Abstract
The fields of neuroscience and bone biology have recently converged following the discovery that bone remodeling is directly regulated by the brain. This work has defined bone remodeling as one of the cardinal physiological functions of the body, subject to homeostatic regulation and integrated with the other major physiological functions by the hypothalamus. Central to this discovery was the definition of the adipocyte-derived hormone leptin as a regulator of both arms of bone remodeling, formation and resorption, through its action on the ventromedial hypothalamus and subsequently via the sympathetic nervous system to osteoblasts. The characterization of the sympathetic nervous system as a regulator of bone remodeling has led to several large clinical studies demonstrating a substantial protective effect of ß-blockers, particularly ß1-blockers, on fracture risk. Studies in model organisms have reinforced the role of the central nervous system in the regulation of bone remodeling in vivo by the identification of several additional genes, namely cocaine and amphetamine regulated transcript (Cart), melanocortin 4 receptor (Mc4R), neuropeptide Y (NPY), Y2 receptor, cannabinoid receptor CB1 (Cnbr1), and the genes of the circadian clock. These genes have several common features, including high levels of expression in the hypothalamus and the ability to regulate other major physiological functions in addition to bone remodeling including energy homeostasis, body weight, and reproduction. We review the major pathways that define the new field of neuroskeletal biology and identify further avenues of inquiry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Beyond development, bone is a physiologically active and reactive tissue responding to dietary, hormonal, paracrine/autocrine, and mechanical signals necessary to release minerals into the bloodstream and to adapt to physiological and mechanical demands. The responsiveness of bone is accomplished by the action of osteocytes that modulate mineral homeostasis and respond to mechanical strain as well as by osteoclasts and osteoblasts through the process of bone remodeling, a mechanism that promotes conservation of an appropriate architecture and stable bone mass during adulthood [1, 2]. Defects in bone remodeling, generally induced by an imbalance between osteoblast and osteoclast activity, lead to pathological conditions such as osteoporosis, a disease characterized by reduced bone mineral density (BMD) and increased fracture risk [3]. This disease, the most prevalent degenerative condition in developed countries, has become of prime importance due to our ever-increasing life span and the as yet poorly understood deterioration of bone quality over time.
Clues about the existence of neuronal and central regulatory components of the bone remodeling process have been reported in the clinical literature for several decades. Those correlations between bone and the nervous system include the reflex sympathetic dystrophy syndrome (or algodystrophy) characterized by localized sympathetic hyperactivity and osteopenia; increased osteogenic activity and BMD associated with severe head injury [4]; the associations between stroke, spinal cord injury, or peripheral neuropathy and osteopenia, bone fragility, or poor fracture healing [5–10]; and observations of robust neurogenesis during fracture healing [6, 11, 12]. It also seems logical that bone physiology would be coupled to the regulation of other homeostatic functions to better integrate skeletal physiology with the daily physiological changes triggered by the environment or by endogenous rhythms. The hypothalamus in that regard would be the preferential center to achieve this function, thanks to its “sensing” properties, its ability to integrate peripheral and central signals, and its ability to send efferent hormonal and neuronal signals in response to stimulation.
The hypothalamus was implicated as a regulator of bone remodeling by melding two independent and well-established epidemiological observations: obese patients are protected from osteoporosis and osteoporosis typically follows gonadal deficiency. These clinical observations, which were previously viewed as unconnected, were synthesized to suggest the existence of a common mechanism regulating reproduction, body weight, and bone remodeling [13]. Based on the fact that reproduction and body weight are centrally regulated, the hypothesis implied the existence of a novel central locus for the regulation of bone remodeling. A number of studies by the Karsenty group using genetic and pharmacological manipulation of mouse models confirmed the notion that bone homeostasis is under the influence of a hypothalamic control center and that the output pathway is neuronal in nature.
Multiple Hypothalamic Pathways Regulate Bone Remodeling
Leptin
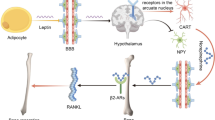
The first experimental evidence of functional regulation of bone remodeling by the hypothalamus was provided by mice that are both hypogonadal and obese. The discovery that absence of leptin (Ob) or its signaling receptor (ObRb) induces a high bone mass in mice, in spite of hypogonadism, suggested a major inhibitory role for leptin in the regulation of bone mass [13]. Leptin is an adipocyte-derived hormone that acts on the brain to reduce food intake, mostly by regulating the activity of neurons in the hypothalamic arcuate nucleus that highly express ObRb. This anorexigenic function of leptin occurs via stimulation of neurons that express the anorexigenic peptide alpha-melanocyte-stimulating hormone (α-MSH) and inhibition of neurons that coexpress the orexigenic peptides neuropeptide Y (NPY) and agouti-related protein (AgRP) [14]. However, along with its better-known role in the regulation of food intake and energy expenditure, leptin also regulates and integrates via the hypothalamus, or by direct peripheral effects, many other physiological functions, including the regulation of lipid and glucose metabolism, cardiovascular function, angiogenesis, reproduction, hematopoiesis, wound healing, and brain development [15].
Experimental evidence that leptin uses a central relay to regulate bone remodeling was provided first in mice and later in rats and sheep by demonstrating that low doses infused intracerebroventricularly (icv) significantly decrease trabecular bone mass [13, 16, 17]. Additional studies using mouse models with high or low serum leptin levels confirmed the pronounced effect of leptin on bone mass. Markedly increasing serum leptin level by transgenic liver overexpression reduced bone mass [18]. In contrast, mice characterized by low serum leptin level, such as A-ZIP lipodystrophic or PPARghyp/hyp mice, displayed a high bone mass phenotype [18, 19]. Follow-up studies using chemical lesioning and icv infusion of leptin defined a population of ventromedial hypothalamic (VMH) neurons as constituting the major center responsive to leptin for its regulation of bone remodeling [20]. Indeed, destruction of VMH neurons, which highly express the leptin receptor ObRb, recapitulated the bone phenotype of mice lacking leptin (ob/ob); and leptin icv infusion could rescue the high bone mass phenotype of ob/ob mice but not that of VMH-lesioned ob/ob mice, thereby convincingly demonstrating that leptin requires VMH neurons to decrease bone mass.
Surprisingly, a recent study using Sf1-cre mice to delete hypothalamic ObRb receptors in a VMH-specific manner reported the absence of a bone density phenotype in these mutant mice [21]. Unfortunately, bone mass was not assessed in this mouse model, and the field awaits a more appropriate histomorphometric or micro-computed tomographic (microCT) analysis. If it turns out to be correct that these mice have no bone phenotype, since 20% of their VMH neurons remained responsive to leptin (i.e., the ObRb deletion was only 80% complete), these findings would suggest that the VMH is highly sensitive to leptin for bone mass regulation.
The strong expression of ObRb in hypothalamic neurons together with the previously described in vivo results allowed elucidation of a novel physiological pathway for the control of bone mass by the brain, a discovery that has led to several controversies. The high bone mass and decreased fracture risk associated with obesity are counterintuitive based on this pathway, where high leptin levels should reduce bone mass. One explanation for this observation invokes the notion of leptin resistance, where leptin levels above a critical threshold create a state of resistance to the hormone. This explanation has been used to explain why obesity is associated with high leptin levels and may hold true to explain the increased bone mass in obese patients who would have a state of low leptin signaling centrally.
Another controversy revolves around the central vs. peripheral effects of leptin. Unfortunately, the literature on the peripheral effects of leptin is difficult to interpret as central leptin resistance may have been caused by the use of pharmacological doses of leptin; e.g., the cumulative dose of leptin that is required in mice to decrease bone mass centrally is approximately 5 μg, whereas the cumulative dose used to demonstrate a peripheral action of leptin on resistance to load [22] was 860 μg, both given over similar time frames. In another example, Burguera et al. [23] showed partial protection against ovariectomy-induced bone loss in rats with 100 μg/day of peripheral leptin administration. Food intake, one of the most important central functions regulated by leptin, was not different between leptin- and vehicle-treated rats in the latter half of the study, suggesting the development of central leptin resistance. This was consistent with the authors’ finding that cerebrospinal fluid leptin levels were virtually identical in the two groups of animals. A second consideration in interpreting this body of work is that some peripheral leptin effects on bone can only be demonstrated in ob/ob mice and not in wild-type (WT) mice [24]. However, the reason leptin produced in vivo by liver overexpression was able to decrease bone mass [18] is at present unclear but may relate to the short half-life of leptin (5 min), to leaky expression centrally, or to inappropriate (asynchronous) peaks of exogenous leptin vis-à-vis its endogenous circadian secretion rhythm.
A separate controversy is whether leptin is produced peripherally. Several groups have shown that leptin (and its signaling receptor ObRb) can be found in human primary trabecular osteoblast cultures [24–26], whereas others have shown the absence of leptin, ObRb, and leptin signaling in mouse primary calvarial osteoblast cultures [12]. A mouse knock-in of ß-galactosidase into the leptin locus demonstrated X-gal staining only in white fat and no other tissue, and overexpression of leptin in mouse osteoblasts in vivo via the 2.3kb α1 collagen promoter did not affect trabecular bone mass [18, 20]. These studies indicate there may be species-specific differences in the production and signaling of leptin. If human leptin is produced in osteoblasts and if it directly and positively regulates bone formation, then clinical studies would be expected to show a positive association between serum leptin and BMD. On the other hand, if leptin mainly signals centrally to inhibit bone formation, then clinical studies should show an inverse association between serum leptin and BMD [27]. As discussed below, a large majority of studies find an inverse association, arguing against the peripheral mode of action. It is, however, possible that in the obese state peripheral production or positive signaling of leptin in bone becomes more prominent and this, along with central leptin resistance effects, could contribute to the higher bone mass and reduced fracture risk seen with obesity. If this is true, then clinical studies limited to obese participants should show a positive association between serum leptin and BMD.
Finally, there has been controversy surrounding the phenotype of ob/ob mice that were reported to have high trabecular bone mass in femora and vertebrae [12]. Bone studies of male ob/ob mice confirmed a high vertebral bone mass but identified low femoral bone mass, both trabecular and cortical, indicating that the response of bone to the absence of leptin signaling varies between the axial and appendicular skeleton in a gender-specific manner [13, 28]. Gender-specific differences in bone mass have been seen by us in several mouse models used to study the leptin pathway, validating the observations of Hamrick et al. [28] and suggesting that this difference is a general feature of the pathway. How this occurs is a fascinating and open research question.
Clinical studies have confirmed a role for leptin in the regulation of bone biology in humans. However, the skeletal sites, age, gender, and strength of the effect(s) of leptin are still being defined. Numerous studies have suggested that serum leptin level is associated with BMD, independently of fat mass and other covariates [27, 29–36]; but the association is not seen in many, often smaller, studies [37–42] and appears stronger for women. The stronger association in women is notable for two reasons, first because women have a two- to threefold higher leptin level than men and second because virtually all of the mouse studies elucidating the role of leptin and its pathway to bone were done in female mice [13, 18, 20, 27]. Moreover, the low bone mass phenotype in the femora of male ob/ob mice may identify a confounding variable that reduced the power to detect an effect in some of the negative clinical studies [39–44]. When detected, the association between serum leptin and BMD is almost always inverse, as would be expected from the mouse data; however, positive associations have occasionally been reported [43, 44]. Despite their hypogonadism, leptin-deficient patients have a high BMD phenotype that is equivalent to that seen in leptin-replete, eugonadal, obese controls [45]. Leptin-deficient patients also have accelerated bone age, indicating that leptin is an inhibitor of both chondrogenesis and osteogenesis in humans, as suggested by animal studies [46]. This effect was more pronounced in younger patients, suggesting that rapidly growing bone may be more sensitive to regulation by leptin [18].
NPY and NPY Receptors
NPY is a target of leptin signaling in the hypothalamus, with the potential to act through at least five receptors (Y1, Y2, Y4, Y5, and Y6) that differ not only in their binding profiles but also in their distribution in the central nervous system (CNS) and the periphery. All of these receptors are expressed in the hypothalamus, and several respond to other ligands. One important ligand for Y receptors, NPY, is widely distributed within the CNS. Strong expression is found in the hypothalamic area, where NPY fibers project from the arcuate nucleus, which integrates emotional, vegetative, sensory, and autonomic functions with endocrine functions.
NPY functions in the CNS include the regulation of anxiety-related behavior, feeding, and cardiovascular and memory functions [47]. Its role at the periphery is still unclear, but immunoreactivity for NPY is found in nerve fibers distributed throughout bone [48, 49]. Strongly supporting a role of NPY receptor signaling in the regulation of bone formation, Y2 −/− mice display an increase in long bone trabecular bone mass that can be reproduced by hypothalamus-specific deletion of Y2, indicating that Y2 signaling in the hypothalamus inhibits bone formation [50]. Similarly, ob/ob mice have a high trabecular bone mass phenotype that can be reproduced by destruction of hypothalamic neurons expressing ObRB [13, 20]. This similarity between ob/ob and Y2 −/− mice and the elevation of NPY levels in the hypothalamus of both mouse models [51, 52] pointed toward a mechanistic link between NPY and leptin for the regulation of bone mass and to the suggestion that NPY may be a common mediator of the high bone mass of these two mouse models [53]. However, a dichotomy between the ObRb and Y2 pathways is supported by comparison of the long bones of male Y2 −/− and ob/ob mice that revealed that Y2 deficiency stimulates both cortical and trabecular bone mass, while leptin deficiency has the opposite effect on both bone compartments, diminishing trabecular and cortical bone mass [54]. Furthermore, absence of the Y2 receptor stimulated cortical bone formation with or without blockade of leptin action [54]. Interestingly, inactivation of both Y2 and Y4 receptors led to a further increase in bone mass compared to Y2 alone, which was accompanied by reduced serum leptin level, suggesting a Y4-mediated additional effect of leptin deficiency on the Y2 −/− bone phenotype [55].
Thus, Y2 receptor signaling clearly regulates, via a hypothalamic relay, the bone formation arm of the bone remodeling process in both femoral trabecular and cortical bone compartments. Whether NPY is the ligand for Y2 receptor for this function on bone mass and whether the role of NPY on bone formation is restricted to long bones is still a question since a bone mass phenotype was observed in NPY-deficient femurs but not vertebrae [56, 57]. The role of other Y receptors in bone remodeling awaits analysis of mutant mice lacking Y1 or Y5. The peripheral function of NPY also remains to be investigated based on its rich expression in the bone microenvironment and during fracture healing. Lastly, one will have to verify whether the role of Y receptor and NPY signaling is conserved from mouse to humans since no human data are available yet to correlate these exciting mouse results with human physiology.
The Cannabinoid System
The cannabinoid system, mostly known for its involvement in psychotropic, analgesic, and orexigenic processes, has also recently been shown to regulate bone mass in vivo. The cannabinoid type 1 (CB1) receptor is expressed in the CNS and sympathetic nervous system (SNS), as well as in osteoclasts. It accounts for most of the CNS actions of cannabinoid drugs and endocannabinoid molecules synthesized by the body. Its absence in mutant animals induces a bone phenotype that is dependent on strain background differences, as has been observed for their other phenotypes [58]. Indeed, CNR1 −/− mice on the outbred CD1 background exhibit a high bone mass phenotype [59], while CNR1 −/− mice on the inbred C57BL6/J background display a low bone mass phenotype, decreased bone formation, and elevated osteoclast number [60]. The molecular explanation for these opposing phenotypes is not known. It is, however, interesting to note that CNR1 −/− mice on a C57BL6/J background are hypersensitive to leptin icv, which could explain at least part of their phenotype [61]. Because it inhibits norepinephrine release, CB1 signaling in peripheral nerves may affect signaling of the SNS to bone (described in more detail below) [62]. However, since CB1 is expressed in both the CNS and peripheral neurons and cells, the contribution of central versus peripheral CB1 receptors in mediating the effect of cannabinoids on bone remains to be determined. Here again, conditional deletion of Cnr1 or increased expression of CB1 ligands through transgenesis will bring meaningful information regarding the importance and site of action of this receptor for its regulatory action on bone mass.
The CB2 receptor encoded by Cnr2 is more specific for peripheral tissues and is notably expressed in immune cells, osteoblasts, and osteoclasts. Mice deficient for the Cnr2 gene display a low bone mass phenotype resulting from high bone turnover, and in vitro analyses demonstrated a direct effect of CB2 agonists on the generation of osteoclasts, indicating a peripheral mode of action [63].
The importance of the cannabinoid system in bone biology is implicated in the bone response to traumatic brain injury (TBI), which enhances osteogenesis and stimulates central endocannabinoid production. In a TBI mouse model, Tam et al. [64] reported that Cnr1 −/− mice, but not Cnr2 −/− mice, are resistant to TBI-induced stimulation of bone formation, suggesting that CB1 signaling mediates the pro-osteogenic state in TBI.
In human studies, a significant association of single-nucleotide polymorphisms and haplotypes encompassing the CB2 gene (CNR2) at the susceptibility locus for low hip BMD [65] on human chromosome 1p36 was found in patients with postmenopausal osteoporosis [66]. An association was not found for CNR1, but based on the mouse results, studies of TBI in humans may be required to demonstrate an effect of this locus.
CART and Melanocortin 4 Receptor
Cocaine and amphetamine-regulated transcript (CART), another neuropeptide whose expression is positively controlled by leptin signaling [67, 68], has recently been shown to regulate the bone resorption arm of bone remodeling [69]. Cart is broadly expressed in the CNS, including in hypothalamic neurons, as well as in peripheral organs such as the pancreas and the adrenal gland. Mice deficient for Cart, although eugonadal, display a low bone mass phenotype solely due to an increase in bone resorption, indicating that CART inhibits bone resorption in vivo [69]. The importance of CART in regulating bone remodeling and the hypothalamic nature of this regulation are supported by animal models characterized by low or high hypothalamic Cart expression and significant bone phenotypes. Low Cart expression in ob/ob mice accompanies the increased bone resorption observed in these mice, while increased hypothalamic Cart expression in obese and hyperleptinemic melanocortin 4 receptor (Mc4r)-deficient mice correlates with their low bone resorption and high bone mass [69]. Furthermore, decreasing Cart by genetic means in Mc4r-deficient mice rescues their resorption phenotype, indicating that Cart overexpression in Mc4r-deficient mice is required for their high bone mass phenotype [70]. Importantly, this CART-mediated regulatory loop of bone resorption may be conserved in humans as people lacking MC4R have increased CART serum levels and a high bone mass phenotype associated with decreased bone resorption [69–71]. The mode of action of CART on bone resorption is not yet defined and awaits identification of the CART receptor.
Neuronal Nitric Oxide Synthase and Central Nitric Oxide Signaling
Nitric oxide (NO) and enzymes producing it (endothelial, inducible, and neuronal NO synthases) play important regulatory roles in multiple cell types, including osteoblasts. In particular, endothelial NO synthase (eNOS) appears to be a positive regulator of osteoblast proliferation and differentiation, based on its predominance in bone cells (compared to other isoforms) and the low bone mass phenotype of mice deficient for eNOS [72, 73]. Inducible NOS (iNOS) activity in osteoblasts is essential for mediating interleukin-1 (IL-1)-induced bone resorption and regulating the effects of proinflammatory cytokines on bone [74–76]. On the other hand, neuronal NOS (nNOS) expression is minimal in bone cells, and osteoblasts derived from nNOS-deficient mice do not display obvious proliferation or differentiation defects, whereas in vitro receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)-induced osteoclastogenesis from bone marrow macrophages is increased in the absence of nNOS [77]. In contrast to these in vitro observations, nNOS-deficient mice display a high bone mass phenotype and low bone turnover. This bone phenotype along with the high expression of nNOS in the CNS, including the hypothalamus, suggest that nNOS might regulate bone homeostasis by a central relay, although a role in the peripheral nervous system cannot be excluded for now. eNOS and iNOS are also expressed in the CNS and could play a role in the regulation of bone mass via a central relay as well. Experimental interventions aimed at modifying NO production or NOS activity specifically in the hypothalamus or other CNS regions are essential to determine whether a central relay involving NO signaling regulates bone homeostasis.
Sympathetic Signaling Downstream of Hypothalamic Leptin-Responsive Neurons Regulates Bone Formation
The identification of a central relay for leptin’s antiosteogenic function led to the question of the nature of the downstream mechanism whereby hypothalamic neurons regulate the activity of distant cells like osteoblasts. One could imagine the existence of two alternate pathways: a humoral pathway involving a molecule secreted by the hypothalamus or pituitary and a neuronal pathway. Parabiosis experiments have argued against a humoral mechanism [20], while several observations support a neuronal pathway: (1) ob/ob mice have a low sympathetic tone, (2) electrical stimulation of the VMH [78] and leptin injection directly into the VMH nucleus result in enhanced sympathetic tone [79], and (3) innervation of bone and the presence of immunological reactivity for diverse neuropeptides within the bone microenvironment were described several decades ago [for review, see 80], although the physiological relevance of these observations remained obscure. Along with these observations, the selective detection, among all postsynaptic adrenergic receptors, of the ß2-adrenergic receptor (β2AR) in mouse primary osteoblasts supported the hypothesis that the SNS could relay leptin signaling from the hypothalamus to osteoblasts [69]. In agreement with this hypothesis, mice lacking dopamine ß-hydroxylase (Dbh), the enzyme generating norepinephrine, were found to have an increased bone mass, as were mice treated with the ß-blocker propranolol. In contrast, mice treated with the adrenergic agonist isoproterenol, used as a surrogate of sympathetic activation, lost bone mass [20]. In rats, a β2AR agonist decreased BMD as well as mechanical strength [81], while ß-blockade had been previously shown to have the opposite effects [82]. These data strongly suggested that ß-adrenergic signaling, downstream of leptin signaling, inhibits bone formation by osteoblasts. However, because of the multiple endocrine abnormalities observed in ob/ob and Dbh mice, these data and concepts have been questioned. Phenotypic analysis of mice lacking Adrβ2 (the β2AR gene), which are not plagued by any of the endocrine abnormalities seen in ob/ob or Dbh mice, provided a definitive answer to this question. Indeed, Adrβ2-deficient mice, as opposed to ob/ob, VMH-lesioned, or Dbh −/− mice, have a normal body weight and normal hormonal status but show an increased trabecular bone formation rate and increased bone mass [69]. Bone marrow transplantation experiments between WT and Adrβ2-deficient mice demonstrated that this bone phenotype was due to a bone cell-autonomous defect and not a central defect, which was a possibility based on the widespread expression of Adrβ2 in the CNS. Importantly, Adrβ2-deficient mice, in contrast to WT littermates, were resistant to leptin icv infusion, indicating that β2AR signaling is required for leptin’s antiosteogenic function [69]. At the cellular level, Adrβ2-deficient mice, like ob/ob mice, showed increased numbers of osteoblasts and an increased bone formation rate, suggesting that inhibition of osteoblast proliferation (or differentiation) and function is the final effect of sympathetic signaling. Together, this in vivo pharmacological and genetic body of evidence demonstrated that leptin regulates bone formation via VMH neurons, the SNS, and β2AR signaling in osteoblasts.
Although mouse osteoblasts do not normally express Adrβ1 and Adrβ1-deficient mice have a normal bone mass, mice deficient for both Adrβ1 and Adrβ2 display a low bone mass phenotype, suggesting an indirect role for β1AR signaling in bone biology. The reduced femur length and low bone mass characterizing Adrβ1/2-deficient mice are accompanied by decreased serum levels of insulin-like growth factor I (IGF-I), suggesting that this indirect role may proceed through an osteoblast-independent mechanism [83].
In addition to the examples cited at the beginning of this article, a role for β-adrenergic signaling in human bone biology is supported by several recent epidemiological studies [84, 85]. A meta-analysis of seven studies assessing fracture risk in patients using β-blockers found their use was associated with a 28% reduction in hip fracture risk (95% confidence interval [CI] 19–37% reduction) and a 14% reduction (95% CI 2–24% reduction) in the risk for any fracture [84]. These case-control studies yielded several important pieces of information. First, it appears that β-blocker effects on BMD, which is different from bone mass, account for only a small portion of the observed fracture protection [77]. Thus, the influence of sympathetic signaling on bone metabolism may be somewhat different for humans and mice, with the caveat that human bone mass has not been properly examined. Second, one of the largest studies [86] found that only β-blockers without intrinsic sympathomimetic activity were protective, indicating that the observed protection was not a side effect of the drugs but rather due to sympathetic blockade. Third, α-adrenergic blockers have no detectable effect on fracture risk, indicating specificity for the β-adrenergic pathway [84]. Fourth, the protective effects of β-blockers appear to be mediated through effects on sympathetic blockade of β1-adrenoceptors, perhaps explaining the failure of one study that excluded β1-blockers to find an effect on fracture risk [87]. Taken together with the mouse data showing that Adrβ1 deficiency rescues the high bone mass of Adrβ2 deficiency, these results point to an as yet unexplored complexity in the regulation of bone metabolism by sympathetic signaling. Long-term prospective randomized trials are required to demonstrate unequivocally a protective effect of β-blockers, but these will take some time to complete. For now, the human data remain quite promising and open to further refinement.
Sympathetic Signaling Regulates Both Arms of Bone Remodeling
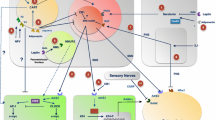
Further analysis of the bone phenotype of Adrβ2-deficient mice revealed that β2AR signaling regulates not only bone formation but also bone resorption. Indeed, Adrβ2-deficient mice display decreased bone resorption due to a decrease in osteoclast differentiation. To understand the cellular and molecular basis of this phenotype, osteoblast-osteoclast coculture assays were performed using combinations of WT and Adrβ2 −/− cells. This strategy demonstrated that β2AR stimulation in osteoblasts indirectly favors osteoclast differentiation. Further biochemical and molecular studies indicated that β2AR stimulation in osteoblasts increases the cyclic adenosine monophosphate (cAMP) level and activates protein kinase A (PKA), which eventually results in the phosphorylation of ATF4, a cAMP response element binding protein (CREB) family member previously shown to regulate osteoblast differentiation and collagen synthesis [88]. Mutation/phosphorylation and promoter studies pinpointed ATF4’s serine 254 as the target of PKA and as a necessary step for ATF4 to bind to and transactivate the Rankl promoter. In agreement with this result, isoproterenol, a surrogate of sympathetic signaling, increased Rankl expression in WT cells but not in Aft4 −/− osteoblasts [69]. These studies thus characterized a new transcriptional target of β2AR signaling in osteoblasts and identified a key role for β2AR signaling in the regulation of bone formation and bone resorption. The in vivo relevance of the role of sympathetic signaling in regulating bone resorption was confirmed in mice and rats by showing that ß-blockers could ameliorate or even prevent bone loss associated with ovariectomy [25, 81]. Moreover, a short-term randomized trial confirmed that ß-blockers could suppress bone resorption in humans [89]. The pathway whereby leptin regulates bone formation and bone resorption is illustrated in Figure 1.
Pathway for the regulation of bone formation and resorption by white adipose tissue. Structures are listed in the central axis of the figure; functions are on the right and molecular mediators on the left of the arrows. Arrows indicate enhancement of function, while the blunted line between SNS and Osteoblast indicates inhibition of osteoblast function and proliferation by the SNS. Question marks indicate regulators that remain to be discovered. Homeostasis requires feedback; therefore, an arrow representing a hypothetical pathway is shown from Osteoblast to Fat, but osteoblasts could just as well provide feedback to the hypothalamus and/or the SNS.
The sympathetic/ß-adrenergic pathway is so far the only identified signaling pathway linking the hypothalamus and bone cells, downstream of leptin. Whether the autonomic nervous system, and in particular β2AR signaling, also mediates hypothalamic NPY or Y2 receptor antiosteogenic function remains unknown. The lack of any changes in plasma total calcium, leptin, hypothalamo-pituitary-corticotropic, -thyrotropic, -somatotropic, or -gonadotropic status in Y2 −/− mice suggests that the Y2 receptor does not modulate bone formation by a known humoral mechanism and, therefore, that alteration of autonomic function through hypothalamic Y2 receptors could possibly play a role in the central regulatory circuit involved in bone formation [50]. Interestingly, viral retrograde neuronal labeling recently confirmed the neuronal nature of the link between bone and brain and, in particular, revealed targeting to the parvocellular nuclei where NPY is released and the Y1 receptor is expressed [90]. Additional studies including infusion of NPY to Adrβ2 −/− mice should address the question of the relationship between NPY signaling in the hypothalamus and β2AR-mediated sympathetic signaling to bone.
The Circadian Clock Regulates Sympathetic Signaling to Bone by Gating Osteoblast Proliferation
Daily variation in bone marrow proliferation with a peak occurring at night has long been known [91], as have daily (circadian) variations in the synthesis of collagen [92] and, more recently, in the variation in bone turnover markers [93–95]. Since the body’s main clock resides in the hypothalamus and leptin-regulated bone remodeling was demonstrated to be under hypothalamic control, these observations suggested that leptin’s effects on bone may be physiologically integrated by the molecular clock.
The molecular clock is evolutionarily conserved from archaebacteria to humans and is found in most organs of the body. It is composed of a positive transcriptional arm that turns on a group of genes termed the negative arm. The negative arm then suppresses the positive arm, with the whole process lasting around 24 hours and ending with reinduction of the positive arm genes to restart the cycle (Fig. 2). The positive arm of the clock is a heterodimeric transcription factor composed of the Clock and BMAL1 (MOP3) proteins. The main negative arm genes are Period (Per) 1 and 2 and Cryptochrome (Cry) 1 and 2 [89].
Simplified view of the molecular clock. BMAL1 and Clock form a heterodimeric transcription factor that stimulates transcription of the major negative arm genes Per1, Per2, Cry1, and Cry2. This stimulation is self-limited because, along with Rev-Erbα, Per and Cry disrupt BMAL1/Clock-mediated transcription. The next phase sees declining Per and Cry levels, and this in combination with their inhibition of Rev-Erbα function relieves the repression of BMAL1/Clock. BMAL1 and Clock are then free to be reinduced by positive regulators like the retinoic acid-related orphan receptor α (Rora) to begin the cycle anew.
To test the role of the molecular clock in the regulation of bone mass, female mice lacking both Per genes, which are molecularly arrhythmic, were examined and found to have a high bone mass phenotype in vertebrae and femora that was due to increased numbers of osteoblasts and an increased bone formation rate [96]. Mice lacking both Cry genes showed the same phenotype, indicating that the molecular clock is indeed an inhibitor of bone mass. Mice lacking a molecular clock were not deficient in leptin or sympathetic tone but were found to be resistant to the antiosteogenic effect of icv leptin infusion. Since increasing sympathetic signaling by icv leptin infusion did not lead to the expected bone loss in these mice, the results suggested an interruption of the leptin-SNS-bone pathway at the level of the bone. Consistent with this, bone was found to harbor all components of the molecular clock with up to 70-fold variation in the level of gene expression over a 24-hour period. Moreover, osteoblast-specific deletion of Per function using the 2.3 kb α1(I)-collagen promoter cre transgene resulted in high bone mass [96].
In WT mice, osteoblasts showed a diurnal variation in proliferation with a peak of DNA synthesis at night, whereas mice lacking a molecular clock showed no variation but instead had a tonically elevated rate of proliferation, as did their osteoblasts in vitro, which showed shortening of the G1 phase of the cell cycle. These results suggested that the molecular clock may mediate the effects of sympathetic signaling on osteoblasts. Consistent with this, β2AR was found to signal via CREB to directly activate the negative arm clock genes Per1 and Per2 in osteoblasts. These clock genes then inhibit c-myc transcription, leading to downregulation of its target cyclin D1, thus describing a complete molecular pathway for leptin’s antiosteogenic function [96]. In addition to the clock genes, β2AR signaling activated gene expression of AP-1, a family of transcription factors known to regulate osteoblast proliferation in part through activation of c-myc and cyclin D1 [90]. Thus, the data are consistent with a model whereby the molecular clock acts downstream of β2AR to gate sympathetic signaling in osteoblasts to ensure that proliferation preferentially occurs at certain times. In its absence, osteoblast proliferation is increased throughout the 24-hour day, and this eventually leads to high bone mass. Looking forward, the molecular mechanisms whereby the clock regulates the synthesis of bone extracellular matrix remain to be elucidated.
The clocks found in peripheral organs are synchronized by the master clock in the hypothalamus [97]. The regulation of bone mass by the clock is therefore likely to be influenced by factors that entrain the master clock, such as day length, and could therefore be one of the mechanisms underlying the seasonal changes observed in bone mass for most mammals, including humans [98, 99]. Intriguingly, peripheral clocks are able to function independently of the master clock under certain circumstances [100, 101]; thus, there may be ways to selectively affect the osteoblast clock to achieve a therapeutic gain in bone mass.
Conclusions
The increasing number of articles reporting bone phenotypes in mutant animal models deficient for neuropeptides or their receptors confirms the physiological relevance of a central and neuronal regulatory arm of bone remodeling and emphasizes the importance of genetically engineered mouse models for understanding bone physiology and homeostasis. Although of major importance, these studies still require follow-up clinical investigation to confirm the conservation of function between murine and human physiology and disease and to allow the design of new therapeutic strategies for the treatment of bone diseases. The results obtained during the last 15 years using genetically engineered mouse models and in vivo studies also highlight by their sometimes contradictory and/or complex results the importance of using appropriate genetic controls (mouse background), technology (e.g., histomorphometry with microCT analyses, which are far superior to BMD analysis in mice), nomenclature, and definition of the sex, bone, bone compartment, and age of the studied animals.
The demonstration that bone remodeling is regulated by hypothalamic centers suggests that this process is an integral part of the complex homeostatic control system that allows the body to respond to internal and external environmental changes, allowing the coordinated integration of bone homeostasis with diet, reproduction, lactation, and physical activity via hypothalamic neuronal networks responsive to mediators generated by each of these physiological processes. One can hypothesize that the evolutionary selection of common mediators for functions of a disparate nature may have occurred as a mechanism to jointly coordinate these functions, via the hypothalamus. This is best exemplified by the complex central effect of the hormone leptin on reproduction, bone remodeling, food intake, energy expenditure, and immunity.
The knowledge brought by these studies is fascinating and allows a deeper understanding of mammalian homeostasis. However, the complexity of hypothalamic signaling networks will make it difficult to intervene therapeutically at the hypothalamic level to modulate any aspect of bone remodeling without affecting other homeostatic processes, such as the regulation of body weight homeostasis or reproduction. An alternative, more feasible strategy may be to intervene downstream of the hypothalamus with drugs that block signals from the hypothalamus to bone cells, the reception of these signals by bone cells, or intracellular transduction pathways in bone cells. ß-Blockers may be one way to achieve such a goal based on their dual ability, demonstrated in mice and rats, to increase bone formation and decrease bone resorption. The existence of ß-blockers that do not cross the brain barrier will also be of interest, to limit their action to peripheral tissues. Although there are no NPY receptor antagonists clinically available, the recent results obtained with mice for the regulation of both body weight and bone mass may trigger more interest in developing such agents.
Since feedback loops are required for homeostasis to occur, these new findings imply the existence of a pathway whereby bone cells can signal back to the hypothalamus (or other brain centers). The nature and existence of this putative signal are still unknown but could involve factors of either a humoral or neuronal nature. The emerging description of neuroskeletal biology is a major conceptual advance in the field of bone biology that is allowing old questions to be asked in new ways, and it has already proven to be promising for identifying new targets for the amelioration of bone disease. What makes it particularly stimulating is not only the chance to identify new therapeutics but also the opportunity to use knowledge, concepts, and tools from other fields and apply them to the field of bone biology.
References
Bonewald LF (2002) Osteocytes: a proposed multifunctional bone cell. J Musculoskelet Neuronal Interact 2:239–241
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Cooper C, Melton LJI (1996) Magnitude and impact of osteoporosis and fractures. In: Marcus R, Feldman D, Kelsey J (eds), Osteoporosis. Academic Press, San Diego, pp 419–434
Perkins R, Skirving AP (1987) Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Joint Surg Br 69:521–524
Freehafer AA, Mast WA (1965) Lower extremity fractures in patients with spinal-cord injury. J Bone Joint Surg Am 47:683–694
Aro H (1985) Effect of nerve injury on fracture healing. Callus formation studied in the rat. Acta Orthop Scand 56:233–237
Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y (1999) Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int 9:269–275
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27:305–309
Poole KE, Reeve J, Warburton EA (2002) Falls, fractures, and osteoporosis after stroke: time to think about protection? Stroke 33:1432–1436
Pearson J, Dancis J, Axelrod F, Grover N (1975) The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol 34:413–424
Hukkanen M, Konttinen YT, Santavirta S, Paavolainen P, Gu XH, Terenghi G, Polak JM (1993) Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 54:969–979
Li J, Ahmad T, Spetea M, Ahmed M, Kreicbergs A (2001) Bone reinnervation after fracture: a study in the rat. J Bone Miner Res 16:1505–1510
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil T, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Zigman JM, Elmquist JK (2003) From anorexia to obesity–the yin and yang of body weight control. Endocrinology 144:3749–3756
Ahima RS, Saper CB, Flier JS, Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307
Guidobono F, Pagani F, Sibilia V, Netti C, Lattuada N, Rapetti D, Mrak E, Villa I, Cavani F, Bertoni L, Palumbo C, Ferretti M, Marotti G, Rubinacci A (2006) Different skeletal regional response to continuous brain infusion of leptin in the rat. Peptides 27:1426–1433
Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, Amling M (2006) Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res 21:1591–1599
Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G (2004) Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 101:3258–3263
Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J (2004) Enhanced bone formation in lipodystrophic PPARγhyp/hyp mice relocates haematopoiesis to the spleen. EMBO Rep 5:1007–1012
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB (2006) Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203
Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175:405–415
Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT (2001) Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142:3546–3553
Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92:73–78
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner O, Gordeladze JO, Drevon CA (2001) Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res 16:1426–1433
Di Monaco M, Vallero F, Di Monaco R, Mautino F, Cavanna A (2003) Fat body mass, leptin and femur bone mineral density in hip-fractured women. J Endocrinol Invest 26:1180–1185
Hamrick MW, Pennington C, Newton D, Xie D, Isales C (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383
Weiss LA, Barrett-Connor E, von Muhlen D, Clark P (2006) Leptin predicts BMD and bone resorption in older women but not older men: the Rancho Bernardo Study. J Bone Miner Res 21:758–764
Chanprasertyothin S, Piaseu N, Chailurkit L, Rajatanavin R, Ongphiphadhanakul B (2005) Association of circulating leptin with bone mineral density in males and females. J Med Assoc Thai 88:655–659
Blum M, Harris SS, Must A, Naumova EN, Phillips SM, Rand WM, Dawson-Hughes B (2003) Leptin, body composition and bone mineral density in premenopausal women. Calcif Tissue Int 73:27–32
Ruhl CE, Everhart JE (2002) Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res 17:1896–1903
Roux C, Arabi A, Porcher R, Garnero P (2003) Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone 33:847–852
Thomas T, Burguera B, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S (2001) Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29:114–120
Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M, Sasaki A, Kawachi S, Yoshino K, Yasuda K (2001) Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab 86:5273–5276
Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, Cowell CT (2004) Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 80:966–972
Roemmich JN, Clark PA, Mantzoros CS, Gurgol CM, Weltman A, Rogol AD (2003) Relationship of leptin to bone mineralization in children and adolescents. J Clin Endocrinol Metab 88:599–604
Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW (2005) The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf) 63:131–138
Papadopoulou F, Krassas GE, Kalothetou C, Koliakos G, Constantinidis TC (2004) Serum leptin values in relation to bone density and growth hormone-insulin like growth factors axis in healthy men. Arch Androl 50:97–103
Zhong N, Wu XP, Xu ZR, Wang AH, Luo XH, Cao XZ, Xie H, Shan PF, Liao EY (2005) Relationship of serum leptin with age, body weight, body mass index, and bone mineral density in healthy mainland Chinese women. Clin Chim Acta 351:161–168
Sahin G, Polat G, Baethis S, Milcan A, Baethdatoethlu O, Erdoethan C, Camdeviren H (2003) Body composition, bone mineral density, and circulating leptin levels in postmenopausal Turkish women. Rheumatol Int 23:87–91
Rauch F, Blum WF, Klein K, Allolio B, Schonau E (1998) Does leptin have an effect on bone in adult women? Calcif Tissue Int 63:453–455
Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K (2001) Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 55:341–347
Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC (2001) Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab 86:1884–1887
Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O’Rahilly S, Trussell RA (2004) Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab 89:4821–4826
Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A (2005) Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 37:607–621
Thorsell A, Heilig M (2002) Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides 36:182–193
Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M (1988) Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst 25:119–125
Hill EL, Elde R (1991) Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264:469–480
Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H (2002) Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109:915–921
Sainsbury A, Schwarzer C, Couzens M, Herzog H (2002) Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes 51:3420–3427
Wilding JP, Gilbey SG, Bailey CJ, Batt RA, Williams G, Ghatei MA, Bloom SR (1993) Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology 132:1939–1944
Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, Enriquez R, During M, Herzog H, Gardiner EM (2005) Hypothalamic control of bone formation: distinct actions of leptin and Y2 receptor pathways. J Bone Miner Res 20:1851–1857
Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, Eisman JA, Gardiner EM, Herzog H (2006) Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21:1600–1607
Sainsbury A, Baldock PA, Schwarzer C, Ueno N, Enriquez RF, Couzens M, Inui A, Herzog H, Gardiner EM (2003) Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol Cell Biol 23:5225–5233
Baldock PA, Allison S, Sainsbury A, Enriquez RF, Gardiner EM, Herzog H, Eisman JA (2006) Hypothalamic neuropeptide Y exerts a negative effect on cortical bone formation. In: Abstracts of the 28th annual meeting of the American Society for Bone and Mineral Research September 2006. JBMR, vol 21 (suppl 1). Abstract no S65, p 1246
Elefteriou F, Takeda S, Liu X, Armstrong D, Karsenty G (2003) Monosodium glutamate-sensitive hypothalamic neurons contribute to the control of bone mass. Endocrinology 144:3842–3847
Lutz B (2002) Molecular biology of cannabinoid receptors. Prostaglandins Leukot Essent Fatty Acids 66:123–142
Idris AI, van’t Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, Ralston SH (2005) Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med 11:774–779
Tam J, Ofek O, Fride E, Ledent C, Gabet Y, Muller R, Zimmer A, Mackie K, Mechoulam R, Shohami E, Bab I (2006) Involvement of neuronal cannabinoid receptor CB1 in regulation of bone mass and bone remodeling. Mol Pharmacol 70:786–792
Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648
Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G (1996) Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118:2023–2028
Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I (2006) Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 103:696–701
Tam J, Alexandrovich A, Di Marzo V, Petrosino S, Trembovler V, Zimmer A, Ledent C, Mackie K, Mechoulam R, Shohami E, Bab I (2006) CB1, but not CB2 cannabinoid receptor mediates stimulation of bone formation induced by traumatic brain injury. Abstracts of the 28th Annual Meeting of the American Society for Bone and Mineral Research September 2006. JBMR, vol 21 (suppl 1). Abstract no 1032, p S10
Devoto M, Shimoya K, Caminis J, Ott J, Tenenhouse A, Whyte MP, Sereda L, Hall S, Considine E, Williams CJ, Tromp G, Kuivaniemi H, Ala-Kokko L, Prockop DJ, Spotila LD (1998) First-stage autosomal genome screen in extended pedigrees suggests genes predisposing to low bone mineral density on chromosomes 1p, 2p and 4q. Eur J Hum Genet 6:151–157
Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A (2005) Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 14:3389–3396
Douglass J, McKinzie AA, Couceyro P (1995) PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci 15:2471–2481
Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G (2006) Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology 147:3196–3202
Orwoll B, Bouxsein ML, Marks DL, Cone RD, Klein RF (2004) Increased bone mass and strength in melanocortin-4 receptor-deficient mice. In: Orthopaedic Research Society/American Academy of Orthopaedic Surgeons Presentations 2003, 71st Annual Meeting of the AAOS. San Francisco, CA
Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M, Huang P, MacIntyre I, Polak J (2001) Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 158:247–257
Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, Ralston SH (2001) Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 142:760–766
van’t Hof RJ, Ralston SH (1997) Cytokine-induced nitric oxide inhibits bone resorption by inducing apoptosis of osteoclast progenitors and suppressing osteoclast activity. J Bone Miner Res 12:1797–1804
van’t Hof RJ, Armour KJ, Smith LM, Armour KE, Wei XQ, Liew FY, Ralston SH (2000) Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc Natl Acad Sci USA 97:7993–7998
Lowik CW, Nibbering PH, van de Ruit M, Papapoulos SE (1994) Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J Clin Invest 93:1465–1472
van’t Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH (2004) Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 145:5068–5074
Perkins MN, Rothwell NJ, Stock MJ, Stone TW (1981) Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 289:401–402
Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K (1999) Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48:1787–1793
Elefteriou F (2005) Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci 62:2339–2349
Bonnet N, Benhamou CL, Brunet-Imbault B, Arlettaz A, Horcajada MN, Richard O, Vico L, Collomp K, Courteix D (2005) Severe bone alterations under beta2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone 37:622–633
Minkowitz B, Boskey AL, Lane JM, Pearlman HS, Vigorita VJ (1991) Effects of propranolol on bone metabolism in the rat. J Orthop Res 9:869–875
Pierroz D, Baldock P, Bouxsein ML, Ferrari S (2006) Low cortical bone mass in mice lacking beta 1 and beta 2 adrenergic receptors is associated with low bone formation and circulating IGF-1. Abstracts of the 28th Annual Meeting of the American Society for Bone and Mineral Research September 2006. JBMR, vol 21 (suppl 1). Abstract no 1091, p S26
Wiens M, Etminan M, Gill SS, Takkouche B (2006) Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260:350–362
Rejnmark L, Vestergaard P, Mosekilde L (2006) Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens 24:581–589
Schlienger RG, Kraenzlin ME, Jick SS, Meier CR (2004) Use of beta-blockers and risk of fractures. JAMA 292:1326–1332
Levasseur R, Marcelli C, Sabatier JP, Dargent-Molina P, Breart G (2005) Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l’Osteoporose prospective study. J Am Geriatr Soc 53:550–552
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 117:387–398
Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB (2005) Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216
Denes A, Boldogkoi Z, Uhereczky G, Hornyak A, Rusvai M, Palkovits M, Kovacs KJ (2005) Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience 134:947–963
Mauer AM (1965) Diurnal variation of proliferative activity in the human bone marrow. Blood 26:1–7
Simmons DJ, Nichols G Jr (1966) Diurnal periodicity in the metabolic activity of bone tissue. Am J Physiol 210:411–418
Schlemmer A, Hassager C, Jensen SB, Christiansen C (1992) Marked diurnal variation in urinary excretion of pyridinium cross-links in premenopausal women. J Clin Endocrinol Metab 74:476–480
Srivastava AK, Bhattacharyya S, Li X, Mohan S, Baylink DJ (2001) Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice. Bone 29:361–367
Ladlow JF, Hoffmann WE, Breur GJ, Richardson DC, Allen MJ (2002) Biological variability in serum and urinary indices of bone formation and resorption in dogs. Calcif Tissue Int 70:186–193
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122:803–815
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Rosen CJ, Morrison A, Zhou H, Storm D, Hunter SJ, Musgrave K, Chen T, Wei W, Holick MF (1994) Elderly women in northern New England exhibit seasonal changes in bone mineral density and calciotropic hormones. Bone Miner 25:83–92
Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G (1991) Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 115:505–512
Ko CH, Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15(special issue 2):R271–R277
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00223-007-9068-3.
Rights and permissions
About this article
Cite this article
Patel, M.S., Elefteriou, F. The New Field of Neuroskeletal Biology. Calcif Tissue Int 80, 337–347 (2007). https://doi.org/10.1007/s00223-007-9015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9015-3