Abstract
Summary
By meta-analysis, the risk of fracture was 15 % lower in patients treated with β-adrenergic blockers compared to controls independent of gender, fracture site, and dose. This might be attributable to β1-selective blockers.
Introduction
The aim of this study is to determine by meta-analysis whether β-adrenergic blockers (BBs) reduce fracture risk and whether the effect, if demonstrable, is dependent upon selectivity, dose, gender, or fracture site.
Methods
A literature search was performed in electronic databases MEDLINE, EMBASE, and reference sections of relevant articles to identify eligible studies. Adjusted estimates of fracture risk effect size (ES) were pooled across studies using fixed or random-effects (RE) meta-analysis as appropriate. Dose-related effects were evaluated using meta-regression. To explore the relative efficacy of β1-selective blockers in comparison to nonselective BBs, adjusted indirect comparison was performed.
Results
A total of 16 studies (7 cohort and 9 case–control studies), involving 1,644,570 subjects, were identified. The risk of any fracture was found to be significantly reduced in subjects receiving BBs as compared to control subjects (16 studies, RE pooled ES = 0.86, 95 % CI 0.78–0.93; I2 = 87 %). In a sensitivity analysis limited to those studies deemed to be most robust, the BB effect to reduce fracture risk was sustained (four studies, pooled ES = 0.79, 95 % CI 0.67–0.94; I2 = 96 %). The risk of a hip fracture was lower in both women and men receiving BBs (women: pooled ES = 0.86, 95 % CI 0.80–0.91; I2 = 1 % and men: pooled ES = 0.80, 95 % CI 0.71–0.90; I2 = 0 %). Similar risk reductions were found for clinical vertebral and forearm fractures, although statistical significance was not reached. The reduction in risk did not appear to be dose-related (test for a linear trend p value 0.150). Using adjusted indirect comparisons, it was estimated that β1-selective agents were significantly more effective than nonselective BBs in reducing the risk of any fracture (six studies, β1-selective blockers vs. nonselective BBs: RE pooled ES = 0.82, 95 % CI = 0.69–0.97).
Conclusions
The findings suggest that the risk of fracture is approximately 15 % lower in patients treated with BBs compared to controls independent of gender, fracture site, and dose. This risk reduction might be associated with the effects of β1-selective blockers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeleton is innervated by the autonomous nervous system (1), and osteoblasts express β2-adrenergic receptors (β2AR) (2, 3). A central regulation of bone mass by the sympathetic nervous system (SNS) via β2AR has been demonstrated in mouse models, an effect thought to be leptin-dependent (4, 5). The interaction between SNS and the skeleton in human subjects is not fully elucidated but may also involve additional factors both at central and peripheral sites (6–8)). In general, SNS activation is considered to contribute to bone loss (9), and, therefore, pharmacological βAR blockade would be expected to have a favorable effect on the skeleton.
Beta-adrenergic receptor antagonists (β-blockers, BBs) are established pharmacological agents, which are being used for a wide variety of indications (e.g., hypertension, arrhythmias, myocardial infarction, congestive heart failure, and angina pectoris). The beneficial effects of BBs are mostly mediated by blockade of presynaptic adrenoceptors that increase the release of norepinephrine from sympathetic nerve terminals and decrease of central vasomotor activity, as well as the inhibition of peripheral catecholamine actions on βARs, leading to a reduction in cardiac output, heart rate, myocardial oxygen demands, renin release, angiotensin II production, and inhibition of vascular smooth muscle cell proliferation (10).
The clinically meaningful effect of BBs on the human skeleton has been estimated by a meta-analysis of observational studies, in which fracture was used as the main outcome. BBs were found to be associated with a significant reduction in risk of any fracture (relative risk 0.86, 95 % confidence interval (CI) 0.70–0.98) (11). However, many relevant studies have been published since (12–21), which collectively are being regarded as inconclusive (22, 23). Moreover, no pooled estimate regarding gender-specific effects of BBs on fracture risk has been reported, even though such an effect has been suggested (24). Gender-specific effects gain biological plausibility in terms of differences among the sex steroids and their respective rates of decline (estrogens vs. androgens), femur geometry (25), volumetric bone mineral density (26), trabecular changes (27), and quantitative trait loci patterns (28). Moreover, a site-specific effect of β-blockers has been suggested (13) as well as a dose–response pattern (24). Also importantly, it has been proposed that any benefit BB may confer to fracture risk reduction is mediated by β1-selective blockers rather than non-selective BBs (19). To address these considerations, a meta-analysis of observational studies, to date, was conducted.
Methods
Search strategy
To identify eligible studies, a computerized literature search was performed in electronic databases MEDLINE and EMBASE over a 7-year period, from January 2006 to January 2013 (English language only). MeSH and free text terms used for the search were combined with methodological filters to limit retrieval of studies to those involving only human subjects (Supplementary appendix). This protocol was complemented by a secondary search involving the scanning of the reference sections of all relevant studies, reviews, and the previous meta-analysis (11). Titles and abstracts were first screened for relevance by two independent reviewers (KAT and SS), and articles deemed potentially relevant were obtained in full. Any disagreements were resolved by discussion and the opinion of a third reviewer, as needed.
Eligibility
To be eligible for inclusion, a study (cohort or case–control, prospective, or retrospective) needed to report an extractable estimate of the fracture risk in patients under treatment with BBs. Predefined exclusion criteria were the following: (1) no control group and (2) treatment with BBs of less than a year. Case–reports, case-series, unpublished studies, and conference abstracts were not considered.
Data extraction and definition of outcomes
Standardized data extraction forms were used independently by two reviewers (SS, KAT). Specific emphasis was placed on the methodology applied in each study for the ascertainment of BB use and fractures, as well as adjustments for confounders. From the within-study reported estimates, the one derived from the model adjusted for the higher number of covariates was considered as the best estimate. Any data on cumulative exposure, BB selectivity, interaction with other agents, and fracture type were also extracted. A subset of studies was characterized as “best available evidence” (higher quality), provided that (1) BB use and fractures were ascertained by an objective (considered to be less vulnerable to bias) method (computerized medical records and/or x-rays as opposed to ascertainment on the basis of interviews/questionnaires), (2) study population was derived from the general population, and (3) reported fractures were rigorously assessed as incident (as opposed to prevalent). The Newcastle–Ottawa scale (NOS) was also used independently by two reviewers to verify the assessment of study quality. If not available, standard errors (SE) were calculated from CIs using the following formula: ln(SE) = [ln(upper 95 % CI) limit – ln (lower 95 % CI limit)] / 3.92. Primary outcome was the risk of any fracture in patients receiving BBs compared to controls. Secondary outcomes were the risk of hip, clinical vertebral, and wrist fractures in female and male patients (gender- and site-specific risk) receiving BBs compared to controls. Risk of any fracture in patients receiving high BB dose compared to that in those receiving low BB dose and in those receiving selective β1-blockers compared to nonselective BB also served as secondary analyses.
Data synthesis
For any of the prespecified outcomes, a relative measure of risk in each study was expressed as an adjusted hazard ratio (aHR) with the corresponding 95 % CI or adjusted odds ratio (aOR) with the corresponding 95 % CI for cohort studies and case–control studies, respectively. Adjusted estimates were used to minimize the potential confounding effect of patient-level characteristics on the risk of fracture. For data synthesis, logarithmic transformation of adjusted estimates was used, back-transformed for reporting. Pooled adjusted estimates were calculated using the generic inverse variance method. Fixed or random-effects (RE) models were used depending on the degree of heterogeneity (random effects used when I2 > 50 %). To obtain an overall pooled effect size (ES) estimate, the most informative of the effect size estimate (that is the estimate derived from the model with the highest number of covariates) was used, and aOR were considered an approximation of aHR, given the expected low incidence and small effect. Pooled ES estimate was then translated to the number-needed-treat (NNT) (using the formula NNT = 1-[PEER*(1-OR)]/(1-PEER)*(PEER)*(1-OR), where PEER = patients expected event rate) to promote interpretation. In addition to the overall pooled estimate, gender- and site-specific estimates were also computed. Sensitivity analysis followed including only those studies characterized as best available evidence. Small study effects (publication bias) were explored by the Egger test (using 0.1 as the p value cutoff, acknowledging the low power of this test). To detect a potential dose-related effect, a meta-regression with linear trend estimation was undertaken (29). To this end, exposure was classified into three categories (low, medium, and high on the basis of the sum of defined daily doses), the sum of prescriptions, the average dose group, or sum of treatment days, and category-specific estimates were used. To explore the relative efficacy of β1-selective blockers in comparison to nonselective BBs, an adjusted indirect comparison was undertaken as explained in (30). Analyses were conducted in Stata/MP 10.0 for Windows (StataCorp LP, 4905 College Station, TX 77845, USA).
Results
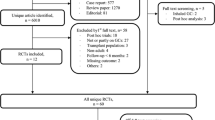
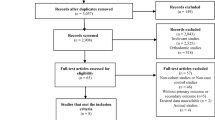
From a total of 949 references identified through the computerized search and the secondary search, 15 studies met the inclusion criteria (12–19, 21, 24, 31–35). In one study (16), results from two distinct populations were reported, and this study is therefore treated as two individual studies. Search results (flow chart) and a list of excluded studies on a full-text basis (n = 16) may be found in Supplementary file 1. The general characteristics of the studies included in the meta-analysis are summarized in Table 1. In all, fracture data regarding 1,644,570 individuals from 16 studies (7 cohort and 9 case–control studies), with a mean age ranging from 43 to 81 years old, were analyzed.
In general, only clinical vertebral (as opposed to morphometric vertebral fractures) were considered with the exception of one study in which this was not clear (18). On the other hand, prevalent fractures (as opposed to incident fractures) and non-vertebral fractures (as opposed to all fracture sites) were used as the main outcome in a subset of the studies (15, 16, 34), and, thus, their effect on the pooled estimate was investigated in the sensitivity analysis.
Meta-analysis
Primary outcome
Overall risk of any fracture
The risk of any fracture was significantly reduced in subjects receiving BBs compared to controls (16 studies, RE pooled ES = 0.86, 95 % CI 0.78–0.93, I2 = 87 %; Fig. 1). This finding was consistent in both cohort (7 studies, pooled aHR = 0.84, 95 % CI 0.71–0.98; I2 = 91 %) and case–control studies (9 studies, pooled aOR = 0.87, 95 % CI 0.79–0.95; I2 = 70 %). No evidence of publication bias was detected (Egger test p = 0.65). Assuming the overall lifetime risk of any osteoporotic fracture at the age of 50 to be 30 % (36), one osteoporotic fracture is prevented for every 30 patients under treatment with BBs.
Sensitivity analyses
In a prespecified sensitivity analysis limited to the best available evidence (12, 13, 19, 24), the risk of any fracture was again found to be significantly reduced in subjects receiving BBs compared to controls (4 studies, pooled ES = 0.79, 95 % CI 0.67–0.94; I2 = 96 %). This finding was also confirmed in the subgroup analysis of those studies designated as of higher quality (above the median) on the basis of NOS (8 studies, pooled ES = 0.84, 95 % CI 0.76–0.92; I2 = 92 %). This was also the case in a sensitivity analysis excluding the two studies with the largest sample size (12, 19) (14 studies RE pooled ES = 0.87, 95 % CI 0.78–0.95; I2 = 66 %). To inspect the effect of duplicate publication bias (37), one of the two studies (16, 24) using General Practice Research Database (GPRD) data was alternately excluded. This finding continued to be robust, excluding either the GPRD study by Schlienger et al. (24) (RE pooled ES = 0.86, 95 % CI 0.78–0.95; I2 = 88 %) or de Vries et al. (16) (RE pooled ES = 0.86, 95 % CI 0.78–0.95; I2 = 88 %). No evidence of publication bias was detected by Egger test in any of the above analyses.
Secondary outcomes
Gender- and site-specific risk of fractures
Gender- and site-specific pooled estimates of the fracture risk are summarized in Table 2. In general, the magnitude of effect remained consistent in men, women, and mixed populations. The estimate of hip fracture risk was lower in both women and men receiving BBs compared to controls (Females: pooled ES = 0.86, 95 % CI 0.80–0.91; I2 = 1 % and males: pooled ES = 0.80, 95 % CI 0.71–0.90, I2 = 0 %; Fig. 2). Exclusion of the two largest studies did not affect the results. Similar risk reductions were suggested for clinical vertebral and forearm fractures, although statistical significance was not reached (Table 2).
Dose-related effects
A potential dose-related effect, expressed in terms of either cumulative or current exposure, was explored in a subset of studies. Exposure was quantified on the basis of the sum of defined daily dose (12, 19), the sum of prescriptions (24), the last prescribed dose (16), dose group and sum of treatment days (17), and the midpoint of the recommended dose range (32). Two studies (one case–control and one cohort) did not support a dose-related pattern (16, 17), while in three studies, a dose–response relationship was evident (12, 19, 24). Another study in which patients were evaluated on duration of BB treatment risk was increased after 8 years (33). Extractable data (adjusted ES estimates across dose categories) were provided only in four case–control studies (including distinctly GPRD and PHARMO studies, reported in De Vries et al. (16)) (12, 16, 24) and one cohort study (19). Pooled effects stratified by dose level (low, medium, or high) revealed no significant difference between medium and high dose compared to low dose (p values 0.786 and 0.161, respectively, Fig. 3). Meta-regression analysis showed no significant differences among exposure categories, although a suggestion for a linear trend, compatible with a dose–response relationship, was noted (p value 0.150). By alternately excluding GPRD studies to inspect the effect of duplicate publication bias (37), findings remained unchanged with the exclusion of either Schlienger et al. (24) (p value 0.167) or de Vries et al. (16) (p value 0.156).
Selectivity
The potential effect of β1-selective blockers on fracture risk as compared to that of nonselective BBs was investigated in a subset of the studies included in the meta-analysis. In three cohort studies (13, 14, 19), the β1-selective agents were significantly more effective in reducing fracture risk than nonselective BBs, in line with a previous report (32). In contrast, no major difference on the basis of BB selectivity was detected in two studies (16, 24). A study in which β1-selective agents were not considered reported no significant difference in fracture between BBs users and controls (34). Using adjusted indirect comparison, it was estimated that β1-selective agents were significantly more effective than nonselective BBs in reducing the risk of any fracture (six studies, β1-selective blockers vs. nonselective BBs: RE pooled ES = 0.82, 95 % CI 0.69–0.97). By alternately excluding GPRD studies to inspect the effect of duplicate publication bias (37), findings remained unchanged with the exclusion of either Schlienger et al. (24) (RE pooled ES = 0.76, 95 % CI 0.62–0.93) or de Vries et al. (16) (RE pooled ES = 0.79, 95 % CI 0.64–0.99).
Interaction with other antihypertensives
The protective effect of BBs was present only in patients who had received or were currently receiving other antihypertensive regimens in both GPRD and PHARMO-RLS analyses (16). On the other hand, the risk reduction (compared to controls) was rather similar in patients only on BB and those on concurrent use of BB and thiazides (24). Unfortunately, thiazide use was investigated as a covariate in the majority of studies, and no further analysis was feasible.
Discussion
The present study suggests that the risk of any fracture is approximately 15 % lower in patients treated with BBs compared to controls. This risk reduction appears to be seen in men and women and for all major fracture sites (hip, vertebral, and forearm) and remained robust in sensitivity analyses. Dose dependency was not established. Finally, it was demonstrated that the reduction in fracture risk was associated with the effects of β1-selective blockers rather than nonselective BBs.
A series of elegant preclinical experiments established the role of β2AR in skeletal biology (5, 38–43), and the reported clinical benefit in terms of fracture risk could be explained within this context. On the other hand, the role of β1AR signaling and its potential interaction with β2AR remain unclear (44). An “unexplored complexity” in the regulation of bone metabolism by sympathetic signaling has been suggested (6). Thus, the present finding that fracture risk reduction is possibly associated with the effects of β1AR rather than β2AR blockade could not have been anticipated intuitively. Such complexity may also explain the counterintuitive epidemiological report that β2-agonists had a rather neutral effect on fracture risk (45). An alternative explanation β1AR blockade’s apparent superiority could be gleaned by an interesting study, in which the acute effects of β1AR blockade on parathyroid hormone (PTH) secretion were investigated (46). In this study, an increase in pulsatile PTH secretion was documented in response to intravenous infusion of a short-acting hydrophilic BB agent (esmolol). Thus, aside from a direct local action on the skeletal β1AR, the beneficial effect of selective β1-blockers on bone metabolism conceivable could be mediated by the osteoanabolic actions of PTH. This speculation will obviously require experimental confirmation.
Assuming the overall lifetime risk of any osteoporotic fracture at the age of 50 to be 30 % (36), 1 osteoporotic fracture is prevented over the life course of every 30 treated patients. This is not a negligible effect and may have important implications for clinical practice and/or health policy. However, the reported anti-fracture potential of BBs should be carefully weighed against the side-effects associated with their use, namely hypotension, dizziness, blurred vision (which collectively might be associated with an increased risk of falls), as well as cold extremities, bradycardia, nausea, insomnia, erectile dysfunction, and negative influence on glucose and lipid metabolism. Moreover, the demonstrated superiority of β1-selective blockers over nonselective agents in terms of skeletal effects may provide additional impetus to explore further the “complex” role of β1AR in the central regulation of the skeleton.
The findings of the present study should be interpreted with caution because of the observational nature of the evidence and significant heterogeneity in the results, which was not explained in the sensitivity analysis. Diversity in study design, study populations, and use of beta-blockers (type, dose, and duration) are plausible explanations for the observed statistical heterogeneity. Another potential source of heterogeneity may be the underlying disease. Heart failure, a treatment indication for BBs, is an established, clinically and densitometrically, independent risk factor for osteoporotic fractures (47). However, the extent to which this may have contributed to the observed heterogeneity could not be quantified. Finally, it should be noted that beta-adrenergic receptor selectivity is rather lost at higher doses (48), an observation that may have a potential confounding effect in the dose-response analysis. On the other hand, (1) the use of adjusted (rather than crude estimates), which has probably minimized the confounding effect of patient-level characteristics (imbalance between groups on a study level) and (2) the analysis of the best available evidence, which confirmed the robustness of the findings, supports the validity of the results. Some of our findings are also in accordance with a recently published meta-analysis by an independent group (49), despite the facts that a different methodology was applied, different populations were considered (18, 19, 34), and that dose and selectivity were not addressed. On the other hand, no difference in fracture risk between carvedilol-treated patients with congestive heart failure and controls could be documented in a report of a pooled analysis of nine relevant trials (22, 32). However, it should be noted that fractures were recorded only as adverse events, an imbalance between study groups in terms of baseline fracture risk could not be excluded, and that the duration of the trials may not have been long enough for the full skeletal effects to take place.
In summary, by this meta-analysis, the risk of any fracture is approximately 15 % lower in patients under treatment with BBs compared to controls independently of gender and site. This risk reduction may be mostly associated with β1-selective blockers.
References
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F (2011) Beta2-adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152:1412–1422
Takeda S, Karsenty G (2008) Molecular bases of the sympathetic regulation of bone mass. Bone 42:837–840
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Patel MS, Elefteriou F (2007) The new field of neuroskeletal biology. Calcif Tissue Int 80:337–347
Elefteriou F (2005) Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci 62:2339–2349
Elefteriou F (2008) Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 473:231–236
Toulis KA, Anastasilakis AD, Polyzos SA, Makras P (2011) Targeting the osteoblast: approved and experimental anabolic agents for the treatment of osteoporosis. Horm (Athens) 10:174–195
Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C (2004) Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 25:1341–1362
Wiens M, Etminan M, Gill SS, Takkouche B (2006) Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260:350–362
Rejnmark L, Vestergaard P, Mosekilde L (2006) Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case–control study. J Hypertens 24:581–589
Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV (2011) Association between beta-blocker use and fracture risk: the Dubbo Osteoporosis Epidemiology Study. Bone 48:451–455
Meisinger C, Heier M, Lang O, Doring A (2007) Beta-blocker use and risk of fractures in men and women from the general population: the MONICA/KORA Augsburg cohort study. Osteoporos Int 18:1189–1195
Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL (2007) Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40:1209–1216
de Vries F, Souverein PC, Cooper C, Leufkens HG, van Staa TP (2007) Use of beta-blockers and the risk of hip/femur fracture in the United Kingdom and The Netherlands. Calcif Tissue Int 80:69–75
Solomon DH, Mogun H, Garneau K, Fischer MA (2011) Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res 26:1561–1567
Sosa M, Saavedra P, Gomez de Tejada MJ, Mosquera J, Perez-Cano R, Olmos JM, Munoz-Torres M, Amerigo MJ, Moro MJ, Diaz-Curiel M, Alegre J, Malouf J, Del Pino J, Nogues X, Torrijos A (2011) Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res 23:112–117
Song HJ, Lee J, Kim YJ, Jung SY, Kim HJ, Choi NK, Park BJ 2012 beta1 selectivity of beta-blockers and reduced risk of fractures in elderly hypertension patients. Bone
Turker S, Karatosun V, Gunal I (2006) Beta-blockers increase bone mineral density. Clin Orthop Relat Res 443:73–74
Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF (2006) Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2. Arch Intern Med 166:241–246
Reid IR (2008) Effects of beta-blockers on fracture risk. J Musculoskelet Neuronal Interact 8:105–110
Ilic K, Obradovic N, Vujasinovic-Stupar N (2013) The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int 92:217–227
Schlienger RG, Kraenzlin ME, Jick SS, Meier CR (2004) Use of beta-blockers and risk of fractures. JAMA 292:1326–1332
Peacock M, Buckwalter KA, Persohn S, Hangartner TN, Econs MJ, Hui S (2009) Race and sex differences in bone mineral density and geometry at the femur. Bone 45:218–225
Riggs BL, Melton Iii LJ III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954
Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ 3rd (2006) Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21:124–131
Peacock M, Koller DL, Lai D, Hui S, Foroud T, Econs MJ (2009) Bone mineral density variation in men is influenced by sex-specific and non sex-specific quantitative trait loci. Bone 45:443–448
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, Bradburn M, Eastwood AJ (2005) Indirect comparisons of competing interventions. Health Technol Assess 9:1–134, iii-iv
Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC (2004) Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res 19:19–24
Reid IR, Gamble GD, Grey AB, Black DM, Ensrud KE, Browner WS, Bauer DC (2005) beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res 20:613–618
Rejnmark L, Vestergaard P, Kassem M, Christoffersen BR, Kolthoff N, Brixen K, Mosekilde L (2004) Fracture risk in perimenopausal women treated with beta-blockers. Calcif Tissue Int 75:365–372
Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Breart G (2005) Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc 53:550–552
Jensen J, Nielsen LH, Lyhne N, Hallas J, Brosen K, Gram LF (1991) Drugs and femoral neck fracture: a case–control study. J Intern Med 229:29–33
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Bazelier M, de Boer A, de Vries F (2012) Acid suppressants and hip fracture: duplicate publication bias? Bone 49:920, author reply 921
Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI (2009) Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys 482:96–103
Kondo H, Togari A (2011) Continuous treatment with a low-dose beta-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int 88:23–32
Nagao M, Feinstein TN, Ezura Y, Hayata T, Notomi T, Saita Y, Hanyu R, Hemmi H, Izu Y, Takeda S, Wang K, Rittling S, Nakamoto T, Kaneko K, Kurosawa H, Karsenty G, Denhardt DT, Vilardaga JP, Noda M (2011) Sympathetic control of bone mass regulated by osteopontin. Proc Natl Acad Sci U S A 108:17767–17772
Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, Nagao M, Saita Y, Hemmi H, Notomi T, Nakamoto T, Schipani E, Takeda S, Kaneko K, Kurosawa H, Karsenty G, Kronenberg HM, Vilardaga JP, Noda M (2012) Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc Natl Acad Sci U S A 109:7433–7438
Rodrigues WF, Madeira MF, da Silva TA, Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ, Barbosa-Neto O, Lopes AH, Napimoga MH (2012) Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br J Pharmacol 165:2140–2151
Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, Pichon C, Courteix D (2008) Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol 217:819–827
Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL (2012) Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res 27:1252–1262
de Vries F, Pouwels S, Bracke M, Leufkens HG, Cooper C, Lammers JW, van Staa TP (2007) Use of beta-2 agonists and risk of hip/femur fracture: a population-based case–control study. Pharmacoepidemiol Drug Saf 16:612–619
Schmitt CP, Obry J, Feneberg R, Veldhuis JD, Mehls O, Ritz E, Schaefer F (2003) Beta1-adrenergic blockade augments pulsatile PTH secretion in humans. J Am Soc Nephrol 14:3245–3250
Majumdar SR, Ezekowitz JA, Lix LM, Leslie WD (2012) Heart failure is a clinically and densitometrically independent risk factor for osteoporotic fractures: population-based cohort study of 45,509 subjects. J Clin Endocrinol Metab 97:1179–1186
Cruickshank JM (1980) The clinical importance of cardioselectivity and lipophilicity in beta blockers. Am Heart J 100:160–178
Yang S, Nguyen ND, Eisman JA, Nguyen TV (2012) Association between beta-blockers and fracture risk: a Bayesian meta-analysis. Bone 51:969–974
Acknowledgments
We are thankful to Professor Dr Alan Reid (Faculty of Medical and Health Sciences, University of Auckland, New Zealand) who kindly provided additional information relevant to this review. This work was conceived during the 2011 Preceptorship Program in Metabolic Bone Diseases held at Columbia University Medical Center, New York, USA.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toulis, K.A., Hemming, K., Stergianos, S. et al. β-adrenergic receptor antagonists and fracture risk: a meta-analysis of selectivity, gender, and site-specific effects. Osteoporos Int 25, 121–129 (2014). https://doi.org/10.1007/s00198-013-2498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2498-z