Abstract
Introduction and hypothesis
Three-dimensional modeling of feminine pelvic mobility is difficult because the sustaining system is not well understood and ligaments are especially difficult to identify on imaging.
Methods
We built a 3-D numerical model of the pelvic cavity, based on magnetic resonance (MR) images and knowledge about anatomy and validated it systematically.
Results
The quantitative results of this model allow for the non-destructive localization of the structures involved in pelvic statics. With a better configuration of the functional pelvis and topological criteria, we can obtain a coherent anatomical and functional model.
Conclusions
This model is the first step in developing a tool to localize and characterize pelvic imbalance in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feminine pelvic statics has challenged medical and scientific experts trying to understand the mechanisms involved. Epidemiology is complex and multifactorial. From a mechanical point of view, physiological mobility is essential to urinary and rectal functions as well as reproduction and sexual intercourse. The stresses experienced by women’s pelvic system when simply standing or participating in physical activities and during different physiological functions are not well understood. Regional tissues are soft, their mechanical behavior is hyperelastic, and their mechanical resistance is not well known.
The mobility of different pelvic organs is linked to the tissues of which these organs are composed, but also the thickness of connective tissues, and the elastic and muscular fibers framing all the organs in diffuse and plane structures play important role as well. These fascias are attached to the pelvic walls by dense structures or ligaments. Ligaments act as suspensions for pelvic organs (vagina, bladder, rectum, and uterus), but their relative contribution is still not well defined. This area is submitted to exceptional stress at the time of childbirth, but local equilibrium may also be disrupted by surgery. With time and the aging of patients, the resistance of pelvic tissues will also vary physiologically. The functional anatomy of this region and the relative importance of these different structures are still largely debated.
The aim of our study was to use a 3-D biomechanical model based on a finite element approach, to define the major contributors to the static suspension of the pelvis. Here, we present the different steps that we followed to build this 3-D biomechanical model of pelvic mobility, which led us to define the major entities of suspension. The anatomy of the pelvic region (organs, bones, and muscles) was built from the magnetic resonance imaging (MRI) scan of a specific patient. The biomechanical properties of pelvic tissues, which have already been reported, are briefly reviewed and taken into account in the anatomical model. Some suspension elements, particularly pelvic ligaments, that are difficult to identify on conventional imaging are incorporated according to anatomical descriptions. The numerical simulations of the mobility of the 3-D biomechanical model are compared to the results of dynamic MRI analysis. The model is then topologically optimized to reduce the mismatch between the two sets of results. Consequently, ligaments or fascias, which are described in the literature but considered as negligible in a first approach in the literature, are progressively introduced and localized. We thus obtained a 3-D biomechanical model, based on finite elements, that anatomically and functionally approximates actual pelvic mobility.
Such a model allows for the accurate simulation of the mobility of the pelvic system and for the definition of the major contributors to the pelvic suspension.
Methods
To develop the 3-D biomechanical model, we obtained MR images of a patient (nulligravida, 20 years old, Caucasian, without any pelvic statics problems upon examination). During the MRI session we performed a static MRI to define the geometry of the pelvic organs. Unfortunately, the MRI did not allow us to localize the suspension elements. To localize them and to define the major contributors to the pelvic statics, we compared the numerical simulation of a 3-D biomechanical model to the dynamic MRI, focusing on the mobility. The mobility for the dynamic MRI was induced by thrusting. During this examination, the patient was asked to not perform pelvic floor contractions and especially use the levator ani muscle.
The finite element method allows, among other things, for the quantification of the displacement under a given. Measuring the displacements of organs under pushing stress using this numerical model, and correlating the results with dynamic MR images, allows for the quantification of the mismatch between the numerical model and the anatomical reality. The discrepancy between the numerical model and MRI measurements with respect to the estimated mobility, in turn, allows then for the localization and definition of the major suspension contributors, which are defined in the literature but not observed on MRI and cannot be neglected to accurately model pelvic mobility.
Building a functional anatomical pelvic model
The construction of the numerical model was performed in several steps. First the geometries of organs and bones were defined using static MR images. During this stage, the suspension contributors could not be observed; thus, they were progressively deduced from a comparison between the numerical simulation and the mobility observed on dynamic MRI. The progressive construction of the functional numerical pelvic model is herein described.
Geometric model
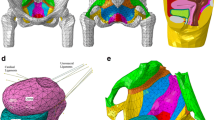
The geometries of the relevant organs and pelvis were determined by the segmentation of static MR images. These were obtained from three different planes (sagittal, axial, and coronal) following the Sigma HD™, 1.5 GEMS protocol with successive contiguous slices 6 mm thick. The surfaces of the organs were rendered by the segmentation of outlines using the software ARTIMED. The surfaces were meshed by triangulation, regularized using the software program BLENDER (Fig. 1a). Finally, the mesh was imported in the finite element software.
a Resulting geometric model. The geometric model is obtained by segmentation of static MRI. It is constituted of bony pelvis, pelvic organs in yellow (bladder, uterus, rectum, and vagina), pelvic floor in red, and pelvic ligaments. b Displacement field measurement on dynamic MRI with optic flow method. Displacement fields are depicted by vectors for each organ on sagittal dynamic MRI. Ve bladder, U uterus, Va vagina, R rectum
The pelvic floor, which is difficult to observe via MRI, is modeled as being close to the pelvic level but with large openings for the vagina and rectum. The different muscles of the pelvis were not differentiated. The ligaments are also difficult to individualize via MRI. They were progressively incorporated, when their contribution was highlighted, in agreement with the anatomical localization presented in the literature.
Finite element model
The finite element model developed featured 4,300 elements and 2,200 nodes inducing 11,867 degrees of freedom. We used the finite element software ABAQUS/Explicit (version 6.101). Explicit resolution was chosen due to its better management of the contacts between organs. The analysis was then conducted using a nearly static approach with progressive loadings.
To complete the finite element problem, the behavior of the different materials involved was introduced but also when the boundary conditions, which define the loading conditions, were introduced.
-
1.
Finite element mesh
The bladder, vagina, rectum, and pelvic floor are represented by deformable shells. The uterus is assumed to be rigid. To model the presence of a contrast gel in the rectum and bladder during MRI, we described these two organs as fluid cavities in the ABAQUS software. The fluid was assumed to be incompressible, allowing for isovolume transformations. Ligaments, when introduced, were assumed to be nonlinear springs with no stiffness during compression and linear behavior when submitted to tension.
-
2.
Mechanical properties of pelvic tissues
To define the mechanical behavior of the pelvic tissues, we used our recent studies on the matter [1–4]. In a first approximation, they were considered to be elastic and isotropic under large transformations. The thicknesses of the shells for the organs and the diameter of the springs for the ligaments are based on anatomical considerations [5, 6]. Table 1 presents all of the mechanical properties of the tissues considered. We assumed that the high content of water in the tissues makes them behave as incompressible solids. Consequently, we set a Poisson coefficient of 0.45 for each deformable shell.
-
3.
Boundary conditions
Very few data are available on the relationship between organs in the pelvic regions. However, according to the anatomical literature, it can be assumed that the circumference of the pelvic floor is steady enough such that its attachment to the bony pelvis and cervix is linked to the upper end of the vagina and that the lower parts of the vagina and rectum are bound to the pelvic floor. Moreover, we assume that the contacts between organs are perfect without any friction.
We simulated a pushing effort with the surface of applied forces inclined at 45° with respect to the patient’s vertical axis, forces being applied to the superior part of the organs [6]. According to the literature [5], the intrapelvic pressure experienced when the patient is lying down is close to 0.01 MPa. When coughing, the intrapelvic pressure is multiplied by a factor of 10–20.
An evaluation tool for numerical modeling of mobilities
As previously explained, this study consisted in determining the major contributors to the pelvic statics and comparing the numerical simulation of a 3-D biomechanical model, defined step by step, to the results measured for patients.
Quantification of displacement on MRI
To define the displacement of organs for the reference patient, we analyzed the dynamic sagittal MRI as the patient exerted a pushing effort. The images were analyzed using an optical flow algorithm implemented in OpenCV (Open Source Computer Vision), which allowed edge displacements of the organs to be measured. The displacement fields are depicted as vectors in Fig. 1b showing the displacement measured for a given image.
Comparing MR images and numerical simulation
To compare the simulated displacements obtained with the numerical 3-D biomechanical model to the MRI analysis, we compared the simulated displacements along the plane of the MRI to the measured displacements. Figure 2a shows the magnitude of the displacements in the MR images. The numerical simulations were compared to the displacements measured along the sagittal via dynamic MRI. Figure 2b illustrates the displacements (depicted as vectors) from the initial model (dotted lines) to the deformed and moved organs. Finally, MRI and numerical simulations were compared; the magnitude of the difference is illustrated in Fig. 2c.
Results: numerical simulation of pelvic mobilities
Many pelvic ligaments are described in anatomy. However, their relative contribution to pelvic equilibrium and the support of organs is not yet well defined. The aim of the numerical model of the pelvic cavity herein described was to determine the major suspension system contributing to the pelvic statics. The proposed model must be anatomically as well as functionally coherent. It does not aim at generating an exact anatomical result but a simplified functional model accounting for the real mobility observed on MRI. The geometry of the organs of the model were defined by the segmentation of static MR images of a reference patient. Connective tissues (ligaments and fascias) were progressively introduced into the model. After each addition, numerical simulations were performed and compared to the results of dynamic MRI analysis (while the patient exerted a pushing effort) of the same reference patient (Fig. 3). Our aim was to build an anatomical model for the numerical simulation of mobility, reducing the error in measurements but still corresponding with the data from the anatomical literature.
Simulations were first realized in decubitus to follow the position of the patient during MRI acquisition. Successive simulations were realized; another suspension (ligament or fascia) was added each time a new simulation was run until the numerical model provided a displacement equivalent to that measured via dynamic MRI (Fig. 4a–f). The geometry of pelvic organs, pelvic bones, and pelvic floor remained fixed during this step-by-step process.
Simulation of mobilities. a Simulation of mobility in the absence of any suspension device: there is movement and the externalization of all organs. b Simulation with the uterosacral ligaments: there is hypermobility of the bladder and rectum, and yet too much mobility of the uterus. c Simulation with the uterosacral and round ligaments: there was a decrease of lateralization of the mobility of the uterus but still excessive mobility of other organs. d Simulation with the uterosacral, round, and broad ligaments: there is a persistence of aberrant mobility of the bladder and rectum. e Simulation with the uterosacral, round, and broad ligaments and vesicopubic apposition and double-sacral: there is a persistent mobility of the bladder, vagina, and uterus. f Simulation of mobility of the complete anatomical model: mobilities are consistent with those measured on MRI
The different steps and configurations involved are described below:
-
Step 1
Only organs defined with static MRI
The segmentation of static MR images performed to define the geometry of pelvic organs does not allow for the easy perception of suspension systems. The first simulation, which was run without the ligaments and with only the organs observed on the MR images, is illustrated in Fig. 4a, which shows that such a situation gives completely aberrant results. Without any supporting structures, all of the organs move. In this case, the uterus is fixed only to the vagina, while the rectum is fixed only to the pelvic floor, and the bladder is not fixed. Figure 4a shows the displacement and exteriorization of all organs. Without support, the bladder falls, and the vagina and rectum turn completely upside down. This solution is not at all adequate.
-
Step 2
Step 1 + uterosacral ligaments
To generate more realistic simulations, we added uterosacral ligaments reported in the literature [6] as one of the major contributors to the pelvic statics. The simulation provides unacceptable mobility. The lateralization of the uterus is reduced but it is still too mobile. This model seems insufficient because the uterosacral ligaments are not sufficient to adequately model mobility (Fig. 4b).
-
Step 3
Step 2 + round ligaments
The addition of round ligaments still did not provide acceptable mobility as the numerical simulation (Fig. 4c) reveals. The uterus is less lateralized but still excessively mobile. This model is also inadequate (Fig. 4c).
-
Step 4
Step 3 + broad ligaments
The addition of broad uterine ligaments, also localized at the time of dissection, allowed for the simulation observed during coughing without excessively large displacements. These simulations reach the levels of effective pelvic pressures and can be quantitatively compared to the results of dynamic MRI analysis. With these three pairs of ligaments, the cervix is supported and uterine and vaginal mobility are more realistic. However, the bladder still falls and the rectum is invaginated, as shown in Fig. 4d. This anatomical model is not satisfactory.
-
Step 5
Step 4 + retropubic and presacral fascia
The quantitative evaluation of the displacements observed on the dynamic MR images of a reference patient (without genital prolapse) exerting a pushing force shows two fixed zones (Fig. 5): the inferior part of the bladder near the pubis and the posterior face of the rectum near the sacrum. This is anatomically reasonable as connective tissue joins organs to pelvis. Because we want an anatomical model that takes into account such observations, we fixed the displacement of the two zones.
Figure 4e illustrates the results obtained with this new anatomical numerical model. In this model, there is no more aberrant mobility. The median error is 12.9 mm which is satisfactory. However, as in the previous model uterine lateralization exceeds that observed in the analysis of MRI displacements.
-
Step 6
Step 5 + paravaginal ligaments and fascias
To reduce uterine lateralization, while still adhering to the anatomical data reported in the literature, we introduced paravaginal ligaments and fascias on each side of the uterus and vagina. The simulation reveals limited uterine deviation, with a mean error between the MRI displacement and simulation of approximately 10.9 mm. This result is satisfying but, as shown for the simulation presented in Fig. 4e, the general shape of the bladder does not correspond to the MRI results and suggests poor anterior fixation.
-
Step 7
Step 6 + umbilical ligament
Finally, we added an umbilical ligament and obtained a more suitable model. Young’s modulus of this last ligament was arbitrarily fixed at 60 MPa. Figure 4f shows the results obtained.
The mobilities of all of the organs are in agreement with MRI observations and measurements. If we compare the numerical results of the simulations with those of the MRI analysis, as shown in Fig. 3, the maximum local error of this model is 9.8 mm. To the best of our knowledge, this final model of functional anatomy seems to be the best and most reliable of those described in the literature. Figure 6 illustrates the evolutions of the functional anatomical model proposed.
Discussion
The definition of a numerical anatomical model of pelvic organs is not new in itself [7–11]. The geometry of organs is always defined in medical images (MRI, scan, etc.). However, such images provide only a basic anatomical reconstruction. A numerical model will be accurate only if suspension elements are added. Unfortunately, these suspension elements are often difficult to describe and their relative contribution to pelvic statics is discussed with no clear anatomical consensus. The suspensions are complex, and though some ligaments are clearly described, diffuse fascias are not as well identified in functional anatomy. Defining a 3-D biomechanical numerical model that simulates the pelvic mobility requires an estimation of the major contributors to pelvic statics and their localization. Such a numerical model must be simple without producing aberrant evaluation of the mobility.
The proposed methodology aims at defining the 3-D geometry of organs by segmentation of static MR images. It defines and locates the major contributors to pelvic statics using an iterative process that involves comparing the simulations of the numerical model with analyzed dynamic MR images. Such a step-by-step optimization process with quantitative evaluation of errors allowed us to validate the major contributors to pelvic statics with respect to the consensus of clinicians as well as anatomical and experimental data.
During the first steps we assessed the requirement of uterosacral ligaments as well as round and broad ligaments. Next, we highlighted the sustaining zones implied in pelvic organ suspension. Fascias fixing the bladder on the one hand and the rectum on the other had to be introduced into retropubic and presacral regions. It also appears that anterior paravaginal suspension should be taken into account to correctly model the mobility of the vagina and uterus. Finally, it was proven that a bladder suspension (urachus suspension) should be introduced to obtain an accurate displacement of the top of the bladder. These elements were progressively added to the model allowing us to observe normal mobility close to that exhibited by the patient studied without any trouble associated with pelvic statics.
The mobility we are now able to simulate with our model is very close to physiological mobility observed. The difference between the numerical model and the MRI measurements is less than a maximum of 1 cm. This model not only largely validates the functional anatomy of the pelvic cavity but also completes it, underlining the importance of less well understood static components.
Models from other healthy patients used to generalize our conclusions should confirm these results. However, this pelvic functional model is, to date, the most suitable available. The proposed approach is actually under development in another medical domain and is becoming a subject of interest for dedicated medical treatments [9–12].
This model, of course, is largely imperfect and should be more precisely defined. A more precise MRI procedure could lead to a better comparison between static and dynamic MR images. Dynamic examination, for example, could immediately follow static examination so repletion of organs would not change. The two series of MRI examinations could then be superposed.
The anatomy of levator muscles is still oversimplified, and the distinction between each group or subgroup could allow for a more suitable perineal evaluation. We have no precise data regarding the stress applied to the pelvic system, the friction between organs, or the contractility of pelvic muscles.
We have at our disposal a numerical model that is anatomically and functionally coherent. The next step should be to analyze the sensitivity of the mechanical parameters (stiffness of ligaments and organs) and topological ones (location of ligaments and fascias) of our models paying special attention to clinicians and researchers. We might then try to develop a better interpretation and understanding of pelvic pathologies by the degradation of the model’s topological or mechanical parameters. This model will also be able to be used to evaluate the impact of delivery on pelvic organs as proposed by Parente et al. [8] with respect to pelvic floor muscles.
References
Rubod C, Boukerrou M, Brieu M, Jean-Charles C, Dubois P, Cosson M (2008) Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct 19:811–816
Gabriel B, Rubod C, Brieu M, Dedet B, de Landsheere L, Delmas V, Cosson M (2011) Vagina, abdominal skin, and aponeurosis: do they have similar biomechanical properties. Int Urogynecol J 22:23–27
Rivaux G, Rubod C, Dedet B, Brieu M, Gabriel B, De Landscheere L, Devos P, Delmas V, Cosson M (2010) Caractérisation biomécanique des ligaments utérins. Implication en statique pelvienne. Pelvi-périnéologie: 1–8
Jean-Charles C, Rubod C, Brieu C, Boukerrou M, Fasel J, Cosson M (2010) Biomechanical properties of prolapsed or non-prolapsed vaginal tissue: impact on genital prolapse surgery. Int Urogynecol J 21:1535–1538
Kamina P (2008) Anatomie clinique du petit bassin et périnée, vol 4. Maloine, Paris
Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JO (2004) Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol 103:447–451
Venugopala Rao G, Rubod C, Brieu M, Bhatnagar N, Cosson M (2010) Experiments and finite element modelling for the study of prolapse in the pelvic floor system. Comput Methods Biomech Biomed Engin 13:349–357
Parente MP, Jorge RM, Mascarenhas T, Fernandes AA, Martins JA (2008) Deformation of the pelvic floor muscles during a vaginal delivery. Int Urogynecol J Pelvic Floor Dysfunct 19:65–71
Epstein FH (2007) MR in mouse models of cardiac disease. NMR Biomed 20:238–255
Majumder S, Roychowdhury A, Pal S (2009) Effects of body configuration on pelvic injury in backward fall simulation using 3D finite element models of pelvis-femur-soft tissue complex. J Biomech 42:1475–1482
Li JM, Bardana DD, Stewart AJ (2011) Augmented virtuality for arthroscopic knee surgery. Med Image Comput Comput Assist Interv 14:186–193
Blemker SS, Asakawa DS, Gold GE, Delp SL (2007) Image-based musculoskeletal modeling: applications, advances, and future opportunities. J Magn Reson Imaging 25:441–451
Acknowledgments
No Institutional Review Board approval was obtained. The MRI of this patient was done for a medical reason and was used with the approval of the patient (signed consent and information letter given) and of the research staff of the unit. Legally it was not necessary to have a specific authorization of the Ethics Committee.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cosson, M., Rubod, C., Vallet, A. et al. Simulation of normal pelvic mobilities in building an MRI-validated biomechanical model. Int Urogynecol J 24, 105–112 (2013). https://doi.org/10.1007/s00192-012-1842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1842-8