Abstract
Introduction and hypothesis
The aim of the study was to assess the interobserver and intraobserver reliability of translabial 3D ultrasound imaging of the urethral sphincter in non-pregnant nulliparous asymptomatic women.
Methods
A study using a 3D translabial ultrasound on thirty-seven women was performed. Urethral sphincter parameters were measured by the same experienced clinician 2 weeks apart. Multiple axial cross-sectional areas at 1-mm distances were used to calculate urethral sphincter volumes. The same measurements were carried out by a second experienced clinician to assess the interobserver reliability.
Results
We found an excellent intraobserver reliability (interclass correlation coefficient, ICC >0.8) and good interobserver reliability (ICC >0.6).
Conclusion
The described technique using multiple axial cross-sectional areas at set distances and a translabial approach is a reliable and accurate tool in the evaluation of the urethral sphincter. This should be used instead of mathematical formulas as the urethral sphincter is not a uniform geometrical sphere. The technique and values reported may help clinicians in the assessment of women with lower urinary tract disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence (UI) is a highly prevalent symptom that affects millions of people worldwide of both sexes and all ages. It has been calculated that 8–13% of men and women worldwide and 43–77% of those in nursing homes are incontinent [1]. UI severely affects patients' quality of life, causing shame, discomfort, embarrassment or loss of self-confidence resulting in withdrawal from social life [2]. It affects physical and mental health as well as sexual relationships [3], thus appearing as one of the most psychologically distressing and socially disruptive problems faced by people of both sexes and all ages.
Urodynamic stress incontinence represents the commonest cause of incontinence in women, affecting up to 60% of those investigated [4]. The underlying pathology has been postulated to involve urethral hypermobility [5], intrinsic urethral sphincter deficiency [5] or any disturbance within the sphincter mechanism [6, 7].
The urethral sphincter is believed to play an important role in the pathogenesis of lower urinary tract symptoms (LUTS) [8], hence information on its structure and function is vital to develop our understanding of urinary incontinence. Currently there are many diagnostic tools available to make functional assessments of the urethra including urodynamics, urethral pressure profilometry, leak point pressures and urethral resistance pressure monitoring. In addition, structural assessments of the female urethra have been made with the use of a variety of imaging, implementing 2D, 3D and 4D ultrasound techniques as well as Magnetic Resonance Imaging (MRI).
MRI has been shown to be an accurate tool in identifying urethral anatomy [9], but despite this, the cost and time involved in this method has to be taken into account when compared to ultrasound imaging. Ultrasound imaging may be performed with 2D, 3D and 4D techniques as well as using a variety of anatomical approaches such as transanal, translabial and endovaginal. However, each method has its own limitations and needs to be carefully considered by the operator.
3D ultrasound imaging has been introduced in the last decade as a novel method to evaluate urethral sphincter volumes using an endovaginal or transanal approach [10]. It has been shown to provide a more accurate measurement of urethral sphincter volumes than conventional 2D ultrasound [10], to correlate with cadaver specimens [11], to be a successful and sensitive tool in pregnant women [12] and in those with and without urinary incontinence [13], and it has also been demonstrated to be a promising preoperative tool in evaluating the outcome of continence surgery [14, 15].
Although the clinical value of accurately and precisely evaluating the urethral sphincter using a 3D ultrasound is evident, its reliability has been poorly studied to date. Hence, the aim of our study was to assess the interobserver and intraobserver reliability of 3D ultrasound imaging of the urethral sphincter in non-pregnant, nulliparous women without LUTS using a translabial approach.
Methods
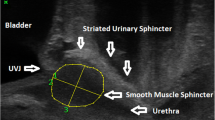
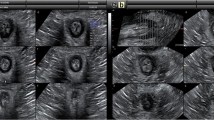
Nulliparous women undergoing ultrasound scan for benign gynaecological conditions were recruited from a tertiary referral teaching hospital. All women were given an information sheet about the study, and consent was obtained. Only those who agreed to participate and did not suffer from any LUTS were included in the study. Menopause, urogenital prolapse, neurological condition, previous continence, pelvic and/or prolapse surgery were considered as exclusion criteria. A 3D translabial ultrasound scan of the urethra was performed with women lying supine with their legs abducted and with an empty bladder. A GE Voluson-i system (GE Healthcare, Austria) with a 5–9 MHz transvaginal 3D/4D ultrasound transducer (RIC 5–9 RS) was used. If the urethral sphincter was not visualised adequately in its entirety with the surrounding tissue, the scan was repeated. The field of view angle was set to its maximum of 70° in the sagittal plane and volume acquisition angle to 85° in the coronal plane. When a clear image of the urethra and rhabdosphincter was obtained in B-mode, a volume box was placed around the urethra, bladder neck and surrounding tissues, and 3D images were then taken using a slow scan time. The volume box used was 8 cm in order to have parallel section increments of less than 0.5 mm. The probe then scanned automatically through an arc of 110° taking 250 images, allowing a simultaneous visualisation of sagittal, transverse and coronal sections. These images were then computer-regenerated into a 3D picture (Fig. 1). In the sagittal plane, the measurements recorded included the distance from the bladder neck to the proximal part of the rhabdosphincter, the bladder neck to the maximal cross-sectional area of the rhabdosphincter and the length of the rhabdosphincter. The cross-sectional area of the total sphincter was serially traced manually, recording each area from one end to the other using 1-mm slice gaps to calculate the area as shown in Figs. 2 and 3. When the entire sphincter was traced, a volume was computed automatically. The process was repeated for the inner core of the sphincter as showed in Fig. 4. Finally the volume of the inner core was subtracted from the total volume to give a measurement of the rhabdosphincter volume. All measurements were taken twice by the same clinician 2 weeks apart to assess the intraobserver reliability. A second blinded experienced clinician remeasured all measurements in order to assess the interobserver reliability. The interclass correlation coefficient (ICC) was calculated to assess limits of agreement. The scale from Altman was used in classification of the reliability values [16]. ICC values under 0.20 were considered poor, 0.21–0.40, fair, 0.41–0.60, moderate, 0.61–0.80, good and 0.81–1.00, excellent.
Three-dimensional translabial image of the female urethra. The volume measurements of the core sphincter and total sphincter were taken in the axial plane (bottom left). The urethra lumen is shown clearly in the rendered volume image (bottom right). B bladder, IC inner core, U urethra lumen, RS rhabdosphincter
Finally, the urethral sphincter volumes were measured using four different separations between the cross-sectional areas: 1 mm, 2 mm, 3 mm, 4 mm and 8 mm. The separation between cross-sectional areas was measured by using displacement along the median sagittal image. The coefficient of variation was calculated for the urethral sphincter volume measurement, and this was plotted against the different axial cross-section separation. All terms and definitions are in accordance with the latest terminology for female pelvic floor dysfunction [17].
A version 19.0 SPSS statistical package was used for statistical analysis (SPSS version 19.0, Chicago, IL). Local ethical approval was obtained for this study from the London–Surrey Borders Research Ethics Committee (10/H0806/21).
Results
A study on thirty-seven asymptomatic nulliparous women (mean age 37 years old, range 24–48) was performed. Twenty-three women were white and 14 were black. White women had a mean age ± SD of 34.8 ± 6.0 vs. 37.5 ± 8.1 for the black women (p = 0.31). Although black women weighed more than white women on average (mean BMI ± SD for black women was 26.5 ± 3.1 vs. 24.9 ± 3.8 for white women), this difference was not statistically significant (p = 0.16). The whole scanning time was 4–6 s.
Table 1 shows the urethral sphincter parameters of the study population, as well as the ICC and limits of agreement of measurements taken 2 weeks apart by the same clinician. The results showed excellent intraobserver reliability (ICC >0.8) in the majority of parameters measured by the same clinician on the two separate occasions. In measuring the total sphincter volumes, the mean difference was 0.154 cm3 with the ICC value showing excellent agreement at 0.948. The mean difference when measuring the internal sphincter volume was 0.0007 cm3, again representing excellent agreement (ICC = 0.993). Measurements of the rhabdosphincter volume, rhabdosphincter length, distance from the bladder neck to proximal rhabdosphincter and distance from the bladder neck to maximal cross-sectional area also all presented excellent ICC agreement (ICC = 0.985, 0.930, 0.991 and 0.975, respectively).
Table 2 shows the ICC and limits of agreement between the two investigators. The results show good to excellent interobserver reliability (ICC >0.6) in all parameters measured by the two different clinicians. In addition, the 95% confidence interval for the limits of agreement of all the volume measurements was less than 10% of the mean volumes. This further confirmed that the measurements were reliable.
The coefficient of variation for a 1-mm separation of axial cross-sectional measurement (mean 1.734, ±SD 0.781) was similar to the coefficient of variation for a 2-mm separation measurement (mean 1.608, ±SD 0.872). The coefficient of variation for wider separations of 4 mm and 8 mm were mean 8.538, ±SD 5.136 and 13.889, ±SD 8.860, respectively, which show an increased variance. There was no difference in the coefficient of variance between volumes measured with the axial cross-sectional separation of 1 mm and 2 mm (p > 0.05).
Discussion
The role of the urethral sphincter in the pathological development of urinary incontinence has been clearly postulated [12–14]. Although the importance of precise structural assessment of the urethral sphincter is paramount, the task of measuring it accurately has recently been met with conflicting opinions.
Evaluation of the rhabdosphincter has been previously reported using MRI and ultrasound imaging with the use of different mathematical formulas to measure its volume. However, this has been based upon assumptions that the shape of the urethra is similar to that of an ellipse. This is incorrect, as the urethral shape is neither elliptical nor spherical, and in view of its atypical geometric shape, we suggest that equations should not be used, since they cannot give an accurate calculation of the urethral volume. Accordingly, we suggest an alternative technique to measure the urethra and rhabdosphincter more precisely using a 3D ultrasound. These measurements are not based upon standardised mathematical equations but on the calculations from 1-mm cross-sectional areas at set distances across the urethra, thus reducing the error in measurements unavoidable with the use of mathematical equations.
Finally, the measurement of the inner core of the urethra (urethral canal) should also be taken into account and subtracted from the measured total urethral volume when assessing the rhabdosphincter. This represents another limitation if mathematical equations are used to measure urethral sphincter volume.
In our study, we decided to use a translabial approach to overcome the limitations of endovaginal and transrectal techniques. Transanal ultrasound approach requires an expensive and dedicated transducer, and it is a more uncomfortable and embarrassing test for the woman. Endovaginal ultrasound scan may inadvertently compress tissues in order to visualise the urethra lying just anterior to it, whereas translabial ultrasound scan provides minimal pressure on local structures and therefore is least likely to alter surrounding anatomy.
In this study, we have shown that there is good interobserver and intraobserver reliability using a translabial 3D ultrasound for measuring the female urethra. It provides both a quick (each scan taking 4–6 seconds) and cheap method of assessment; therefore, it is a practical tool when studying this complex anatomy. This is of significant clinical value as until recently, we have relied mainly upon functional assessments. However, with the progression of simple structural assessments (undertaken by accurate imaging techniques), we may be able to combine this information to influence our choice of management and predict surgical outcomes, as has been previously suggested [14]. However, ours is only a validation study showing normative values in asymptomatic non-pregnant, nulliparous women, and further research in women with lower urinary tract symptoms is needed to assess the clinical role of 3D imaging of the urethral sphincter.
The measurements were performed with an empty bladder to avoid overestimating the sphincter volume due to rhabdosphincter contraction when the bladder is full [15].
In conclusion, the potential for accurate assessment of the urethral morphology with a 3D ultrasound is evident and supported by this study's excellent interobserver and intraobserver reliability. We have shown that 3D ultrasound imaging using multiple axial cross-sectional areas at set distances and a translabial technique is a reliable and accurate tool in the evaluation of the female urethral sphincter. The described technique should be used instead of mathematical formulas as the sphincter is not a uniform geometrical sphere. 3D ultrasound imaging is gaining an increasingly prominent role in urogynaecology and by providing an accurate structural assessment, in addition to the functional assessment gained from established urodynamic studies, we may be able to develop a reliable and comprehensive pre-operative assessment for women with urinary incontinence.
References
Irwin DE, Milsom I, Hunskaar S et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50(6):1306–1314
Trutnovsky G, Tamussino K, Greimel E, Bjelic-Radisic V (2011) Quality of life after periurethral injection with polyacrylamide hydrogel for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 22(3):353–356
Kammerer-Doak D (2009) Assessment of sexual function in women with pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct 20(Suppl 1):S45–S50
Andrada Hamer M, Larsson PG, Teleman P, Etén-Bergqvist C, Persson J (2011) Short-term results of a prospective randomized evaluator blinded multicenter study comparing TVT and TVT-Secur. Int Urogynecol J Pelvic Floor Dysfunct 22(7):781–787
Haliloglu B, Karateke A, Coksuer H, Peker H, Cam C (2010) The role of urethral hypermobility and intrinsic sphincteric deficiency on the outcome of transobturator tape procedure: a prospective study with 2-year follow-up. Int Urogynecol J Pelvic Floor Dysfunct 21(2):173–178
Yang JM, Yang SH, Yang SY, Yang E, Huang WC, Tzeng CR (2010) Clinical and pathophysiological correlates of the symptom severity of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 21(6):637–643
Ashton Miller JA, DeLancey JOL (2007) Functional anatomy of the female pelvic floor. Ann Y Acad Sci 1101:266–296
Wang HJ, Chuang YC, Chancellor MB (2011) Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 22(9):1075–1083
Tan JL, Stoker J, Zwamborn AW (1998) Female pelvic floor: endovaginal MR imaging of normal anatomy. Radiology 206:777–783
Derpapas A, Digesu GA, Fernando R, Khullar V (2011) Imaging in urogynaecology. Int Urogynecol J Pelvic Floor Dysfunct 28. doi:10.1007/s00192-011-1462-8
Strohbehn K, Quint LE, Prince MR, Wojno KJ, Delancey JO (1996) Magnetic resonance imaging anatomy of the female urethra: a direct histologic comparison. Obstet Gynecol 88(5):750–756
Toozs-Hobson P, Khullar V, Cardozo L (2001) Three-dimensional ultrasound: a novel technique for investigating the urethral sphincter in the third trimester of pregnancy. Ultrasound Obstet Gynaecol 17:421–424
Mitterberger M, Pinaggera GM, Mueller T et al (2006) Dynamic transurethral sonography and 3-dimensional reconstruction of the rhabdosphincter and urethra initial experience in continent and incontinent women. J Ultrasound Med 25:315–320
Digesu G, Robinson D, Cardozo L, Khullar V (2009) Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn 28:90–94
Chaliha C, Digesu GA, Hutchings A, Khullar V (2005) Changes in urethral function with bladder filling in the presence of urodynamic stress incontinence and detrusor overactivity. Am J Obstet Gynecol 192(1):60–65
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Haylen BT, de Ridder D, Freeman RM et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct 21(1):5–26
Acknowledgement
The authors are grateful for the support from the NIHR Biomedical Research Centre funding scheme.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Digesu, G.A., Calandrini, N., Derpapas, A. et al. Intraobserver and interobserver reliability of the three-dimensional ultrasound imaging of female urethral sphincter using a translabial technique. Int Urogynecol J 23, 1063–1068 (2012). https://doi.org/10.1007/s00192-012-1669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1669-3