Abstract
The aim of the present study was to evaluate the effect of long-term leflunomide and methotrexate (MTX) therapy during the course of rheumatoid arthritis (RA) estimated by digital X-ray radiogrammetry (DXR) and computer-aided joint space analysis (CAJSA) as diagnostic tools for the quantification of disease-related periarticular osteoporosis and joint space narrowing. Fourty matchable patients with verified RA were treated with leflunomide or MTX during an observation period of 2.5 years. All patients underwent complete computerized calculations of bone mineral density (BMD) and metacarpal index (MCI) by DXR as well as semi-automated measurements of joint space widths (JSW) at the metacarpophalangeal articulations (MCP, thumb to small finger) and proximal interphalangeal joints (PIP, index finger to small finger) using digitized hand radiographs. DXR-BMD revealed an increase of 0.4% (leflunomide-group) versus a reduction of −9.1% (MTX-group). Regarding DXR-MCI, a reduction of −1.1% (leflunomide-group) and −5.3% (MTX-group) was observed. The CAJSA parameters showed a decline of −2.7% (JSW-MCP) versus −2.1% (JSW-PIP) in patients treated with leflunomide. An accentuated joint space narrowing was revealed (JSW-MCP: −5.7%; JSW-PIP: −6.2%) in the MTX group. Digital X-ray radiogrammetry and CAJSA could discriminate the influence of different therapeutic regimes on periarticular osteoporosis and joint space narrowing showing a less accentuated radiographic progression in patients treated with leflunomide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic disease characterized by the juxtaarticular inflammatory involvement of cartilage and bone tissue, frequently and predominantly affecting the small joints of the hand [1, 2]. The extent of joint damage is successfully assessed by hand radiographs which still represent the “gold standard” for the evaluation of disease progression and effectiveness of therapy [3]. However, periarticular osteoporosis and joint space narrowing are the first disease-related morphological signs in RA before bone erosions occur. Quantitative hand bone estimates, which capture periarticular osteoporosis, have been proposed as outcome measures in RA [4, 5]. The manifestation of osteoporosis in RA is based on two forms: periarticular osteoporosis in near proximity to the inflamed joints, which is a typical phenomenon in early RA, and generalized osteoporosis affecting the axial and appendicular bones occurring during the prolonged course of RA [6, 7]. Recently, receptor activators of the nuclear κB ligand (RANKL) and osteoprotegerin (OPG), a decoy receptor for RANKL, have been identified as central regulators of osteoclast recruitment and activation. OPG and RANKL production is modulated by various cytokines, growth factors and hormones. In the affected synovium both fibroblasts and activated T-cells express RANKL and maintain osteoclast recruitment and activation. OPG and RANKL are thus important molecular agents with a lasting effect on bone resorption focusing on the periarticular cortical and trabecular bone matrix [8].
Two new computer-aided diagnosis (CAD) systems have been developed in the last five years for the quantification of metacarpal bone mineral density (BMD) and joint space width of the peripheral finger joints. Digital X-ray radiogrammetry (DXR) is a new operator-independent CAD tool, providing automated measurements of cortical BMD on the metacarpals by means of digitized radiographs. The computer-aided joint space analysis (CAJSA) is a recently available approach based on semi-automated measurements of joint space widths at the metacarpophalangeal articulation (JSW-MCP) of the first to fifth finger and at the proximal interphalangeal joints (JSW-PIP, index finger to small finger).
In recent studies DXR and CAJSA have been able to detect and quantify disease-related periarticular cortical bone loss caused by RA, which is accelerated in early RA [9, 10]. For earlier identification of inflammation-related changes enabling a better prognosis and improved success of treatment strategies in patients suffering from RA, the availability of CAD techniques provides the opportunity for quantitative measurements of radiogeometric features [11, 12].
Disease-modifying antirheumatic drugs (DMARD) have been shown to suppress symptoms and clinical signs as well as radiographic progression in RA [13, 14]. Methotrexate (MTX) is probably the most frequently applied DMARD in RA. It is also prescribed for other rheumatic and non-rheumatic disorders. Leflunomide, an oral immunosuppressive agent, actually plays a major role in the therapy of RA, while several studies described the reduction of structural joint damage as shown in hand radiographs with reduced evidence of erosions and joint space narrowing [13, 14].
The objective of this longitudinal study design was the quantification of RA progression by means of DXR and CAJSA in patients treated with leflunomide compared to those treated with MTX.
Patients and methods
Patients
Forty Caucasian patients (31 female and 9 male) were enrolled without preselection regarding the grade of RA. The patients included in the study were 32–72 years of age (mean age: 57.9 ± 12.5 years). RA was diagnosed according to the American College of Rheumatology criteria [15]. 20 patients were treated with 15 mg of MTX per week and 10 mg folic acid per day. The second group (20 patients) was treated with 20 mg of leflunomide per day. The initial digital radiographs of the non-dominant hand were timed 1.6 ± 0.8 years after commencing the MTX versus the leflunomide therapy. The second radiograph was performed 2.5 years ± 0.7 years after the first radiograph (see Table 1 for detailed information).
Subjects with hormone replacement/bisphosphonate therapy or with other conditions known to affect the bone metabolism were excluded as were patients with signs of fracture and visible osteosynthetic material in the right and left upper extremities (including ulna, radius and hand).
Disease activity was assessed by means of the erythrocyte sedimentation rate and C-reactive protein levels.
The severity of RA was evaluated using the Larsen score [16] which evaluates 32 joints of the feet and hands (total sum of points: 160): score 0 = normal joint; score 1 = periarticular demineralization, soft tissue affection, initial reduction of the joint space width; score 2 = initial erosions and reduction of the joint space width; score 3 = multiple erosions and advanced reduction of the joint space width; score 4 = partial ankylosis; score 5 = ankylosis or mutilation. The individual sum of scoring points was then divided by the evaluated joints. Each X-ray was independently scored by two experienced musculoskeletal radiologist blinded to each other. In cases of ambiguity, a third highly experienced musculoskeletal radiologist reviewed the radiographs before making the final decision.
Methods
Acquisition of hand radiographs
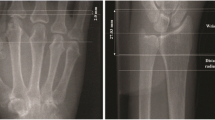
Digital X-ray radiogrammetry and CAJSA were used to determine DXR-BMD and DXR-MCI as well as JSW-MCP and JSW-PIP based on digital radiographs of the hand. All plain radiographs in anterior-posterior projection were acquired by means of digital X-ray equipment (Siemens Multix, 1994, Erlangen, Germany) under the following standardized conditions: filter 1.0 (aluminium 80), film-focus distance 1 meter, aluminium 80, tube voltage 42 kV, exposure level 4 mA per second. The digital radiographs were printed with a laser printer (Agfa Drystar 5500, 508 ppi, pixel size 50 μm, 12 bit/4,096 grey levels; Agfa, Cologne, Germany). The used film brand was AGFA Scopix Laser 2 B 400 (Agfa, Cologne, Germany).
The printouts of the digital radiographs were subsequently scanned into the system at a resolution of 300 dots per inch, corresponding to 5.5 line pairs/mm.
Calculation of DXR-BMD and DXR-MCI (by digital X-ray radiogrammetry)
Digital X-ray radiogrammetry (Pronosco X-Posure System™, Version 2.0; Sectra; Sweden) was applied to determine the BMD (in g/cm²), cortical thickness (CT in cm), metacarpal bone width (W in cm) and metacarpal index (MCI; a dimensionless parameter based on the mean cortical thickness normalized with the mean outer bone diameter of the metacarpals), requiring radiographs of the non-dominant hand. The digital radiographs were printed and subsequently scanned into the system. The system itself checked the quality of the scanned images and interrupted the examination in case of inadequate quality (i.e. technical lack of exposure and focus as well as incorrect illustration of the relevant anatomical structures). The computer algorithms automatically defined regions of interest around the narrowest bone parts of the metacarpals II, III and IV and subsequently determined the outer and inner cortical edges of the studied cortical bone parts. There is no operator interaction connected to the DXR measurement. The analyzed images and their regions of interest were displayed on the computer monitor.
The mean of the cortical thickness and overall cortical thickness of the second, third and fourth metacarpal were estimated. The cortical volume per area (VPA) was subsequently calculated for each bone. Based on the mean VPA, the DXR-BMD was computed with a correction for the estimated porosity index. The porosity index is a technical parameter given as a value between 1 and 19 and derived from the area percentage of local intensity minima found in the cortical part of the bone relative to the entire cortical area [17].
Measurement of joint spaces (by means of computer-aided joint space analysis)
The CAJSA (Radiogrammetry Kit, Version 1.3.6; Sectra; Sweden) could estimate all visible JSW-MCP and JSW-PIP without distinction including severely altered joints with subluxation and partial ankylosis. Only joints with complete ankylosis could not be considered. This technique performed a joint space analysis of a finger joint by detecting the joint edges within a rectangular region of interest defined by the user. The positioning of the region of interest to identify the favored joint represented the unique operator-dependent interaction during the whole measurement. The software based on an edge filtering of the region of interest automatically detected the tips of the two involved bones. A 1.5 cm long edge path across each bone was also determined and the distance between the two edges was measured as a function of the horizontal position. The mean average and standard deviation of the distance over a moving interval of 0.8 cm was calculated. The distance between the bones was defined to be above the edge interval, for which the standard deviation was minimal.
The measurement of the joint spaces was methodically established for the MCP (thumb to small finger) and proximal interphalangeal articulation (index finger to small finger). The joint space width was expressed as the mean value of the MCPs (thumb to small finger) and the proximal interphalangeal articulations (index finger to small finger). The joint space widths was given in mm.
Taking into account the age- and gender-dependent reduction of DXR and CAJSA parameters, z- and t-scores were calculated as follows:
CAJSA technique
DXR technique
Ethics
All examinations were performed in accordance with the rules and regulations of the local human research and ethics committee. On a special note, the authors emphasize that all radiographs used for DXR and CAJSA calculations were performed as part of routine clinical care. No additional radiographs were obtained only for study purposes.
Reproducibility data
The intra-radiograph reproducibility (ten measurements of the same image of the same hand) of the DXR and CAJSA parameters showed the following coefficients of variation:
DXR-BMD: | 0.05% | DXR-MCI | 0.13% | DXR-CT | 0.09% |
DXR-W: | 0.15% | JSD-MCP | 0.53% | JSD-PIP | 0.86% |
Statistical analysis
The objective of the statistical analysis was to quantify the changes of BMD and JSW in patients with RA under therapy with MTX versus leflunomide. The changes were verified using the t-test for paired groups (significance level: P value < 0.05). The statistical analysis was performed using SPSS® version 14.0 (SPSS, Chicago, Illinois, USA), for Windows respectively.
Results
Leflunomide group (see Tables 2 and 3)
DXR-BMD increased (+0.4%; P = not significant) from 0.480 ± 0.095 g/cm² (initial measurement) to 0.482 ± 0.098 g/cm² (second measurement). A reduction from 0.370 ± 0.104 (initial measurement) to 0.366 ± 0.100 (second measurement) was observed for DXR-MCI (−1.1%; P = not significant) and from 0.152 ± 0.039 cm to 0.149 ± 0.040 cm (second measurement) for DXR-CT (−2.0%; P = not significant). Furthermore, DXR-W increased (+4.9%; P = not significant) from 0.821 ± 0.040 cm (initial measurement) to 0.825 ± 0.068 cm.
JSW-MCP was not significantly reduced (−2.7%) from 1.46 ± 0.27 mm (initial measurement) to 1.42 ± 0.30 mm (second measurement). The z-score of JSW-MCP also revealed a minor, insignificant reduction from −0.41 ± 1.11 SD to −0.57 ± 1.37 SD (second measurement). Equivalent results were obtained for JSW-PIP (−2.1%) with an insignificant decrease from 0.97 ± 0.20 mm (initial measurement) to 0.95 ± 0.16 mm and for the corresponding z-score from −0.58 ± 1.06 SD to −0.74 ± 0.91 SD (second measurement).
Methotrexate group (see Tables 4 and 5)
DXR-BMD was significantly reduced in the MTX group, with −9.1% (P < 0.05) from 0.481 ± 0.104 g/cm² (initial measurement) to 0.437 ± 0.082 g/cm² (second measurement). Regarding DXR-MCI (−5.3%) and DXR-CT (−10.8%), a significant decrease from 0.361 ± 0.081 (initial measurement) to 0.342 ± 0.071 and from 0.148 ± 0.040 cm to 0.132 ± 0.031 cm (second measurement) was revealed. DXR-W presented no significant reduction (−0.8%; P = not significant) from 0.783 ± 0.160 cm (initial measurement) to 0.777 ± 0.077 cm.
JSW-MCP decreased (−5.7%; P < 0.05) from 1.41 ± 0.33 mm (initial measurement) to 1.33 ± 0.14 mm (second measurement). JSW-PIP also showed a significant reduction (−6.2%; P < 0.05) from 0.97 ± 0.20 mm (initial measurement) to 0.91 ± 0.16 mm. The z-score decreased for JSW-MCP from −0.28 ± 1.40 SD (initial measurement) to −0.39 ± 0.75 SD (second measurement) and for JSW-PIP from −0.27 ± 0.97 SD to −0.42 ± 0.76 SD (second measurement).
Discussion
Most patients with early RA are characterized by apparently normal hand radiographs despite clinical involvement of the finger joints. Since differences exist between the clinical and biochemical disease activity and the occurrence of bone defects and joint destruction [6], the acute phase response (i.e. CRP and ESR), known to correlate with disease activity, is often not sensitive enough. Conventional radiography has always played a major role in the diagnosis and follow-up of RA. The disadvantage of conventional imaging is the limited sensitivity in detecting early periarticular demineralization and narrowing of the JSW [18].
Digital X-ray radiogrammetry and computer-aided joint space analysis
The availability of CAD systems in the diagnosis of RA provides the opportunity for quantitative measurements of radiographically detectable periarticular demineralization and disease-related changes of joint space width. DXR, which operator-independently identifies periarticular demineralization, and semi-automated CAJSA measurements of JSW offer an advantage for prospective and retrospective sampling of quantitative data based on digitized hand radiographs. The DXR technique shows an excellent intra- (0.05–0.33%) and inter-radiograph reproducibility (0.26–1.54%) [19]. The CAJSA technology provides measurements of JSW with high precision and reproducibility [20]. In addition, most of the image-capturing conditions (film-focus distance, film sensitivity, film brand, exposure level) reveal no influence regarding DXR and CAJSA estimates [19]. Due to the high reproducibility, the results of both diagnostic tools indicate that estimated demineralization and joint space narrowing is in fact disease-related and not based on the precision error of the CAD methods itself.
Scoring in rheumatoid arthritis
Early diagnosis of RA is not only essential for the optimal and well timed treatment of osteoporosis, but also for delaying or stopping inflammatory damage of the affected joints [7]. Different scoring methods have been validated and established [21–23] which are based on conventional radiography as the common imaging technique to evaluate the progression of RA. Scoring methods are designed to semiquantitatively measure radiographically visible alterations, in particular erosions and joint space narrowing caused by cartilage damage.
A major limitation of scoring methods is the subjective evaluation of disease-related changes in RA, implying a limited inter- and intra-observer reproducibility, particularly when clinicians with different levels of experience use these established tools [4].
Computerized measurements of JSW are also complicated by the asymmetrical affection of inflamed joints, by subluxation and overlying soft tissue [3]. A possible limitation of DXR may be the estimate of the cortical partition of the bone only, since the cortical bone implies minor bone metabolism compared to trabecular bone tissue. Otherwise cortical thinning of periarticular bone, enhanced by the inflammation process, is a typical phenomenon of bone destruction in RA [24] which can be assumed due to a very high bone turnover on the inner bone surface [25].
In addition, several studies could confirm the diagnostic value of both CAD tools leading to a better discrimination of severity-dependent demineralization as well as narrowing of the JSW in patients suffering from RA.
For the Steinbroker stage, DXR-BMD significantly decreased in an extent of −32.7%. The relative reduction of DXR-MCI was −36.6%. In this context JSW-MCP (mean) showed a significant narrowing of −52.9% [10].
With regard to the modified Larsen score, DXR-BMD showed a significant decline of −27.7% and DXR-MCI also revealed a reduction of −27.5%. A decrease of −41.2% is also verified for JSW-MCP (mean) [26]. Using the Sharp scores (Sharp joint space narrowing score and Sharp erosion score), DXR-BMD was notedly diminished (−27.7% vs. −20.4%), whereas JSW-MCP showed a narrowing of JSW of up to −36.2% [27].
In a longitudinal study Böttcher et al. (2005) revealed a significant decrease of DXR-BMD and DXR-MCI as well as JSW-MCP (total) in RA within a disease duration of six years. A reduction of −32.1% and −33.3% was observed for DXR-BMD versus DXR-MCI. The JSW-MCP (total) showed a relative reduction of −23.5%. An annual DXR-BMD loss of −3.6%, an annual reduction of DXR-MCI of −3.2% and an annual narrowing of JSW-MCP (mean) with −2.0% could be demonstrated on average [10].
Therapeutic effects of leflunomide and methotrexate on periarticular demineralization
It is well recognized that irreversible joint damage in RA occurs soon after the onset of symptoms, often within the first 2 years. Early consistent treatment, for instance with DMARDs, is required to prevent joint destruction. Among the different DMARDs currently used in the treatment of RA, MTX and sulfasalazine are most frequently prescribed. Several studies also confirmed the efficacy of leflunomide in RA. Our data revealed a relative significant reduction of DXR-BMD (−9.1%) and DXR-MCI (−5.3%) in the MTX group during an observation period of 2.6 years, whereas no significant demineralization could be verified for the leflunomide group (DXR-BMD: +0.4%; DXR-MCI: −1.1%).
Some cross-sectional and longitudinal studies evaluated the impact of MTX treatment resulting in therapy-induced osteoporosis of RA patients [28–34]. Buckley et al. (1997) observed BMD changes (measured by dual X-ray absorptiometry (DXA) at the lumbar spine) in both male and female RA patients (n = 68) treated with MTX. Patients treated with prednisone (5 mg/day) and MTX showed an additional bone loss of −8.08% compared to patients treated with a similar dose of prednisone without MTX. This suggests that MTX may increase trabecular bone loss in glucocorticoid-treated patients [30]. The dual X-ray absorptiometry revealed no difference in the vertebral BMD values of 12 patients suffering from polymyalgia rheumatica and treated with prednisone and MTX (10 mg per week) compared to the initially established values. In fact, significant bone loss occurred in further 12 patients with sole prednisone administration (−4.9%, P < 0.05) [31]. Mazzantini et al. [32] published the data of a 2-year longitudinal study evaluating lumbar BMD changes assessed by dual X-ray absorptiometry in female RA patients who had recently started a therapy with DMARDs. After 2 years, 22 patients treated with MTX and 18 patients treated with other DMARD had lost a comparable amount of bone mass, while no correlation was found between the cumulative dose of MTX and the BMD changes within the 2-years observation period [32]. Cranney et al. [33] compared BMD, assessed also by means of DXA, at the lumbar spine and total femur in male and female RA patients treated with MTX (n = 30; mean cumulative dose: 2,810 mg) or with another DMARD (n = 30) observing no significant difference between both groups [33].
Minaur et al. [34] prospectively assessed BMD changes after 1 year in RA patients treated with MTX (n = 64) or other DMARDs (n = 52). Univariate analysis of covariance revealed that MTX at baseline was associated with reduced BMD at the femoral neck [34]. In vitro data pointed at the fact that MTX reduces the number of human osteoblasts [35]. Preston et al. [36] confirmed that MTX had a dose-dependent toxic effect on osteoblastic cells which could already be identified at concentrations used in the treatment of RA [36]. The findings of Cegiela et al. [37] elucidated the disturbed process of bone remodeling in rats with administration of MTX which inhibits the synthesis and mineralization of new osseous matrix. This inhibition resulted in a reduced width of osteoid (non-mineralized bone matrix) of the periosteum and the endosteum [37]. The inhibitory effect of MTX on the osteoblastic cells could be reproduced in in vitro trials, where MTX suppressed bone formation by limited differentiation of early osteoblastic cells [38] and functional inhibition of mature osteoblasts [39]. May et al. [40, 41] found that prolonged administration of low-dose MTX in female rats caused significant osteopenia via suppression of osteoblast activity and stimulation of osteoclast recruitment, thus provoking an increased bone resorption [40, 41].
In contrast to MTX, leflunomide is not associated with an accentuated periarticular demineralization. Leflunomide could ameliorate the course of arthritis by its inhibitory effect on T cells; Urushibara et al. [42] also found that leflunomide induced a marked suppressive effect on RANKL activated intracellular signaling and osteoclast differentiation. Using a bone destruction model characterized by the absence of T cells, Urushibara et al. [42] demonstrated the direct effect of leflunomide in the down-regulation of osteoclastogenesis, supplementing its suppressive effect on T cells [42].
Focusing on the central role of RANKL in arthritic bone destruction, antirheumatic drugs such as leflunomide, which inhibit RANKL signaling, will contribute to the maintenance of an intact joint structure by the direct inhibition of osteoclasts [42].
Therapeutic effects of leflunomide and methotrexate on joint space narrowing
A joint space narrowing could be quantified for JSW-MCP (leflunomide group: −2.7%; MTX group: −5.7%) and JSW-PIP (leflunomide group: −2.1%; MTX group: −6.2%) showing significant results in patients with MTX therapy.
In a longitudinal study Sharp et al. [13] demonstrated a reduced structural damage in RA patients under leflunomide therapy. The leflunomide therapy started with a 3-day loading dose of 100 mg/day, followed by leflunomide daily doses of 20 mg. The trial consisted of two study cohorts: US301 (leflunomide or MTX administered for 4–12 months at an initial MTX dose of 7.5 mg/week which was increased to 15 mg/week over weeks 6–9 in 60% of the patients) and MN302 (treatment of leflunomide and MTX for 12 months, with an initial MTX dose of 7.5 mg/week which was increased to 10 mg/week at week 4 and to 15 mg/week at or after week 12). Changes in the Sharp joint space narrowing score were observed in the US301 study with 0.31 (leflunomide) versus 0.41 (MTX). The data of the MN302 study showed increased values of 1.48 for leflunomide and 1.08 for MTX estimated by means of the Sharp joint space narrowing score [13].
Taking into consideration the radiogeometric assessment of RA progression, the results from the study conducted by van der Heijde et al. [43] in 128 patients with a mean leflunomide treatment duration of 4.3 years (100 mg loading dose for 3 days, followed by 20 mg/day afterwards) demonstrated no radiographically visible progression in 33% of the RA patients during the leflunomide therapy [43].
Larsen et al. [14] radiographically assessed the RA progression in patients treated with leflunomide versus sulfasalazine during a treatment duration of 24 months and demonstrated a delay in disease progression under leflunomide application compared to sulfasalazine (observed as early as 6 months after the start of the treatment and still effective after the 24-month treatment) [14].
A limitation of the study is the difference of age between the MTX and leflunomid group. Furthermore, the study is limited by the small number of included patients. Additionally, further prospective studies are necessary with an increased number of participants to verify the results of this initial study.
Conclusion
The refinement of CAD techniques has promoted the precise measurements of several radiogeometric features in RA using digitized hand radiographs. Possible applications and the clinical importance of DXR and CAJSA might result in a retrospective and also prospective BMD calculation and quantification of joint space narrowing in routinely performed follow-up radiographs in order to monitor the progression of RA under different therapeutic regimes and to confirm the reparative changes after DMARD treatment. For this reason, the operator-independent and widely available DXR and CAJSA technology could be important diagnostic tools considering the individual severity and disease activity of RA based on quantitative data on periarticular osteoporosis and joint space narrowing. The clinician is able to consecutively optimize the therapeutic strategies in order to prevent joint destruction which will inevitably lead to high disability and morbidity.
Abbreviations
- BMD:
-
Bone mineral density (g/cm²)
- CAJSA:
-
Computer-aided joint space analysis
- CRP:
-
C-reactive protein
- CV:
-
Coefficient of variation
- DMARD:
-
Disease-modifying anti-rheumatic drug
- DXA:
-
Dual X-ray absorptiometry
- DXR:
-
Digital X-ray radiogrammetry
- DXR-BMD:
-
Bone mineral density (g/cm²), estimated by means of digital X-ray radiogrammetry
- DXR-MCI:
-
Metacarpal index, estimated by means of digital X-ray radiogrammetry
- ESR:
-
Erythrocyte sedimentation rate
- JSW:
-
Joint space width
- JSW-MCP:
-
Joint space width of the metacarpophalangeal joint
- JSW-PIP:
-
Joint space width of the proximal interphalangeal joint
- JSW-DIP:
-
Joint space width of the distal interphalangeal joint
- MCP:
-
Metacarpophalangeal joint
- NS:
-
Not significant
- NSAID:
-
Non-steroidal anti-inflammatory Drug
- PIP:
-
Proximal interphalangeal joint
- RA:
-
Rheumatoid arthritis
- SD:
-
Standard deviation
- VPA:
-
Volume per area
- W:
-
Bone width
References
Blair WF (1996) An approach to complex rheumatoid arthritis hand and wrist problems. Hand Clin 12:615–628
Gravallese EM (2002) Bone destruction in arthritis. Ann Rheum Dis 61:84–86
Sharp JT (2000) An overview of radiographic analysis of joint damage in rheumatoid arthritis and its use in metaanalysis. J Rheumatol 27:254–260
Rau R, Wassenberg S (2003) Imaging techniques in rheumatology: scoring methods in rheumatoid arthritis. Z Rheumatol 62:555–565. doi:10.1007/s00393-003-0516-9
Hoff M, Haugeberg G, Kvien TK (2007) Hand bone loss as outcome measure in established rheumatoid arthritis: a two-year observational study comparing cortical and total bone loss. Arthritis Res Ther 9:R81. doi:10.1186/ar2280
Haugeberg G, Orstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK (2002) Bone loss in patients with rheumatoid arthritis. Results from a population-based cohort of 366 patients followed up for two years. Arthritis Rheum 46:1720–1728. doi:10.1002/art.10408
Lodder MC, Haugeberg G, Lems WF, Uhlig T, Orstavik RE, Kostense PJ et al (2003) Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: The Oslo-Truro-Amsterdam (OSTRA) collaborative study. Arthritis Rheum 49:209–215. doi:10.1002/art.10996
Hofbauer LC, Heufelder AE (2001) Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med 79:243–253. doi:10.1007/s001090100226
Böttcher J, Malich A, Pfeil A, Petrovitch A, Lehmann G, Heyne JP et al (2004) Potential clinical relevance of digital radiogrammetry for quantification of periarticular bone demineralization in patients suffering from rheumatoid arthritis depending on severity and compared with DXA. Eur Radiol 14:631–637. doi:10.1007/s00330-003-2087-1
Böttcher J, Pfeil A, Rosholm A, Petrovitch A, Seidl BE, Malich A et al (2005) Digital X-Ray Radiogrammetry combined with semi-automated analysis of joint space distances as a new diagnostic approach in rheumatoid arthritis–A cross-sectional and longitudinal study. Arthritis Rheum 52:3850–3859. doi:10.1002/art.21606
James MF, Heald G, Shorter JH, Turner RA (1995) Joint space measurement in hand radiographs using computerized image analysis. Arthritis Rheum 38:891–901. doi:10.1002/art.1780380704
Sharp JT, Gardner JC, Bennett EM (2000) Computed-based methods for measuring joint space and estimating erosion volume in the finger and wrist joints of patients with rheumatoid arthritis. Arthritis Rheum 43:1378–1386. doi :10.1002/1529-0131(200006)43:6<1378::AID-ANR23>3.0.CO;2-H
Sharp JT, Strand V, Leung H, Hurley F, Loew-Friedrich I (2000) Treatment with leflunomide slows radiographic progression of rheumatoid arthritis. Results from three randomized controlled trials of leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum 43:495–505. doi :10.1002/1529-0131(200003)43:3<495::AID-ANR4>3.0.CO;2-U
Larsen A, Kvien TK, Schattenkirchner M, Rau R, Scott DL, Smolen JS et al (2001) Slowing of disease progression in rheumatoid arthritis patients during long-term treatment with leflunomide or sulfasalazine. Scand J Rheumatol 30:135–142. doi:10.1080/030097401300162897
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fies FJ, Cooper NS (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324. doi:10.1002/art.1780310302
Larsen A, Thoen J (1987) Hand radiography of 200 patients with rheumatoid arthritis repeated after an interval of one year. Scand J Rheumatol 16:395–401
Rosholm A, Hylsdrup L, Baeksgaard L, Grunkin M, Thodberg HH (2001) Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int 12:961–969. doi:10.1007/s001980170026
Kalla AA, Meyers OL, Laubscher R (1995) Prevalence of metacarpal osteopenia in young rheumatoid arthritis patients. Clin Rheumatol 14:617–625. doi:10.1007/BF02207926
Böttcher J, Pfeil A, Rosholm A, Malich A, Petrovitch A, Heinrich B et al (2005) Influence of image-capturing parameters on Digital X-Ray Radiogrammetry. J Clin Densitom 8:87–94. doi:10.1385/JCD:8:1:087
Pfeil A, Böttcher J, Seidl BE, Schäfer ML, Hansch A, Heyne JP et al (2007) Computer-aided joint space analysis (CAJSA) of the proximal-interphalangeal joint-normative age-related and gender specific data. Acad Radiol 14:594–602
Steinbroker O, Traeger CH, Batterman RC (1949) Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662
Larsen A, Dale K, Eek M (1977) Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 18:481–491
Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M et al (1985) How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 28:1326–1335. doi:10.1002/art.1780281203
Kalla AA, Meyers OL, Chalton D, Heath S, Brown GM, Smith PR et al (1991) Increased metacarpal bone mass following 18 months of slow acting antirheumatic drugs for rheumatoid arthritis. Br J Rheumatol 30:91–100. doi:10.1093/rheumatology/30.2.91
Jergas M (2003) Conventional radiographs and basic quantitative methods. In: Grampp S (ed) Radiology of osteoporosis. Springer, Berlin, pp 62–64
Böttcher J, Pfeil A, Rosholm A, Schäfer ML, Malich A, Petrovitch A et al (2006) Computerized digital imaging techniques provided by digital radiogrammetry as new diagnostic tool in rheumatoid arthritis. J Digi Imag 19:279–288. doi:10.1007/s10278-006-0263-y
Böttcher J, Pfeil A, Rosholm A, Sörös P, Petrovitch A, Schaefer ML et al (2006) Computerized quantification of joint space narrowing and periarticular demineralization in patients with rheumatoid arthritis based on Digital X-ray Radiogrammetry. Invest Radiol 41:36–44. doi:10.1097/01.rli.0000191594.76235.a0
Katz JN, LeBoff MS, Wade JP, Brown EM, Liang MH (1989) Effect of methotrexate on bone density and calcium homeostasis in rheumatoid arthritis. Clin Res 37:509A abstract
Carbone LD, Kaeley G, McKown KM, Cremer M, Palmieri G, Kaplan S (1999) Effects of long-term administration of methotrexate on bone mineral density in rheumatoid arthritis. Calcif Tissue Int 64:100–101. doi:10.1007/s002239900585
Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM (1997) Effects of low dose methotrexate on the bone mineral density of patients with rheumatoid arthritis. J Rheumatol 24:1489–1494
Ferraccioli G, Salaffi F, De Vita S, Casatta L, Bartoli E (1996) Methotrexate in polymyalgia rheumatica: preliminary results of an open, randomized study. J Rheumatol 23:624–628
Mazzantini M, Di Munno O, Incerti-Vecchi L, Pasero G (2000) Vertebral bone mineral density changes in female rheumatoid arthritis patients treated with low-dose methotrexate. Clin Exp Rheumatol 18:327–331
Cranney AB, McKendry RJ, Wells GA, Ooi DS, Kanigsberg ND, Kraag GR et al (2001) The effect of low dose methotrexate on bone density. J Rheumatol 28:2395–2399
Minaur NJ, Kounali D, Vedi S, Compston JE, Beresford JN, Bhalla AK (2002) Methotrexate in the treatment of rheumatoid arthritis. II. In vivo effects on bone mineral density. Rheumatology 41:741–749. doi:10.1093/rheumatology/41.7.741
Scheven BA, Van der Veen MJ, Damen CA, Lafeber FP, Van Rijn HJ, Bijlsma JW et al (1995) Effects of methotrexate on human osteoblasts in vitro: modulation by 1, 25-dihydroxyvitamin D. J Bone Miner Res 10:874–880
Preston SJ, Clifton-Bligh P, Laurent MR, Jackson C, Mason RS (1997) Effects of methotrexate and sulphasalazine on UMR 106 rat osteosarcoma cells. Br J Rheumatol 36:178–184. doi:10.1093/rheumatology/36.2.178
Cegiela U, Sliwinski L, Kaczmarczyk-Sedlak I, Folwarczna J (2005) In vivo effects of high-dose methotrexate on bone remodeling in rats. Pharmacol Rep 57:504–514
Uehara R, Suzuki Y, Ichikawa Y (2001) Methotrexate (MTX) inhibits osteoblastic differentiation in vitro: possible mechanism of MTX osteopathy. J Rheumatol 28:251–256
Davies JH, Evans BAJ, Jenney MEM, Gregory JW (2002) In vitro effects of chemotherapeutic agents on human osteoblast-like cells. Calcif Tissue Int 70:408–415. doi:10.1007/s002230020039
May KP, West SG, McDermott MT, Huffer WE (1994) The effect of low-dose methotrexate on bone metabolism and histomorphometry in rats. Arthritis Rheum 37:201–206. doi:10.1002/art.1780370208
May KP, Mercill D, McDermott MT, West SG (1996) The effect of methotrexate on mouse cells in culture. Arthritis Rheum 39:489–494. doi:10.1002/art.1780390317
Urushibara M, Takayanagi H, Koga T, Kim S, Isobe M, Morishita Y et al (2004) The antirheumatic drug leflunomide inhibits osteoclastogenesis by interfering with receptor activator of NF-kB ligand-stimulated induction of nuclear factor of activated T cells c1. Arthritis Rheum 50:794–804. doi:10.1002/art.20206
van der Heijde D, Kalden J, Scott D, Smolen J, Strand V (2004) Long term evaluation of radiographic disease progression in a subset of patients with rheumatoid arthritis treated with leflunomide beyond 2 years. Ann Rheum Dis 63:737–739. doi:10.1136/ard.2003.010983
Acknowledgments
The authors thank Monika Arens (managing director, Arewus GmbH) and Jacob Algulin (Sectra, Sweden) for the use of digital X-ray radiogrammmetry, Anders Rosholm (PhD) for the use of the computer-aided joint space analysis and Rüdiger Vollandt (PhD) for the statistical advice. Finally the authors would also like to thank Dieter Felsenberg, MD (Berlin, Germany) and Claus C. Gluer, PhD (Kiel, Germany) for their comments regarding the study design.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfeil, A., Lippold, J., Eidner, T. et al. Effects of leflunomide and methotrexate in rheumatoid arthritis detected by digital X-ray radiogrammetry and computer-aided joint space analysis. Rheumatol Int 29, 287–295 (2009). https://doi.org/10.1007/s00296-008-0682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-008-0682-9