Abstract
Rheumatoid arthritis (RA) is associated with local and systemic inflammation that induces many changes in the skeletal health. Locally, periarticular bone loss and juxta-articular bone erosions may occur while joint ankylosis, generalized bone loss, osteoporosis, and fractures may develop secondary to inflammation. The aim of this narrative review is to summarize the clinical evidence for abnormal skeletal health in RA, the effects of disease modifying anti-rheumatic drugs (DMARDS) on bone health, and the effects of drugs for the prevention or treatment of osteoporosis in the RA population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
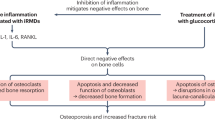
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases affecting nearly 1% of the population and is associated with disability and systemic complications. The pathogenesis of RA is not fully understood but likely results from a combination of factors including environmental (smoking, periodontitis, or gut microbiome), genetic (susceptibility genes), and epigenetic modifications that promote loss of tolerance [1]. RA is characterized by chronic, symmetrically small, and large joint synovitis leading to progressive inflammatory polyarthritis. The majority of RA patients have autoantibodies, including rheumatoid factors (RF) and anti-citrullinated protein antibodies (ACPA) [2]. RA patients may develop periarticular osteopenia, erosions of the subchondral bone of the joint margins, and systemic osteoporosis with increased risk of fractures (Table 1). Prior to effective DMARDS, joint fusion was a significant complication of RA synovitis but is now less common. Skeletal complications of RA are declining with utilization of DMARDS likely secondary to decreasing the pro-inflammatory cytokines driving the chronic inflammation. However, despite significant progress in the treatment of RA, some skeletal manifestations of disease including fractures and osteoporosis still occur at high frequencies.
Localized periarticular bone erosions occur early in rheumatoid arthritis
Juxta-articular bone erosions, breaks in the cortical bone with loss of underlying trabecular bone, are a hallmark of RA and occur early in disease. Bone erosions, visible by ultrasound, high-resolution computerized tomography (CT), magnetic resonance imaging (MRI), and plain radiography, are typically found on the radial aspects of the finger joints at sites where the synovium contacts the bone. Erosions are predictive of more severe course of rheumatoid disease with increased disability and mortality [3]. Within 3 years, 63% of RA patients will have erosions with 74% of these occurring in the first year and 97% within the second [4]. For some patients, erosions may be present within the first 3 months of clinical disease [4]. ACPA antibodies are the strongest predictor of clinical radiographic progression [5, 6]. A recent study evaluated the contribution of RF to erosions and joint space narrowing and found that RF positivity strongly predicted radiographic progression in early RA patients recruited from 1986 to 2001; however, in a modern early RA cohort recruited from 2002 to 2013, the presence of RF failed to predicted radiographic progression [7]. Other RA-associated autoantibodies including anti-carbamylated protein antibodies are also associated with increased erosions and joint damage [8]. Smoking is another strong risk factor for radiographic progression of RA with an adjusted OR = 2.17, 95% confidence intervals (CI 1.06–4.45) [9]. Smoking also induces ACPA autoantibodies possibly further increasing the risk for erosive RA [10]. Fortunately, treatment with conventional and biologic DMARDS stabilizes and limits joint erosions and damage [11].
Erosions occur secondary to pannus formation at the interconnection of the synovium with the cartilage and bone and are most frequently found on the radial surface sparing the palmar and volar surfaces [12, 13]. Pannus is the result of synovial fibroblast proliferation and infiltration of the synovial lining with inflammatory cells including T and B cells, plasma cells, dendritic cells, monocytes, and macrophages [2]. The synovial fibroblasts within the pannus are inappropriately activated through unknown mechanisms and secrete numerous pro-inflammatory cytokines and metalloproteinases leading to sustained synovial inflammation. These metalloproteinases further promote cartilage catabolism at the synovium cartilage interface leading to the classic juxta-articular bone erosion. A unique feature of RA bone erosions is their relative resistance to fully repair even when disease is well controlled. Failure to repair is largely due to inhibition of bone formation by the upregulation of inhibitors of WNT signaling preventing osteoblast differentiation. Potent WNT inhibitor, Dickkopf-related protein 1 (Dkk-1), is upregulated in the synovial tissue by pro-inflammatory cytokines like TNFα [14]. Elevated serum DKK-1 levels are associated with radiographic progression in the 2-year prospective ESPOIR cohort of 813 patients with early RA [15]. Other WNT inhibitors including frizzled-related protein-1 and sclerostin are also increased by synovial inflammation and participate in suppression of bone formation [16, 17]. While treatment of RA with DMARDS promotes stabilization of bone erosions, total erosion healing is still controversial [18, 19]. Anti-sclerostin antibody treatment of osteoporosis is currently in phase III trials, and it will be of great interest to see if WNT-inhibition will promote RA erosion healing.
Joint ankylosis may occur in long-standing rheumatoid arthritis
Spontaneous fusion of the small joints of the hand, wrists, ankle, and forefoot is frequently seen in long-standing RA, especially prior to widespread use of DMARDS. Detected by conventional radiography, bone ankylosis occurs infrequently (0.8%) and is found almost exclusively in long-standing disease [20]. In a Swedish cohort of 325 RA patients with long-standing disease (≥9 years) recruited between 1998 and 2001, only six (1.8%) had ankylosis of the small joints of the hands whereas no fusion was found in a second cohort of 310 patients with median disease duration of 4 years (range 1.5–10) [21]. Using MRI, ankylosis was detected in 10.6% of RA patients and was strongly associated with longer duration of disease [20].
Cervical spine ankylosis also occurs in RA in approximately 9–80% of patients depending on the imaging modality leading to instability, stenosis, and cervical myelopathy [22, 23]. The presence of wrist joint ankylosis is strongly associated with cervical ankylosis (p < 0.01) [24]. Meta-analysis reveals other risk factors for cervical spine involvement in RA include female gender, seropositivity, joint erosions, younger age, long RA duration, long-term corticosteroids, and higher markers of disease activity [25]. Non-biological and biological DMARDS can decrease the incidence of initial cervical spine involvement; however, in contrast to their success in treating peripheral joint manifestations, DMARDS may not prevent progression of cervical disease once it occurs [23].
Rheumatoid arthritis increases fracture risk
RA patients have approximately doubled the risk of fractures compared to gender and age-matched control patients [26, 27] (Table 2). Meta-analysis of seven observational studies including >600,000 patients found a pooled RR of vertebral fractures in RA to be 2.34 (95% CI 2.05–2.63, p < 0.0001) [28]. Fracture risk increases with disease activity and duration, with vertebral fractures approaching a sixfold increase with long-standing RA disease [29]. Having RA for >10 years increased the risk of hip fracture as well (RR 3.4, 95% CI 3.0–3.9). However, young women diagnosed with RA prior to age 50, but not similar young men, have increased non-pathologic fractures (RR 1.63, 95% CI 1.36–1.96) with hip and spine fractures being the most prevalent [30]. Low body mass index (BMI) (RR 3.9, 95% CI 3.1–3.9) and use of oral glucocorticoids (RR 3.4, 95% CI 3.0–4.0) were also associated with substantially elevated risk of hip fracture. Clinical osteoporotic fractures (RR 1.3, 95% CI 1.2–1.4) and hip fractures (RR 1.7, 95% CI 1.5–2.0) remained elevated even in patients that had not received glucocorticoids, consistent with underlying RA itself contributing to fracture risk. Another large study from the Women’s Health Initiative found that RA increased the risk of hip fracture compared to women without RA (RR 3.03; 95% CI 2.03–4.51, p < 0.001) [31]. Opioid use appears to further increase fracture risk in RA perhaps secondary to increased falls. A large nested control study of Canadian RA patients and controls found a significant increase in the odds ratio for nonvertebral fractures highest in the first 20 days of use OR 11.49 (CI 8.81–14.99) but persisting in chronic use as well >356 days OR 1.73 (CI 1.31–2.30) [32]. Taken together, these data indicate that RA is associated with significant increases in fractures and accordingly is included as a separate risk factor in the fracture risk assessment tool (FRAX) used to determine 10-year probability of fracture [33].
Rheumatoid arthritis is associated with osteoporosis
Factors predisposing RA patients to increased fracture including osteoporosis, chronic inflammation, immobility, increased fall risks, vitamin D deficiency, and glucocorticoid use [26, 34]. Generalized osteoporosis (bone mineral density (BMD) T-score <−2.5) is very common and likely is related to an imbalance in bone remodeling resulting in net bone loss. Most studies evaluating bone loss in RA have focused on lumbar spine BMD measurements and have found osteoporosis in 17 to 32% of RA patients with similar numbers if hip BMD is evaluated (15–36%) [35, 36]. Even in the modern era of early diagnosis and treatment, osteoporosis is still prevalent with 26.5% found in a recent cohort of RA patients recruited in 2009 and 2010 [37]. Although glucocorticoid (GC) use significantly increases the risk of BMD loss in RA patients, studies have shown that low BMD occurs in the absence of GC [38]. Loss of BMD occurs early in RA and increases with disease activity [39,40,41,42]. However, even recently diagnosed RA patients with disease for <2 years may have osteoporosis (11%) or osteopenia (24.7%) [43]. A retrospective study evaluating the timing of transition to osteoporosis in 360 female RA patients found that this population of women, with a mean age of 53.7 ± 10.2 years, had osteoporosis at baseline (15%) and another 23.2% of premenopausal and 25.9% of postmenopausal women transitioned to osteoporosis during the follow-up period (mean of 7.4 ± 5.0 years) [44]. The difference between pre-and postmenopausal women transitioning to osteoporosis was not significant and low baseline T-score positively correlated with transition to osteoporosis independent of menopausal status. However, other common risk factors for osteoporosis including low BMI, high RA disease activity, high RF titer, and GC use failed to predict transition to osteoporosis [44].
Mechanistically, generalized osteoporosis and fracture risk may be accelerated due to pro-inflammatory systemic cytokines overexpressed in RA. Pro-inflammatory cytokines including TNFα, IL-1, IL-6, and IL-17 induce the expression of receptor activator of NF-kB ligand (RANKL) leading to increased osteoclast development and activation causing generalized osteopenia and osteoporosis [45]. These same pro-inflammatory cytokines negatively impact osteoblast differentiation and their ability to produce mineralized matrix [46]. Increased disease activity, often associated with increased pro-inflammatory cytokines, as well as markers of bone turnover, significantly increases the risk of osteoporosis [47]. Duration of disease may be a more important indicator of accelerated bone loss in RA [48]. Vitamin D deficiency has been found to be extremely prevalent in RA patients ranging from 35 to 76% of RA patients [49, 50]. More recently, ACPA antibodies have also been associated with lower BMD in early untreated RA patients [51]. Interestingly, this study found that RA patients with high titer ACPA and rheumatoid factor had the most reduction in BMD. These findings, coupled with ACPA antibodies preceding clinical RA by up to 10 years, implicate ACPA in directly promoting dysregulated bone remodeling and the presence of early erosions in newly diagnosed patients. Interestingly, healthy subjects with ACPA also have dysregulated bone metabolism and develop bone loss prior to clinical disease [52]. Mechanistically, ACPA antibodies appear to directly activate osteoclasts and induce bone resorption and erosions by binding to citrullinated vimentin present on pre-osteoclasts and osteoclasts [53]. Additional risk factors for osteoporosis include sarcopenia, opioid use, and smoking. Female but not male RA patients have a high prevalence of sarcopenia (30–40%) that may further contribute to acceleration of bone loss [54,55,56,57].
Secondary osteoarthritis and RA
RA-induced joint destruction is one of the leading causes for total knee replacement (TKR) and total hip replacement (THR). RA severity and length of disease contribute to approximately 80% of patients with RA having TKR [58]. Although TKR and THR significantly improve mobility and quality of life, RA patients have increased risk of complications including infections and need for revisions [58, 59]. Based on a prospective population-based study of >30,000 RA patients recruited beginning in 1978, the risk of revision of TKR was 1.6 times higher for RA than osteoarthritis [60]. Meta-analysis of studies between 1990 and 2011 revealed an increased risk of dislocation following THR, risk of infection, and risk of early revision following TKA in RA versus OA [61]. Late revisions, 90-day mortality, or rates of venous thromboembolic events following THA or TKA in patients with RA versus OA were similar [61]. Tight control of RA appears to decrease or delay the need for TKR and THR [62,63,64].
Rheumatoid arthritis and osteonecrosis of the jaw
Given the significant burden of osteoporosis and fracture risk, many RA patients receive anti-osteoporosis therapies including oral or intravenous bisphosphonates, teriparatide, or denosumab. Although medication-induced osteonecrosis is most frequently seen in patients with malignancy, RA has been suggested to be a risk factor for osteonecrosis of the jaw (ONJ). There are numerous case reports of ONJ in RA patients but limited data suggests that RA is a risk factor for ONJ. Several retrospective studies have failed to find a difference in ONJ disease spectrum, clinical course, or outcomes between patients with RA or without RA [65,66,67]. In a large Japanese study with 5696 RA patients, only five confirmed cases were reported leading to a prevalence of 0.94% for all RA patients and 0.26% among females with RA ≥65 years of age [68]. Similar to the general population, risk factors for ONJ in RA include long duration of osteoporosis treatment, age, glucocorticoid use, and recent tooth extraction.
Reduction in RA disease activity with DMARDS may improve skeletal health
DMARDS have significant ability to control the inflammation associated with RA, and many studies suggest that individual drugs or combinations of DMARDS that reduce RA disease activity stabilizes or improves skeletal health (recently reviewed in [47, 69]). The skeletal effects of DMARDS include stopping the progression of periarticular erosions, altering bone remodeling to favor bone formation, and stabilizing or improving BMD (Table 3). Although many clinical trials confirm prevention of erosion progression, controversy still exists in the literature concerning the role of DMARDS in promoting full bone erosion “healing” versus erosive regression [19, 86, 87]. In either case, it is clear that biologic or nonbiologic DMARDS can retard local bone destruction if low disease activity is achieved. However, several studies have shown by power Doppler ultrasound or MRI that even patients in clinical remission (28-joint Disease Activity Score-erythrocyte sedimentation rate DAS ≤2.6) may continue to have bone marrow edema or synovitis associated with progression of erosions [88, 89]. Overall, there are a number of trials indicating that controlling RA disease activity with one or more DMARDS might have a protective effect on localized hand BMD or generalized BMD including the spine and hip (Table 3). However, additional studies are required to determine if stabilization of BMD is sufficient to reduce fracture risk in RA patients.
Glucocorticoids are unique in ability to control the symptoms of RA, reduce the rate of erosion progression, but also increase osteoporosis and fracture risk [90]. Meta-analysis reveals that daily GC as low as 2.5 mg prednisolone daily significantly increases the risk of vertebral fractures and doses of 7.5 mg leading to a RR of hip fracture of 1.77 (CI 1.55–2.02) and a RR of vertebral fracture of 5.18 (4.25–6.31) [91]. However, in the setting of early RA with high disease activity, GC treatment has been shown to stabilize BMD in multiple studies (Table 3). The BeSt study analyzed BMD in recent onset RA patients over 2 years with goal-directed therapy including GCs or steroid sparing DMARDS. After 2 years, BMD was similar in patients receiving GCs compared to those that did not receive GCs [92]. Another similar trial followed early RA patients on methotrexate-based treatment and randomized them to receive either 10 mg of prednisone daily or placebo in presence of bisphosphonates and calcium and vitamin D and found an increase in BMD in both treatment groups over the 2-year study, with no difference in BMD among the prednisone versus placebo-treated groups [93]. Taken together, these studies indicate that RA disease control is likely a significant factor for preserving bone mass and that GCs in early RA may be beneficial in achieving this goal. Unfortunately, fracture has not been an end point for these studies, so our understanding if the BMD stabilization or improvement translates into fracture reduction is limited. Additionally, GC-induced fractures occur at higher BMD than seen in postmenopausal osteoporosis [34]; thus, given the high risk of fracture in RA patients, efforts should be made to reduce the daily doses of GC as quickly as possible and preventive measures including calcium and vitamin D supplementation and medications, including bisphosphonates, teriparatide, or denosumab, should be used when appropriate according to recent American College of Rheumatology guidelines on the prevention and treatment of GC-induced osteoporosis [94].
Treatment of RA-associated osteoporosis
Because of the increased risk of fractures, RA patients should have routine assessment for osteoporosis at the time of diagnosis and periodically to review disease activity, review other medications that may increase the risk of bone loss [95], and monitor other conditions that promote osteoporosis including increasing age, menopausal status, and functional status. Similar to osteoporosis assessment in the general population, fracture risk may be assessed with a BMD measurement obtained via dual-energy X-ray absorptiometry (DXA), quantitative computerized tomography (QCT) or ultrasound, or with the FRAX instrument with or without a BMD. Vitamin D stores should be evaluated and replaced and calcium supplementation given. Unfortunately, physician compliance with current guidelines remains low with less than 50% of RA patients taking GC being prescribed therapy to prevent or treat osteoporosis [96].
Multiple studies have shown a BMD protective effect of bisphosphonates in RA patients even when receiving GC [97,82,99]. A recent study compared the spine BMD of 192 RA patients with age and sex-matched volunteers to determine whether bisphosphonate use was still needed to improve BMD if the patients had tight control of RA disease activity [100]. Compared to controls, RA patients had lower BMD at all sites at the time of enrollment. At 3 years, well-controlled RA patients receiving bisphosphonates had significantly higher percent change in spine BMD than well-controlled RA patients not receiving bisphosphonates (6.2 vs. 1.8%, p = 0.0001). Bisphosphonate use significantly increased spine BMD with an OR of 2.13 (CI 1.03–4.38); however, use of biologic agents, reducing GC dose, and tight disease control did not significantly increase BMD at 3 years. These data indicate that even with our improving ability to get low RA disease activity, RA patients are still at risk for low BMD and fracture. Risk factors for treatment failure of osteoporosis in RA include noncompliance with bisphosphonates, daily GC dose ≥7.5 mg/day before the first BMD measurement, immobilization >3 months, and high disease activity score [101].
Other osteoporosis treatments appear to be effective in the treatment of osteoporosis in RA. The discovery of RANKL as a key factor driving the formation and function of osteoclasts has led to the therapeutic targeting of this pathway with anti-RANKL antibody, denosumab. RANKL is upregulated by a variety of stimuli that contribute to excessive bone remodeling including several RA-associated cytokines including IL-1, IL-6, Il-17, and TNFα [102]; thus, therapeutic targeting of RANKL should inhibit osteoclastogenesis and prevent RA bone erosions and osteoporosis. Denosumab significantly improves BMD in RA patients especially if coupled with vitamin D and calcium supplementation [103, 104]. A small study of 49 patients treated with bisphosphonates and 49 treated with denosumab found no significant difference in BMD at 1 year, indicating that one drug is not more effective than the other at 1 year [105]. Several studies have shown that the addition of denosumab to methotrexate or biologics improves bone erosions and BMD (reviewed in [47]). When added to methotrexate, denosumab, but not alendronate, induced partial repair of bone erosions at 1 year when evaluated with high-resolution peripheral quantitative computed tomography [106]. There has been concern about increasing infections in patients receiving immunosuppression with DMARDS or biologics concomitantly with denosumab. However, a recent study analyzed the risk of hospitalization for serious infection in RA patients concurrently treated with biologics and denosumab and found that serious infection risk was not increased in those patients receiving biologics with denosumab compared to zoledronate [107]. These studies support the use of RANKL inhibition to prevent and treat generalized bone loss in RA.
Anabolic agent, teriparatide has also been found to be effective in RA-associated postmenopausal osteoporosis [108]. A recent randomized control trial evaluated the effect of teriparatide on joint erosions in RA [109]. Despite improvement in BMD at the femur and spine, established patients controlled on TNF-inhibitors failed to have a significant reduction in erosion volume in the hands and wrists with the addition of teriparatide for 1 year. These data support the hypothesis that the erosion bone matrix or microenvironment is no longer conducive to anabolic bone remodeling. However, this was a small study and treatment was limited to 1 year instead of the typical 2-year teriparatide course. Additional studies are needed to fully determine the benefits of teriparatide treatment in RA.
Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis
The new American College of Rheumatology guidelines for the prevention and treatment of GC-induced osteoporosis encourage early assessment of fracture risk including a clinical assessment of dose, duration, and pattern of GC use, evaluation of falls, frailty, fractures, malnutrition, significant weight loss, low body weight, family history of hip fracture, secondary causes of osteoporosis, ≥3 alcohol drinks per day, height, weight, muscle strength testing, and clinical findings of prior silent vertebral fractures [94]. Patients ≥40 should also have FRAX analysis with or without BMD testing; whereas patients <40 years of age, BMD testing is recommended only if the patient has multiple risk factors or prior osteoporotic fracture. Importantly, FRAX risk should be increased by 20% for GC-dosages ≥7.5 mg, as it may underestimate fracture risk at higher GC doses [110]. Additionally, the guidelines recommend calcium (800–1000 mg/day) and vitamin D (600–800 IU/day) supplementation as well as lifestyle modifications for all patients. The new guidelines, developed using GRADE methodology, focused on making recommendations based on the balance of relative benefits and harms of treatment, quality of the evidence, and patient preferences.
In the new guidelines, patients can be categorized as low risk, moderate risk, or high fracture risk based on the clinical fracture risk assessment. Low-risk patients include younger patients <40 with no history of osteoporotic fracture and no additional risk factors receiving <7.5 mg/day of GC. Additional low-risk patients include those ≥40 years of age with a GC-adjusted FRAX risk for major osteoporotic fracture of <10% or hip fracture risk <1%. For these low-risk patients, there is a conditional recommendation for oral bisphosphonates, teriparatide, or denosumab. Moderate-risk patients include patients ≥40 years of age with a GC-adjusted FRAX risk of major fracture 10–19% and hip fracture >1% but <3%, as well as younger patients <40 years of age with a Z-score <−3 or rapid bone loss in a year and predicted to take GC ≥7.5 mg for ≥6 months. Treatment with oral bisphosphates is conditionally recommended over alternative medications, including intravenous bisphosphonates, teriparatide, denosumab, or raloxifene, based on safety, cost, and lack of superior anti-fracture benefits from these other medications. High-risk patients include any patient with a prior osteoporotic fracture independent of age and those ≥40 years of age who have GC-adjusted FRAX for major osteoporotic fracture of ≥20% or hip fracture risk ≥3% or a BMD T-score ≤−2.5. For high-risk patients, there is a strong recommendation for treatment with the preferred oral bisphosphonates followed by alternative agents listed above. Repeated BMD testing is recommended every 2–3 years for moderate and high-risk patients remaining on GC. Based on the high risk of fracture in RA that is exacerbated by use of GC, many RA patients are moderate to high risk and treatment with oral bisphosphonates, calcium, vitamin D, and lifestyle modifications are recommended.
Conclusions
In summary, RA-associated systemic and local inflammation leads to generalized bone loss, fractures, juxta-articular bone erosions, joint fusion, and secondary osteoarthritis. Patients with ACPA are likely to have early generalized bone loss and bone erosions at the time of diagnosis. In many but not all instances, tight control of RA disease activity with conventional, biologic, or synthetic DMARDS can prevent or stabilize bone erosions, joint ankylosis, and secondary osteoarthritis but may have limited ability to significantly improve generalized bone loss. Recognition of high fracture risk and osteoporosis in RA should prompt clinicians to evaluate fracture risk by BMD or FRAX analysis. While GC in high disease states may be beneficial to local and generalized bone loss in RA, the prolonged use of GC especially at doses higher than 7.5 mg daily increases fracture risk. For patients taking GC, early evaluation of fracture risk with BMD or FRAX assessment is recommended within 6 months. In the RA population, GC-associated osteoporosis may be managed by bisphosphonates, denosumab, or teriparatide at the discretion of the physician.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibodies
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CI:
-
95% confidence intervals
- CT:
-
Computerized tomography
- DMARDS:
-
Disease modifying anti-rheumatic drugs
- DKK-1:
-
Dickkopf-related protein 1
- FRAX:
-
Fracture risk assessment tool
- GC:
-
Glucocorticoids
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of NF-kB ligand
- RF:
-
Rheumatoid factor
- RR:
-
Relative risk
- TNFα:
-
Tumor necrosis factor alpha
- TKR:
-
Total knee replacement
- THR:
-
Total hip replacement
References
Bellucci E, Terenzi R, La Paglia GM, Gentileschi S, Tripoli A, Tani C et al (2016) One year in review 2016: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 34(5):793–801
Veale DJ, Orr C, Fearon U (2017) Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol 39:343–354
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8(11):656–664
Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G et al (2007) Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford, England) 46(2):342–349
Koga T, Okada A, Fukuda T, Hidaka T, Ishii T, Ueki Y et al (2017) Anti-citrullinated peptide antibodies are the strongest predictor of clinically relevant radiographic progression in rheumatoid arthritis patients achieving remission or low disease activity: a post hoc analysis of a nationwide cohort in Japan. PLoS One 12(5):e0175281
Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M et al (2000) The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 43(8):1831–1835
Carpenter L, Norton S, Nikiphorou E, Jayakumar K, McWilliams DF, Rennie KL et al (2017) Reductions in radiographic progression in early RA over 25-years: changing contribution from RF in 2 multi-centre UK inception cohorts. Arthritis Care Res (Hoboken). doi:10.1002/acr.23217
Kumar S, Pangtey G, Gupta R, Rehan HS, Gupta LK (2017) Assessment of anti-CarP antibodies, disease activity and quality of life in rheumatoid arthritis patients on conventional and biological disease-modifying antirheumatic drugs. Reumatologia 55(1):4–9
Saevarsdottir S, Rezaei H, Geborek P, Petersson I, Ernestam S, Albertsson K et al (2015) Current smoking status is a strong predictor of radiographic progression in early rheumatoid arthritis: results from the SWEFOT trial. Ann Rheum Dis 74(8):1509–1514
Fisher BA, Bang SY, Chowdhury M, Lee HS, Kim JH, Charles P et al (2014) Smoking, the HLA-DRB1 shared epitope and ACPA fine-specificity in Koreans with rheumatoid arthritis: evidence for more than one pathogenic pathway linking smoking to disease. Ann Rheum Dis 73(4):741–747
Ciubotariu E, Gabay C, Finckh A (2014) Joint damage progression in patients with rheumatoid arthritis in clinical remission: do biologics perform better than synthetic antirheumatic drugs? J Rheumatol 41(8):1576–1582
Martel W, Hayes JT, Duff IF (1965) The pattern of bone erosion in the hand and wrist in rheumatoid arthritis. Radiology 84:204–214
McGonagle D, Tan AL, Moller Dohn U, Ostergaard M, Benjamin M (2009) Microanatomic studies to define predictive factors for the topography of periarticular erosion formation in inflammatory arthritis. Arthritis Rheum 60(4):1042–1051
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D et al (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163
Seror R, Boudaoud S, Pavy S, Nocturne G, Schaeverbeke T, Saraux A et al (2016) Increased Dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Data from the ESPOIR Cohort. Sci Rep 6:18421
Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA (2000) Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A 97(6):2791–2796
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y et al (2013) Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal 25(10):2069–2078
Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y (2006) Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther 8(3):R76
Sharp JT, Van Der Heijde D, Boers M, Boonen A, Bruynesteyn K, Emery P et al (2003) Repair of erosions in rheumatoid arthritis does occur. Results from 2 studies by the OMERACT subcommittee on healing of erosions. J Rheumatol 30(5):1102–1107
Barbieri F, Zampogna G, Camellino D, Paparo F, Cutolo M, Garlaschi G et al (2016) Ankylosis of the wrist bones in patients with rheumatoid arthritis: a study with extremity-dedicated MRI. Clin Exp Rheumatol 34(1):49–52
Leden I, Theander J, Svensson B (2008) Small joint ankylosis in rheumatoid arthritis: a vanishing phenomenon or a pathogenetic clue, or both? Ann Rheum Dis 67(12):1786–1787
Eulderink F, Meijers KA (1976) Pathology of the cervical spine in rheumatoid arthritis: a controlled study of 44 spines. J Pathol 120(2):91–108
Gillick JL, Wainwright J, Das K (2015) Rheumatoid arthritis and the cervical spine: a review on the role of surgery. Int J Rheumatol 2015:252456
Iizuka H, Iizuka Y, Okamura K, Yonemoto Y, Mieda T, Takagishi K (2016) Bony ankylosis of the facet joint of the cervical spine in rheumatoid arthritis: its characteristics and relationship to the clinical findings. Mod Rheumatol:1–5
Zhu S, Xu W, Luo Y, Zhao Y, Liu Y (2017) Cervical spine involvement risk factors in rheumatoid arthritis: a meta-analysis. Int J Rheum Dis 20:541–549
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54(10):3104–3112
Brennan SL, Toomey L, Kotowicz MA, Henry MJ, Griffiths H, Pasco JA (2014) Rheumatoid arthritis and incident fracture in women: a case-control study. BMC Musculoskelet Disord 15:13
Chen B, Cheng G, Wang H, Feng Y (2016) Increased risk of vertebral fracture in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) 95(45):e5262
Ghazi M, Kolta S, Briot K, Fechtenbaum J, Paternotte S, Roux C (2012) Prevalence of vertebral fractures in patients with rheumatoid arthritis: revisiting the role of glucocorticoids. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 23(2):581–587
Amin S, Gabriel SE, Achenbach SJ, Atkinson EJ, Melton LJ 3rd (2013) Are young women and men with rheumatoid arthritis at risk for fragility fractures? A population-based study. J Rheumatol 40(10):1669–1676
Wright NC, Lisse JR, Walitt BT, Eaton CB, Chen Z (2011) Women’s Health Initiative I. Arthritis increases the risk for fractures—results from the Women’s Health Initiative. J Rheumatol 38(8):1680–1688
Acurcio FA, Moura CS, Bernatsky S, Bessette L, Rahme E (2016) Opioid use and risk of nonvertebral fractures in adults with rheumatoid arthritis: a nested case-control study using administrative databases. Arthritis Rheumatol 68(1):83–91
Klop C, de Vries F, Bijlsma JW, Leufkens HG, Welsing PM (2016) Predicting the 10-year risk of hip and major osteoporotic fracture in rheumatoid arthritis and in the general population: an independent validation and update of UK FRAX without bone mineral density. Ann Rheum Dis 75(12):2095–2100
Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48(11):3224–3229
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County rheumatoid arthritis register. Arthritis Rheum 43(3):522–530
Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, Di Munno O et al (2000) A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian study group on bone mass in rheumatoid arthritis. J Rheumatol 27(11):2582–2589
Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH (2014) Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology (Oxford, England) 53(10):1759–1766
Lane NE, Pressman AR, Star VL, Cummings SR, Nevitt MC (1995) Rheumatoid arthritis and bone mineral density in elderly women. The study of osteoporotic fractures research group. J Bone Miner Res 10(2):257–263
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344(8914):23–27
Als OS, Gotfredsen A, Riis BJ, Christiansen C (1985) Are disease duration and degree of functional impairment determinants of bone loss in rheumatoid arthritis? Ann Rheum Dis 44(6):406–411
Sambrook PN, Ansell BM, Foster S, Gumpel JM, Hesp R, Reeve J (1985) Bone turnover in early rheumatoid arthritis 2. Longitudinal bone density studies. Ann Rheum Dis 44(9):580–584
Sambrook PN, Eisman JA, Champion GD, Yeates MG, Pocock NA, Eberl S (1987) Determinants of axial bone loss in rheumatoid arthritis. Arthritis Rheum 30(7):721–728
Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ronday HK, Peeters AJ et al (2007) Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis 66(11):1508–1512
Hwang J, Lee EK, Ahn JK, Cha HS, Koh EM, Lee J (2017) Bone-density testing interval and transition to osteoporosis in patients with rheumatoid arthritis. Osteoporos Int 28(1):231–237
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7(6):429–442
Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB et al (2009) Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res 24(9):1572–1585
Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Uña CR, Adachi JD, Lems WF, Cooper C, Lane NE (2017) IOF Chronic Inflammation and Bone Structure (CIBS) Working Group. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int 28(2):429-446. doi:10.1007/s00198-016-3769-2
Deodhar AA, Woolf AD (1996) Bone mass measurement and bone metabolism in rheumatoid arthritis: a review. Br J Rheumatol 35(4):309–322
Di Franco M, Barchetta I, Iannuccelli C, Gerardi MC, Frisenda S, Ceccarelli F et al (2015) Hypovitaminosis D in recent onset rheumatoid arthritis is predictive of reduced response to treatment and increased disease activity: a 12 month follow-up study. BMC Musculoskelet Disord 16:53
Raczkiewicz A, Kisiel B, Kulig M, Tlustochowicz W (2015) Vitamin D status and its association with quality of life, physical activity, and disease activity in rheumatoid arthritis patients. J Clin Rheumatol Pract Rep Rheum Musculoskeletal Dis 21(3):126–130
Bugatti S, Bogliolo L, Vitolo B, Manzo A, Montecucco C, Caporali R (2016) Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther 18(1):226
Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F et al (2014) Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis 73(5):854–860
Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E et al (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 122(5):1791–1802
Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M et al (2008) Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 59(6):807–815
Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj-Hassouni N (2017) Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 37(6):1015–1020
Okano T, Inui K, Tada M, Sugioka Y, Mamoto K, Wakitani S et al (2017) High frequency of vertebral fracture and low bone quality in patients with rheumatoid arthritis—results from TOMORROW study. Mod Rheumatol 27(3):398–404
El Maghraoui A, Sadni S, Rezqi A, Bezza A, Achemlal L, Mounach A (2015) Does rheumatoid cachexia predispose patients with rheumatoid arthritis to osteoporosis and vertebral fractures? J Rheumatol 42(9):1556–1562
Rodriguez JA, Saddler S, Edelman S, Ranawat CS (1996) Long-term results of total knee arthroplasty in class 3 and 4 rheumatoid arthritis. J Arthroplast 11(2):141–145
Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY (2004) Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 86-A(5):963–974
Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI et al (2010) Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian arthroplasty register. Arthritis Care Res (Hoboken). 62(4):473–479
Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E et al (2012) A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum 64(12):3839–3849
Jamsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU (2013) The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop 84(4):331–337
Moura CS, Abrahamowicz M, Beauchamp ME, Lacaille D, Wang Y, Boire G et al (2015) Early medication use in new-onset rheumatoid arthritis may delay joint replacement: results of a large population-based study. Arthritis Res Ther 17:197
Asai S, Kojima T, Oguchi T, Kaneko A, Hirano Y, Yabe Y et al (2015) Effects of concomitant methotrexate on large joint replacement in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a multicenter retrospective cohort study in Japan. Arthritis Care Res (Hoboken) 67(10):1363–1370
Lescaille G, Coudert AE, Baaroun V, Javelot MJ, Cohen-Solal M, Berdal A et al (2013) Osteonecrosis of the jaw and nonmalignant disease: is there an association with rheumatoid arthritis? J Rheumatol 40(6):781–786
Conte-Neto N, Bastos AS, Spolidorio LC, Marcantonio RA, Marcantonio E Jr (2011) Oral bisphosphonate-related osteonecrosis of the jaws in rheumatoid arthritis patients: a critical discussion and two case reports. Head Face Med 7:7
Di Fede O, Bedogni A, Giancola F, Saia G, Bettini G, Toia F et al (2016) BRONJ in patients with rheumatoid arthritis: a multicenter case series. Oral Dis 22(6):543–548
Furuya T, Maeda S, Momohara S, Taniguchi A, Yamanaka H (2017) Dental treatments, tooth extractions, and osteonecrosis of the jaw in Japanese patients with rheumatoid arthritis: results from the IORRA cohort study. J Bone Miner Metab 35(3):344–350
Barreira SC, Fonseca JE (2016) The impact of conventional and biological disease modifying antirheumatic drugs on bone biology. Rheumatoid arthritis as a case study. Clin Rev Allergy Immunol 51(1):100–109
Rexhepi S, Rexhepi M, Sahatciu-Meka V, Mahmutaj V, Boshnjaku S (2016) The Impact of Low-Dose Diseasemodifying Anti-rheumatics Drugs (DMARDs) on Bone Mineral Density of Premenopausal Women in Early Rheumatoid Arthritis. Med Arch. 70(2):101–103
di Munno O, Mazzantini M, Sinigaglia L, Bianchi G, Minisola G, Muratore M et al (2004) Effect of low dose methotrexate on bone density in women with rheumatoid arthritis: results from a multicenter cross-sectional study. J Rheumatol. 31(7):1305–1309
Ornbjerg LM, Ostergaard M, Jensen T, Horslev-Petersen K, Stengaard-Pedersen K, Junker P et al (2017) Hand bone loss in early rheumatoid arthritis during a methotrexate-based treat-to-target strategy with or without adalimumab-a substudy of the optimized treatment algorithm in early RA (OPERA) trial. Clin Rheumatol. 36(4):781–789
Pfeil A, Lippold J, Eidner T, Lehmann G, Oelzner P, Renz DM et al (2009) Effects of leflunomide and methotrexate in rheumatoid arthritis detected by digital X-ray radiogrammetry and computer-aided joint space analysis. Rheumatol Int. 29(3):287–295
Vis M, Havaardsholm EA, Haugeberg G, Uhlig T, Voskuyl AE, van de Stadt RJ et al (2006) Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFkappaB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 65(11):1495–1499
Guler-Yuksel M, Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Groenendael JH, Mallee C et al (2009) Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 68(3):330–336
Siu S, Haraoui B, Bissonnette R, Bessette L, Roubille C, Richer V et al (2015) Meta-analysis of tumor necrosis factor inhibitors and glucocorticoids on bone density in rheumatoid arthritis and ankylosing spondylitis trials. Arthritis Care Res (Hoboken). 67(6):754–764
Eekman DA, Vis M, Bultink IE, Kuik DJ, Voskuyl AE, Dijkmans BA et al (2011) Stable bone mineral density in lumbar spine and hip in contrast to bone loss in the hands during long-term treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 70(2):389–390
Marotte H, Miossec P (2008) Prevention of bone mineral density loss in patients with rheumatoid arthritis treated with anti-TNFalpha therapy. Biologics : targets & therapy. 2(4):663–669
Seriolo B, Paolino S, Sulli A, Ferretti V, Cutolo M (2006) Bone metabolism changes during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 1069:420–427
Briot K, Rouanet S, Schaeverbeke T, Etchepare F, Gaudin P, Perdriger A et al (2015) The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine. 82(2):109–115
Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, Hatta K et al (2014) The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology (Oxford, England) 53(5):900–903
Hein G, Eidner T, Oelzner P, Rose M, Wilke A, Wolf G et al (2011) Influence of Rituximab on markers of bone remodeling in patients with rheumatoid arthritis: a prospective open-label pilot study. Rheumatol Int. 31(2):269–272
Boumans MJ, Thurlings RM, Yeo L, Scheel-Toellner D, Vos K, Gerlag DM et al (2012) Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann Rheum Dis. 71(1):108–113
Coulson KA, Reed G, Gilliam BE, Kremer JM, Pepmueller PH (2009) Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) registry. J Clin Rheumatol. 15(4):155–160
Roussy JP, Bessette L, Bernatsky S, Rahme E, Lachaine J (2013) Biologic disease-modifying antirheumatic drugs and the risk of non-vertebral osteoporotic fractures in patients with rheumatoid arthritis aged 50 years and over. Osteoporos Int. 24(9):2483–2492
Moller Dohn U, Boonen A, Hetland ML, Hansen MS, Knudsen LS, Hansen A et al (2009) Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann Rheum Dis 68(10):1585–1590
Gravallese EM, Walsh NC (2011) Rheumatoid arthritis: repair of erosion in RA—shifting the balance to formation. Nat Rev Rheumatol 7(11):626–628
Ramirez J, Narvaez JA, Ruiz-Esquide V, Hernandez-Ganan J, Cuervo A, Inciarte-Mundo J et al (2017) Clinical and sonographic biomarkers of structural damage progression in RA patients in clinical remission: a prospective study with 12 months follow-up. Semin Arthritis Rheum. doi:10.1016/j.semarthrit.2017.04.007
Lisbona MP, Pamies A, Ares J, Almirall M, Navallas M, Solano A et al (2014) Association of bone edema with the progression of bone erosions quantified by hand magnetic resonance imaging in patients with rheumatoid arthritis in remission. J Rheumatol 41(8):1623–1629
Kirwan JR, Bijlsma JW, Boers M, Shea BJ (2007) Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev 24(1):Cd006356
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporosis Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 13(10):777–787
Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Hulsmans HM, de Beus WM et al (2008) Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Ann Rheum Dis 67(6):823–828
van der Goes MC, Jacobs JW, Jurgens MS, Bakker MF, van der Veen MJ, van der Werf JH et al (2013) Are changes in bone mineral density different between groups of early rheumatoid arthritis patients treated according to a tight control strategy with or without prednisone if osteoporosis prophylaxis is applied? Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 24(4):1429–1436
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE et al (2017) American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. doi:10.1002/acr.23279
Panday K, Gona A, Humphrey MB (2014) Medication-induced osteoporosis: screening and treatment strategies. Ther Adv Musculoskelet Dis 6(5):185–202
Watt J, Thompson A, Le Riche N, Pope J (2014) There is still a care gap in osteoporosis management for patients with rheumatoid arthritis. Joint Bone Spine 81(4):347–351
Ebina K, Noguchi T, Hirao M, Hashimoto J, Kaneshiro S, Yukioka M et al (2016) Effects of switching weekly alendronate or risedronate to monthly minodronate in patients with rheumatoid arthritis: a 12-month prospective study. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 27(1):351–359
Shin K, Park SH, Park W, Baek HJ, Lee YJ, Kang SW et al (2017) Monthly oral ibandronate reduces bone loss in Korean women with rheumatoid arthritis and osteopenia receiving long-term glucocorticoids: a 48-week double-blinded randomized placebo-controlled investigator-initiated trial. Clin Ther 39(2):268–278 e2
Lems WF, Lodder MC, Lips P, Bijlsma JW, Geusens P, Schrameijer N et al (2006) Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low-dose prednisone: a randomized, double-blind, placebo-controlled trial. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 17(5):716–723
Tada M, Inui K, Sugioka Y, Mamoto K, Okano T, Anno S et al (2017) Use of bisphosphonate might be important to improve bone mineral density in patients with rheumatoid arthritis even under tight control: the TOMORROW study. Rheumatol Int 37(6):999–1005
Wen L, Kang JH, Yim YR, Lee JW, Lee KE, Park DJ et al (2016) Risk factors for treatment failure in osteoporotic patients with rheumatoid arthritis. Mod Rheumatol 26(2):194–199
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7(4):292–304
Nakamura Y, Suzuki T, Yoshida T, Yamazaki H, Kato H. (2017) Vitamin D and Calcium are required during denosumab treatment in osteoporosis with rheumatoid arthritis. Nutrients 9(5)
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T et al (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (denosumab) in patients with rheumatoid arthritis on methotrexate to validate inhibitory effect on bone erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75(6):983–990
Kinoshita H, Miyakoshi N, Kashiwagura T, Kasukawa Y, Sugimura Y, Shimada Y (2016) Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod Rheumatol 23:1–5
Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J et al (2016) Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. Arthritis Care Res (Hoboken). doi:10.1002/acr.23133
Curtis JR, Xie F, Yun H, Saag KG, Chen L, Delzell E (2015) Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol 67(6):1456–1464
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2014) Comparison of the effect of 18-month daily teriparatide administration on patients with rheumatoid arthritis and postmenopausal osteoporosis patients. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 25(12):2755–2765
Solomon DH, Kay J, Duryea J, Lu B, Bolster MB, Yood RA et al (2017) Effects of teriparatide on joint erosions in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol
Kanis JA, Johansson H, Oden A, McCloskey EV (2011) Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 22(3):809–816
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Heinlen, L., Humphrey, M.B. Skeletal complications of rheumatoid arthritis. Osteoporos Int 28, 2801–2812 (2017). https://doi.org/10.1007/s00198-017-4170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4170-5