms.
Effect of pH
The polymer films of PVC and polyethylene with the fungal and bacterial strains after keeping at an incubation period of 4 weeks were studied for different pH: 5, 7, and 9 for evaluating the growth of microorganisms at best-optimized pH condition (Khan et al. 2017).
Effect of inoculum concentration
The polymeric films were inoculated with various bacterial and fungal strains then incubated for 4 weeks. The different inoculum concentrations used of 3 ml/100 ml, 5 ml/100 ml, and 7 ml/100 ml and 10 ml/100 ml respectively. Different concentrations of inoculum was used to achieve the optimal concentration of inoculum dosage for best microbial growth and their effect on the polymer surfaces (Sarkhel et al. 2020).
Statistical analysis
The experiments were done in triplicate with the results presented as a mean value with standard deviation (Mean ± SD). The data were analyzed using analysis of variance, and the significant difference between the means was compared using T test at a significance level at p < 0.050. The GraphPad Prism 9.1.0 was used for statistical analysis.
Results and discussion
Molecular identification
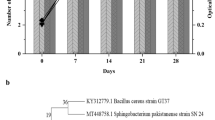
The four microbial isolates (2 bacterial strains and 2 fungal strains) shown the growth with the wide clear zone around them after 5–7 days of incubation in primary screening. These microbial strains were selected in this preliminary screening and further used for biodegradation assays. The molecular identification of the fungal species based on the 18S rRNA sequencing and 16S rRNA sequencing methods made bacterial identification. The bacterial strain Sb1 and Sb2 were identified as Bacillus licheniformis (Accession number NR_118996.1) and Achromobacter xylosoxidans (Accession number NR_118403.1). The fungal strains Sf1 and Sf2 were identified as Aspergillus niger (Accession number NG_065763.1) and Aspergillus glaucus (Accession number NG_063391.1). The phylogenetic analysis was made by Neighbor-joining method on MEGA7 using the bootstrap value of 500 replicates (Fig. 1). The potential fungal and bacterial strains were selected for the experimental study based on biodegradation screening results.
Representation of Phylogenetic tree for bacterial strains SB1 (A) and SB2 (B), fungal strains Sf.1 (C) and Sf.2 (D) by Neighbor-joining Method on MEGA 7 along with the type strains retrieved from the gene bank NCBI. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches
Biodegradation study in liquid medium by fungus and bacteria
For the assessment of deterioration of plastics by these microbial isolates, weight, loss percentage was calculated. The degradation efficacy of PVC and PE films was assessed by comparing dry weight loss percentages. The bacterial strain Sb.1 and Sb.2 indicated the weight loss percentage 32.2 ± 3.3% and 30 ± 3.3%, respectively for polyethylene. For PVC films, weight loss percentage of Sb.1 and Sb.2 is 15 ± 3.3% and 17 ± 3.3%, respectively. The fungal isolates Sf.1 and Sf.2 indicated the weight loss percentage of 40 ± 3.3% and 25 ± 3.3%, respectively for polyethylene. The weight loss percentage of PVC for fungal strains Sf.1 and Sf. 2 was 10 ± 3.3% and 32 ± 3.3%, respectively (Fig. 2). Reduced weight loss indicated that bacterial and fungal strains used polymers PVC and polyethylene were used by bacterial and fungal strains as a sole carbon source. The weight reduction and surface erosion process involved in the degradation of plastic products is most likely due to bacterial and fungal enzymatic reactions (Skariyachan et al. 2016). The use of weight loss as a metric of polymer biodegradation has been extensively acknowledged and employed by numerous studies (Muhonja et al. 2018).

source in lab. B Degradation potential of fungal and bacterial isolates in Soil burial test in terms of weight loss percentage. The results shown are the average of three independent experiments. Error bars indicate standard deviation (± SD). *p < 0.05
The degradation profile of polymers in terms of weight loss percentage. A Fungal and bacterial isolates were grown in a minimal salt medium with the sole carbon
Biodegradation study in soil
Biodegradation assessment in soil burial experiment
The degradation efficiency of microbial isolates in aseptically sterilized soil determined by weight loss percentage. The bacterial isolate Sb.1 and Sb.2 exhibited the deterioration of PVC with 43 ± 3.3% and 32.1 ± 3.3% weight loss percentage, respectively. The degradation efficiency of fungal isolates Sf.1 and Sf.2 for PVC with reference to weight loss percentage was 31 ± 3.3% and 32 ± 3.3%, respectively. The bacterial isolates Sb.1 and Sb.2 revealed the deterioration of polyethylene polymers with 20 ± 3.3% and 13 ± 3.3% weight loss percentage, respectively. The degradation efficiency of fungal isolates Sf.1 and Sf.2 for polyethylene in terms of weight loss percentage was 12 ± 3.3% & 15 ± 3.3%, respectively. In a previous study (Hikmah et al. 2018) weight, loss percentage for polyethylene was 7.5 ± 3.3% in 35 days. In current research fungal treatment, increase the percentage by 10–12% in 28 days (Fig. 2). The fungal and bacteria degradation results are in accordance to the study conducted by (Muhonja et al. 2018) who reported the degradation of virgin Polyethylene.
Scanning electron microscopy
The SEM analysis used to provide all the structural changes observed on the plastics triggered by the microbial isolates. With the application of scanning electron microscopy, the initial biodegradation tests were further confirmed. Corrosion of the surface morphology of the plastic film after treatment with the purified microbial isolate was observed. SEM analysis revealed major changes in the physical structure of the PVC film and polyethylene when they exposed to fungal filtrate and bacterial culture. (Figs. 3, 4, 5, 6). The presence of multiple holes/cracks and changes in the plastic film surface structure was observed to help the biodegradation of these plastics due to the treatment of microbial enzymes. The surface of polymer films was smooth and shiny before the treatment with microbial isolates. After the degradation assay, PVC and polyethylene film surface lost their smoothness and SEM analysis confirmed that. The variations like erosion, pits, and cracks were observed on the polymer's surface area due to attachment of fungal and bacterial cells by the formation of biofilms. Many studies reported that variation is may be due to the activity of intercellular or extracellular enzymes of microbes (Skariyachan et al. 2016, 2017). Microbial strains used these polymers as the only carbon source, which was indicated by the morphological changes on the surface of polymer films.
The Study by (Bhatia et al. 2014) documented that the surface erosion process involved in the deterioration of plastic products is most likely due to bacteria and fungus enzymatic reaction. Anwar et al. (2016) also reported the progressive surface degradation of PVC by bacterial strains using the SEM analysis.
Fourier-transform infrared spectroscopy (FTIR)
Changes in bond breaking, chemical transfer and the development and absence of the areas of interest that allow us to assess the degree of biodegradation of polymers with the help of FTIR are the latest functional groups. When the rate of degradation increases over time, as many monomeric and oxidative forms of polyethylene and PVC are obtained and the peaks becomes wider (Bonhomme et al. 2003).
The FTIR spectrum of polyethylene film treated with the sample Sb.1 revealed some structural changes. A new peak emerged in the peak region 1025 cm−1 corresponds to the stretching of C–N (amine) bond. A shift in peaks can be seen in the region 1470–1461 cm−1 indicated the C–H methyl group) bending. The Sb1 treated PVC film spectra that a new peak emerged in the 600–700 cm-1 corresponds to the C–H bond's bending. The PVC spectra also revealed the formation of a few new peaks in the region of 900–1500 cm−1, which indicates C=C bending and O–H bending. The peaks removal observed at the region of 1900–2200 cm−1 in the treated PVC film, which was present in the virgin (untreated) film. These results are in accordance with the study conducted by (Sarkhel et al. 2020) and showed many similar peaks.
The new functional groups that are not observed in the virgin PE film indicate biodegradation by the bacterial strain Sb.2. It was revealed by many structural variations between the peaks region 700–1500 cm−1 compared with the control. The new functional groups appeared which are not observed in the virgin PE film is the indication of biodegradation by the bacterial strains. The new peaks indicated the strong C=C bending, C–O (aromatic ester) stretching and O–H (carboxylic acid) bending. The FTIR spectra of bacterial treated PVC film indicating the degradation evidence. The new peaks observed in the region 1022 and 1639 cm−1 indicating the O–H bending and N–H (amine) bending. In addition, the appearance of new peaks in the spectra of fungal-treated PVC sheets revealed a chemical shift of peaks caused by microbial biodegradation activity (Ali et al. 2014).
The analysis of FTIR spectrum of PE film treated with the fungal strain Sf.1 indicated the shift of peak from the regions 717.45 cm−1 to 718.10 cm−1 and 1470.99 cm-1 to 1461.86 cm−1 respectively. The new functional group can be observable in the peak region 1031.94 cm−1 in the treated film that cannot be observed in the virgin PE film. The new peak at 1461.86 and 1031 cm−1 indicated the C–H bending and S=O (sulfoxide) stretching respectively (See Table.1).
The FTIR spectra of Sf1 treated PVC film demonstrated the functional groups removal in the peak region from 700 to 750 cm−1 compared with the control. The new peak appeared at the region of 900–1450 cm−1 indicating the C=C (alkene) and O–H bending. Another shift of peaks can be observed in the region of 1900–2100 cm−1 r and 2900–3000 cm−1 revealing the N=C=S (isothiocyanate) stretching. The similar kind of variations in wavenumbers were also reported by (El-Sayed et al. 2021) using the microbial species in PVC degradation.
The FTIR spectrum of Sf.2 treated PE film clearly shows new peaks' formation and shift at different regions. The shift of peak can be seen from 717.45 to 717.73 cm−1 and another shift is at the region of 1470.99–1461.61 cm−1 in the treated PE film compared with the untreated film. The formation of new functional groups can be seen in the region 1076.88 cm-1 that is not present in the virgin PE film. The new groups appeared in the region of 1076 and 1461 cm−1 reveled the C-H bending and C=C bending respectively. The occurrence of new peaks and degradation of some peaks confirmed the biodegradation of PE by fungal mediated enzymes and a study conducted by (Das et al. 2018) reported similar results.
The fungal-treated PVC film’s spectrum shown the formation of three new peaks at the region between 900 cm−1 and 1500 cm−1. The first new peak emerge in the region 956 cm−1 indicated the C=C bending in the treated PVC film. The new peaks in the region 1251 and 1426 cm−1 indicated the C–O (aromatic ester) stretching O–H bending respectively. The major structural changes C–H bond bending, C–O stretching, ester ethylene and C–H stretching are the clear indications of degradation in compared to the control (Roy et al. 2021) (See Table. 2) (Figures in supplementary data).
Effect of different growth conditions on the degradation of plastics
Temperature plays a critical role in degradation as it can affect microbial growth. The rate of deterioration was analyzed using a range of temperatures from 25 to 45 °C. The better results in terms of weight loss percentage were obtained at 37 °C after 4 weeks of incubation of bacterial and fungus in the presence of polymeric films (Fig. 7). The reason may be that, at this temperature the microbial growth was higher than the other temperatures, which results the higher degradation rate (Muhonja et al. 2018). The pH also plays a very important role in the growth of microorganisms (Fig. 8). The study also evaluated the effect of pH on the rate of degradation of plastics. The best degradation rate was observed at alkaline pH. The microbial growth was low at the acidic pH, that was may be the reason for lower degradation which was also reported by (Skariyachan et al. 2017). The current study found that the isolates could be effectively grown at pH 8.5, implying that vast plastic waste dumps raise soil pH, allowing bacteria to adapt to such conditions. The degradation rate was also evaluated at different dose of inoculum concentration. The amount of inoculum concentration plays a significant role in the biodegradation system. In some cases, a small dose of inoculum is insufficient to degrade the polymers. The inoculum doses that; are too high may result in poor degradation of the material (polymer). Thus, the right amount of inoculum is important for the setup of degradation systems. The degradation showed an increasing trend as inoculum dose increased (Fig. 9). The results are in accordance with the study conducted by (Sarkhel et al. 2020) the increase in inoculum concentration signified the rate of degradation.
A The PVC degradation profile in terms of weight loss percentage by bacterial and fungal strains at various temperatures (25, 37 and 45). B PE degradation in terms of weight loss percentage at various temperatures (25, 37 and 45). The results represent the average of three independent experiments. The error bar indicates the standard deviation (± SD). *p < 0.005
A The PVC degradation profile in terms of weight loss percentage by bacterial and fungal strains at various pH (5, 7 and 9). B Polyethylene (PE) degradation in terms of weight loss percentage at various pH (5, 7 and 9). The results represent the average of three independent experiments. The error bar indicates the standard deviation (± SD). *p < 0.005
A The PVC degradation profile in terms of weight loss percentage by bacterial and fungal strains at inoculum dosage (3, 5, 7, and 10 ml). B Polyethylene (PE) degradation in terms of weight loss percentage at various inoculum doses (3, 5, 7, and 10 ml).The results represent the average of three independent experiments. The error bar indicates the standard deviation (± SD). *p < 0.005
Conclusion
Bacterial and fungal strains have the potential to be used in the management of plastic waste. The current study deals with the influence of microbial strains on the degradation of polymer films obtained from PVC and polyethylene plastics. When microbial treated samples were compared to control samples, polymers' mechanical strength and stabilityk both reduced. The degradation was further confirmed by scanning electron microscopy and Fourier-transform infrared spectroscopy (FTIR). The present study evaluated the potential of the fungal and bacterial strains in the degradation of plastic in in-vitro and in soil burial methods. These findings suggest that bacteria and fungus could be utilized to generate inocula for biodegradation trials to improve PVC and PE decomposition under aerobic circumstances. The findings of this study could be useful for removing polyethylene and PVC safely in the presence of microorganisms and could be a viable solution to the society's plastic waste issue.
References
Ali MI, Ahmed S, Robson G, Javed I, Al N, Atiq N, Hameed A (2014) Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J Basic Microbiol 54(1):18–27. https://doi.org/10.1002/jobm.201200496
Alvarez-Barragan J et al (2016) The biodegradative activity of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl Environ Microbiol 82:5225–5235. https://doi.org/10.1128/AEM.01344-16
Anwar MS, Kapri A, Chaudhry V, Mishra A, Ansari MW, Souche Y, Goel R (2016) Response of indigenously developed bacterial consortia in progressive degradation of polyvinyl chloride. Protoplasma 253(4):1023–1032. https://doi.org/10.1007/s00709-015-0855-9
Awasthi S, Srivastava N, Singh T, Tiwary D, Mishra PK (2017) Biodegradation of thermally treated low-density polyethylene by fungus Rhizopus oryzae NS 5. 3Biotech 7(1):73–80. https://doi.org/10.1007/s13205-017-0699-4
Begum MA, Varalakshmi B, Umamagheswari K (2015) Biodegradation of polythene bag using bacteria isolated from soil. Int J Curr Microbiol Appl Sci 4(11):674–680
Bhatia M, Girdhar A, Tiwari A, Nayarisseri A (2014) Implications of a novel pseudomonas species on low density polyethylene biodegradation: an in vitro to in silico approach. Springerplus 3:497. https://doi.org/10.1186/2193-1801-3-497
Bonhomme S, Cuer A, Delort AM, Lemaire J, Sancelme M, Scott G (2003) Environmental biodegradation of polyethylene. Polym Degrad Stab 81(3):441–452. https://doi.org/10.1016/S0141-3910(03)00129-0
Chaudhary AK, Vijayakumar RP (2019) Studies on biological degradation of polystyrene by pure fungal cultures. Environ Dev Sustain. https://doi.org/10.1007/s10668-019-00394-5
Das MP, Kumar S, Das J (2018) Fungal-mediated deterioration and biodegradation study of low-density polyethylene (LDPE) isolated from municipal dump yard in Chennai, India. Energy Ecol Environ 3(4):229–236. https://doi.org/10.1007/s40974-018-0085-z
El-Sayed MT, Rabie GH, Hamed EA (2021) Biodegradation of low-density polyethylene (LDPE) using the mixed culture of Aspergillus carbonarius and A. fumigates. Environ Dev Sustain. https://doi.org/10.1007/s10668-021-01258-7
European Parliament (2020) plastics—the facts 2020. An analysis of European plastics production, demand, and waste data. Available from: https://www.plasticseurope.org/en/resources/market-data (Accessed 07 March 2021).
Hikmah M, Setyaningsih R, Pangastuti A (2018) The potential of lignolytic trichoderma isolates in LDPE (low-density polyethylene) plastic biodegradation. IOP Conf Ser: Mater Sci Eng 333:012076. https://doi.org/10.1088/1757-899X/333/1/012076
Jain K, Bhunia H, Reddy MS (2021) Degradation of polypropylene-poly-L-lactide blends by bacillus isolates: a microcosm and field evaluation. Bioremediat J. https://doi.org/10.1080/10889868.2021.1886037
Khan S, Nadir S, Shah ZU, Shah AA, Karunarathna SC, Xu J, Khan A, Munir S, Hasan F (2017) Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ Pollut 225:469–480. https://doi.org/10.1016/j.envpol.2017.03.012
Kumari R, Singh S, Pradhan N, Chandni S, Karthik L, Kumar G, Rao KB (2014) RSM optimized media to increase the antibacterial activity of wild and mutated strain of Nocardiopsis VITSRTB. Res J Pharm Tech 7(2):213–220
Mansoor A (2019) Environment: how plastic is killing US dawn. https://www.dawn.com/news/1477373. Accessed 22 Nov 2019
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 13(7):e0198446. https://doi.org/10.1371/journal.pone.0198446
Nanda S, Sahu S, Abraham J (2010) Studies on the biodegradation of natural and synthetic polyethylene by pseudomonas spp. J Appl Sci Environ Manag. https://doi.org/10.4314/jasem.v14i2.57839
Odusanya SA, Nkwogu JV, Alu N, Udo GE, Ajao JA, Osinkolu GA, Uzomah AC (2013) Preliminary studies on microbial degradation of plastics used in packaging potable water in Nigeria. Niger Food J 31(2):63–72. https://doi.org/10.1016/S0189-7241(15)30078-3
Ritchie H (2018) Plastic pollution. Published online at Our World in Data.org. Retrieved from: https://ourworldindata.org/plastic-pollution
Roy R, Mukherjee G, Gupta AD, Tribedi P, Sil AK (2021) Isolation of a soil bacterium for remediation of polyurethane and low-density polyethylene: a promising tool towards sustainable cleanup of the environment. 3Biotech 11(1):1–14. https://doi.org/10.1007/s13205-020-02592-9
Sarkhel R, Sengupta S, Das P, Bhowal A (2020) Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine sources. J Polym Res 27(1):16. https://doi.org/10.1007/s10965-019-1973-4
Skariyachan S, Manjunatha V, Sultana S, Jois C, Bai V, Vasist KS (2016) Novel bacterial consortia isolated from plastic garbage processing areas demonstrated enhanced degradation for low-density polyethylene. Environ Sci Pollut Res 23(18):18307–18319. https://doi.org/10.1007/s11356-016-7000-y
Skariyachan S, Setlur AS, Naik SY, Naik AA, Usharani M, Vasist KS (2017) Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ Sci Pollut Res 24(9):8443–8457. https://doi.org/10.1007/s11356-017-8537-0
Sowmya HV, Ramalingappa MK, Thippeswam B (2014) Biodegradation of polyethylene by Bacillus cereus. Adv Polym Sci Technol Int J 4(2):28–32
Tamnou EB, Arfao AT, Nougang ME, Metsopkeng CS, Ewoti OV, Moungang LM, Nana PA, Takang-Etta LR, Perrière F, Sime-Ngando T, Nola M (2021) Biodegradation of polyethylene by the bacterium Pseudomonas aeruginosa in acidic aquatic microcosm and effect of the environmental temperature. Environ Chall 3:100056. https://doi.org/10.1016/j.envc.2021.100056
Williams M, Gower R, Green J, Whitebread E, Lenkiewicz Z, Schroder P (2019) No time to waste: tackling the plastic pollution crisis before it’s too late. Tearfund, London
Acknowledgements
The Authors would like to thank, Department of Microbiology and Molecular Genetics, The Women University Multan and Higher Education Commission Pakistan for providing support and all the facilities.
Funding
No funds have been received.
Author information
Authors and Affiliations
Contributions
SS: Conceived idea and design the study, collected the data from experimental methodology, wrote the paper final draft. AI: Design the study; provide analysis tools and other facilities, contributed the data. FD: Analysis of the study, Proof reading of the final draft contributed the data.
Corresponding author
Ethics declarations
Conflict of interest
Authors reported no conflict of interest.
Ethical approval
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saeed, S., Iqbal, A. & Deeba, F. Biodegradation study of Polyethylene and PVC using naturally occurring plastic degrading microbes. Arch Microbiol 204, 497 (2022). https://doi.org/10.1007/s00203-022-03081-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03081-8