Abstract
Introduction and hypothesis
The objective was to determine age-related changes in measurements of urethral sphincter complex components in asymptomatic nulliparous women.
Methods
Eighty nulliparous women ≥18 years underwent 3D ultrasound of the anterior pelvic compartment in a cross-sectional study. Measurements of the urethral sphincter components (smooth muscle sphincter [SMS] and striated urinary sphincter [SUS]) and urethra including area, length, width, and distance of the SUS and SMS from the urethrovesical junction were obtained. The women were grouped into four age groups: < 30 years (group A), 30 to < 45 (group B), 45 to < 60 (group C), and ≥ 60 years (group D). Age-related differences in the measurements were determined. Inter-rater and intra-rater agreement were performed for 20 nulliparous women.
Results
There were 24, 18, 26, and 12 women in groups A, B, C, and D respectively. None of the urethral sphincter complex measurements was significantly associated with age (p > 0.05). No differences were found between the groups for any measurements using one-way ANOVA and multiple comparison pairwise comparison (p > 0.05) other than width of SMS (C > A), urethral length (C > A), and distance of SUS from urethrovesical junction (C > D). Inter-rater and intra-rater agreement were moderate for area, length, and width of SUS (intraclass correlation 0.6) and good (intraclass correlation above 0.8) for the remaining measurements.
Conclusion
Other than width of SMS, urethral length, and distance of SUS from urethrovesical junction, the dimensions of urethral sphincter complex components, as visualized by 3D endovaginal ultrasound, do not vary with age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last two decades, the investigation of women with urinary incontinence has included pressure measurements and radiological imaging. Although these are useful in diagnosing the type of urinary incontinence, the anatomical and pathological etiologies for lower urinary tract and pelvic floor dysfunction remain poorly understood. Hence, additional diagnostic modalities that further our understanding of the problem would be beneficial.
Evidence points to a reduction in striated urinary sphincter size in women with stress urinary incontinence (SUI) [1, 2]. Women with smaller preoperative striated urinary sphincter volume have been found to have poorer surgical outcomes [3]. Therefore, determining the sphincter complex dimensions in patients with SUI may potentially help to define diagnostic cutoffs for patients with SUI, help to confirm the severity of SUI, plan surgery and prognosticate outcomes following surgery. However, age is a potential confounding factor. In a study of 25 female cadavers (age 15–80 years), there was a decrease in urethral striated muscle fiber density in the ventral urethra with aging, without a corresponding decrease in fiber size [4]. Fourteen cadavers in this study were, however, parous [4]. Although the impact of parity on urethral sphincter complex is not entirely known, vaginal childbirth seems to increase urethral mobility by about 20% for all urethral segments [5]. There is thus a need for a larger study in nulliparous women that determines the changes in the urethral sphincter parameters associated with age.
Advanced endovaginal ultrasound provides detailed reliable information on the anatomy and morphology of the female urethral complex [6]. Cadaveric and histological correlation has been previously made to validate the use of three-dimensional endovaginal ultrasound (3D EVUS) using the 8848 transducer in visualizing anterior pelvic compartment structures [7]. The aim of this study was to determine whether there are age-related changes in the measurements of the urethral sphincter complex components in asymptomatic nulliparous women.
Materials and methods
This is an IRB-approved cross-sectional pilot study from July 2009 until December 2012 including 80 nulliparous volunteers recruited in the gynecology clinics at Cleveland Clinic Florida and University of Oklahoma Health Sciences Center using uniform inclusion criteria. Our null hypothesis is that, in nulliparous women, in whom the effects of childbearing have been removed, there are no significant changes over time in urethral sphincter complex anatomy. Inclusion criteria included nulliparity and age ≥ 18 years. Exclusion criteria included presence of symptoms related to urinary incontinence and chronic pelvic pain, presence of pelvic organ prolapse on POP-Q examination ≥ stage 1 and history of prior pelvic floor surgery or radiation to the pelvis. The patients attending the gynecology clinics of the two study sites who met the inclusion criteria were enrolled using consecutive sampling (total enumerative sampling) methodology after informed consent was taken. All women underwent a standardized history and urogynecological examination including assessment of pelvic organ prolapse according to the Pelvic Organ Prolapse Quantification system (POP-Q).
All participants underwent 3D EVUSwith the BK Medical Profocus Ultraview machine (Peabody, MA, USA) using the 8848 12-mHz transducer. The women underwent ultrasound examination in the dorsal lithotomy position with the hips flexed and abducted. The women were recommended to have a comfortable volume of urine in the bladder and did not undergo any pre-procedure preparation. No rectal or vaginal contrast agent was used. Care was taken to insert the transducer in the neutral position to avoid exerting excessive pressure on the surrounding structures and distorting anatomy. For each patient, a length of 6 cm was scanned every 0.25° for a total of 180° starting at the 3 o’clock position and ending at the 9 o’clock position, with 720 cumulative radial scans from which a 3D-rendered cube was calculated [6]. The 3D cubes obtained were digitally catalogued for future analysis.
The 3D cubes were assessed by the first author in the sagittal, coronal, and axial planes to obtain various measurements of the urethral sphincter complex. One measurement per sphincter dimension per patient was taken in the plane most suitable for taking a particular measurement and was obtained only in the plane described. The assessor was blinded to the age of the enrolled women.
-
1
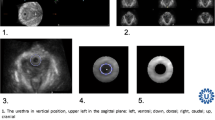
Manipulation in the sagittal plane: the 3D cube was manipulated in the sagittal plane to reach the mid-sagittal plane. The urethral lumen could be seen in its entirety from the urethrovesical junction until the external urethral meatus. The smooth muscle sphincter could be seen as a hypoechoic area within the proximal part of the urethra (Fig. 1). The striated urinary sphincter could be seen along its length as a hyperechoic structure anterior to the smooth muscle sphincter (Fig. 1). The following measurements were taken:
-
a
Smooth muscle sphincter area
-
b
Smooth muscle sphincter length
-
c
Smooth muscle sphincter width
-
d
Distance of the smooth muscle sphincter from the urethrovesical junction
-
e
Urethral length
-
f
Distance of the striated urinary sphincter from the urethrovesical junction
-
a
-
2.
Manipulation in the coronal plane: the 3D cube was manipulated in the coronal plane parallel to the length of the striated urinary sphincter until the level of the sphincter. The striated urinary sphincter could be seen as a hypoechoic sphincter medially with the symphysis pubis on either side (Fig. 2). The following measurements were taken:
-
1
Striated urinary sphincter area
-
2
Striated urinary sphincter length
-
3
Striated urinary sphincter width
-
1
-
c
Manipulation in the axial plane: a line was drawn perpendicular to the urethral lumen at the midpoint of the urethral length in the mid-sagittal plane. The 3D cube was then manipulated in the axial plane parallel to the line drawn until the midpoint of the urethral length (Fig. 3). The following measurements were taken in the axial plane of the mid-urethra:
-
1
Urethral area
-
2
Horizontal diameter of the urethra
-
3
Vertical diameter of the urethra
-
1
Urethral sphincter anatomy in the mid-sagittal plane. The 3D cube was manipulated in the sagittal plane until the largest area of the smooth muscle sphincter (hypoechoic) was seen. The hyperechoic striated urinary sphincter can also be seen along its sagittal length anterior to the smooth muscle sphincter. UVJ urethrovesical junction, 1 area of the smooth muscle sphincter in the sagittal plane, 2 length of the smooth muscle sphincter in the sagittal plane, 3 width of the smooth muscle sphincter in the sagittal plane
Striated urinary sphincter in the coronal plane. The 3D cube was manipulated in the sagittal plane until the striated urinary sphincter could be seen along its sagittal length anterior to the smooth muscle sphincter. The cube was then manipulated in the coronal plane parallel to the striated urinary sphincter until the largest area of the sphincter was seen in the coronal plane. SP symphysis pubis, 1 area of the striated urinary sphincter in the coronal plane, 2 length of the striated urinary sphincter in the coronal plane, 3 width of the striated urinary sphincter in the coronal plane
Urethra in the axial plane. The 3D cube was manipulated in the sagittal plane until the entire extent of the urethral lumen was seen from the urethrovesical junction until the external urethral meatus. The midpoint of the urethral length was determined, and a vertical line was drawn perpendicular to the urethra at that point. The cube was then manipulated in the axial plane up to the vertical line to demonstrate the urethra. U urethra, 1 urethra, 2 vertical diameter of the urethra in the axial plane (at the midpoint of the urethral length), 3 horizontal diameter of the urethra of the urethra in the axial plane (at the midpoint of the urethral length)
The enrolled women were divided into four age groups (using age as a categorical variable): < 30 years (group A), 30 to < 45 years (group B), 45–59 years (group C), and ≥ 60 years (group D). BMI, race, menopausal status, and prevalence of medical disorders were compared among the four groups. The various parameters of the urethra and urethral sphincter complex measured as described above were compared among the four groups.
The inter-rater agreement was determined. The 3D cubes obtained in 20 women were read independently by the second author who was blinded to the age of the women and the measurements obtained by the first author. Intra-rater agreement was also determined in 20 women. The first author reread the 3D cubes in these women a month after the first series of measurements were taken.
This is a pilot study for the measurement of urethral sphincter dimensions. Although the urethral sphincter components have been visualized previously using ultrasound [7], the dimensions of all components and the changes expected in them with age have not been studied previously. Until the changes in the dimensions of urethral sphincter components are known, it is difficult to determine which dimension should constitute the primary outcome for sample size estimation. Also, although striated urethral sphincter has been assessed in prior studies [1], the plane used for measurement and the parameters measured differ from those in our paper. Hence, the sample size could not be estimated.
Statistical analysis was performed using SPSS Statistics 20 (IBM, Chicago, IL, USA). Pearson’s correlation was used to determine the correlation of the measured urethral parameters with age (when used as a continuous variable). One-way ANOVA was used to determine the differences in measurement of each urethral parameter among the four age groups. Multiple comparison pairwise comparison (with post hoc Tukey’s HSD test) was performed to compare the different age groups for the variables that showed significant differences using one-way ANOVA. Intraclass correlation (ICC) was used to determine inter-rater agreement (ICC 2.2) and intra-rater agreement (ICC 2.1).
Results
There were 24 women in group A, 18 in group B, 26 in group C, and 12 in group D. The median (IQR) ages in the four groups were 25 (3), 39 (5.5), 52 (9), 66.5 (9.5) respectively. The four groups were comparable with respect to the demographic data of BMI (median [IQR] BMI was 23.8 (12.1), 24.2 (5.6), 24.1 (5.9), and 25.1 (6) in the four groups respectively; p = 0.051), race (all white), and smoking (all non-smokers). Seventeen women in group C (65.4%) and all women in group D were menopausal (p = 0.001). None of the women were on hormonal treatment. The prevalence of medical disorders (hypertension, diabetes) was significantly higher in group D: none of the patients in groups A and B had medical disorders, 4 (3.8%) patients in group C had a medical disorder, and 4 (25%) of patients in group D had hypertension and/or diabetes; p = 0.04. None of the measured urethral sphincter complex parameters were found to be correlated with age, when age was considered as a continuous variable (p > 0.05, Pearson’s correlation; Table 1).
Age (when used as a categorical variable) did not have a significant effect on any of the measured smooth muscle sphincter parameters, except for smooth muscle sphincter width, in the four age groups (one-way ANOVA test, Table 2). There was a significant effect of age on the smooth muscle sphincter width for the four age groups (F [3, 61] = 2.909, p = 0.04). Post hoc multiple comparison pairwise comparison using the Tukey HSD test for smooth muscle sphincter width indicated that the mean score for group A was significantly lower than that for group C (p = 0.047). However, the other pairwise comparisons between the four groups were not significant (p > 0.05).
Also, age did not have a significant effect on any of the measured striated urinary sphincter parameters, except for distance of the sphincter from the urethrovesical junction, in the four age groups (one-way ANOVA test, Table 2). There was a significant effect of age on the distance of the sphincter from the urethrovesical junction for the four age groups (F [3, 58] = 3.396, p = 0.024). Post hoc multiple pairwise comparison using the Tukey HSD test for the distance of the striated urinary sphincter from the urethrovesical junction indicated that the mean score for group C was significantly higher than that for group D (p = 0.015). However, the other pairwise comparisons among the four groups were not significant (p > 0.05).
Lastly, age did not have a significant effect on any of the measured urethral parameters, except for urethral length, in the four age groups (one-way ANOVA test, Table 2). There was a significant effect of age on the urethral length for the four age groups (F [3, 60] = 3.461, p = 0.022). Post hoc multiple comparison pairwise comparison using the Tukey HSD test for the urethral length indicated that the mean score for group A was significantly lower than that for group C (p = 0.035). However, the other pairwise comparisons among the four groups were not significant (p > 0.05).
Inter-rater agreement (intraclass correlation 2,2; consistency) was good for all the measured urethral sphincter and urethral parameters (> 0.75; Table 3), except for the striated urinary sphincter area and width, for which it was moderate (0.612 and 0.607 respectively). Intra-rater agreement (intraclass correlation 2,1; consistency) was good for all measured urethral sphincter and urethral parameters (> 0.75).
Discussion
None of the urethral sphincter complex measurements were found to be significantly associated with age (p > 0.05). No differences were found between the groups for any of the measurements using one-way ANOVA and on multiple comparison pairwise comparison (p > 0.05) other than width of SMS, urethral length, and distance of the SUS from the urethrovesical junction (p < 0.05). However, no obvious trends were noted in the latter. Inter-rater and intra-rater agreement were moderate for area, length, and width of the SUS (ICC 0.6) and good (ICC above 0.8) for the remaining measurements.
Urethral sphincter muscles are important determinants of the continence mechanism, although commonly ignored [8]. Urethral function measures have been found to be more strongly associated with the presence of stress incontinence than those of urethral support [9]. Women with stress incontinence were found to have lower urethral closure pressure (34 cm H2O) than a group of age-matched continent women (68 cm H2O) [10].
A study on urethral sphincter morphology and function in women with and without stress incontinence using MRI found that a smaller striated urinary sphincter is associated with stress incontinence and poorer pelvic floor muscle function [1]. A study using three-dimensional transvaginal ultrasound in 1999 had previously reported that the striated urinary sphincter had a smaller volume in women with genuine stress incontinence than in continent women [2]. Women who failed continence surgery have been found to have significantly smaller preoperative urethral sphincter volumes than those who had an objective cure [3]. These studies pave the way for larger studies that aim to define diagnostic cutoffs for urethral sphincter dimensions in women with SUI with/without intrinsic sphincter deficiency and exploring the prognostic possibilities of these defined cutoffs following continence surgery.
A possible confounding factor is age. In a study of 25 female cadavers with age ranging from 15 to 80, Perucchini et al. reported a decrease in urethral striated muscle fiber density in the ventral urethra with aging, without a corresponding decrease in fiber size [4]. The 65% loss in total number of fibers found in this study between the third and eighth decade of life is consistent with the 54% loss in urethral closure pressure reported by Rud [11] indicating that these two biological phenomena are of the same magnitude [4]. In a succeeding study in which the authors investigated the precise location of striated sphincter muscle fiber loss, it was found that muscle loss occurred at the bladder neck and along the dorsal wall of the urethra as women aged [12]. The ventral wall of the distal urethra, corresponding to the anatomically distinct compressor urethrae and urethrovaginal sphincter, was spared the muscle loss [12]. The studies, however, have several limitations. The sample size is small and there is a likelihood of artifacts due to postmortem changes and fixation. Fourteen of the 25 women were parous and in 4 patients, parity could not be ascertained [4]. The lack of reliable information regarding continence prior to death is also a severe limitation. The authors concluded that larger samples with older nulliparous women may clarify the relationship between age and parity [12].
To our knowledge, our study is the largest reported study in nulliparous women in which the urethral sphincter complex parameters were not seen to vary with age. In a study involving MRI by Morgan et al. [1], aging was found to correlate with a shorter striated urinary sphincter and a longer vesical neck, but the striated urinary sphincter thickness and area were larger in older women [1]. Since the association of aging with length and area of the striated urinary sphincter were in approximately equal and opposite directions respectively, there was no discernible relationship found between aging and striated urinary sphincter volume [1].
Three-dimensional ultrasound is an accessible imaging modality that overcomes some of the limitations of conventional B-mode sonography and offers an unlimited number of ultrasound sections, even in planes perpendicular to the axis of the ultrasound beam. Wiezoreck et al. [6] have shown that three-dimensional endovaginal ultrasound with the 360° rotational transducer (2052 probe) with automated 3D acquisition is suitable for the reliable assessment of the urethral complex. Three-dimensional endovaginal 180° ultrasound of the anterior compartment with the 8848 probe that we have used in this study could prove to be an important modality for the anatomical investigation of the urethral sphincter complex, because validation of the various components of the sphincter complex, as seen with the use of the modality has been done previously with histological correlation [7]. The 3D software is easy to navigate and work with [7] and cumbersome 3D modeling is not needed. 3D cubes are obtained that can be stored and analyzed later. There are also virtually no gaps in the cubes, which results in fluent and representative views of the anatomical structures in any plane.
Our study has certain limitations. The measured parameters are snapshots of the urethral sphincters only in certain planes. We have evaluated the measurement of the striated urinary sphincter in the coronal plane, the smooth muscle sphincter in the mid-sagittal plane, and the urethra in the axial plane and correlated the measurements with age. Therefore, only the area, length, and width can be measured, but not the volume. Second, ultrasound cannot differentiate between muscle and fibrous tissue. Therefore, if muscle is replaced with fibrous tissue, ultrasound is unable to detect this. We have no knowledge on the impact of hormonal changes on urethral sphincter dimensions. Hence, to preclude any confounding effect on the urethral sphincter measurements, we divided the women into the following age groups: early reproductive; late reproductive; perimenopausal and early menopausal; and late menopausal age groups. Last, in the absence of power calculations, the magnitude of type II error cannot be determined. The study does have several strengths: it is the largest study, with a representative sample size of a homogenous population (nulliparous women). We have used standardized measurements and tested both inter-rater and intra-rater reliability. This study forms the basis to determine the dimensions for the urethral sphincter complex for nulliparous women and understand the changes that occur in patients with SUI with/without ISD and patients with voiding dysfunction. All women in this series were continent. It will be interesting to evaluate parous healthy women who are stress incontinent to better understand the impact of parity and age on the urethral sphincter. Finally, we intend to follow up SUI patients long term to determine the prognostic value of these measurements following surgery.
In conclusion, most of the dimensions of the urethral sphincter complex components, as visualized by 3D endovaginal ultrasound do not appear to vary with age. Although there were significant age-related changes noted with respect to the width of the SMS, urethral length, and distance of the SUS from the urethrovesical junction, no obvious trends were seen.
References
Morgan DM, Umek W, Guire K, Morgan HK, Garabant A, Delancey JOL. Urethral sphincter morphology and function with and without stress incontinence. J Urol. 2009;182(1):203–9.
Athanasiou S, Khullar V, Boos K, Salvatore S, Cardozo L. Imaging the urethral sphincter with three dimensional ultrasound. Obstet Gynecol. 1999;94:295.
Digesu GA, Robinson D, Cardozo L, Khullar V. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2008;28:90–4.
Perucchini D, DeLancey JOL, Ashton-Miller JA, Peschers U, Kataria T. Age effects on urethral striated muscle. I. Changes in number and diameter of striated muscle fibers in the ventral urethra. Am J Obstet Gynecol. 2002;186:351–5.
Dickie KJ, Shek KL, Dietz HP. The relationship between urethral mobility and parity. BJOG. 2010;117(10):1220–4.
Wiezoreck AP, Wozniak MM, Stankiewicz A, Santoro GA, Bogusiewicz M, Rechberger T. 3-D high-frequency endovaginal ultrasound of female urethral complex and assessment of inter-observer reliability. Eur J Radiol. 2012;81:e7–e12.
Shobeiri SA, White D, Quiroz LH, Nihira MA. Anterior and posterior compartment 3D endovaginal ultrasound anatomy based on direct histological comparison. Int Urogynecol J. 2012;23:1047–53.
DeLancey JO. The pathophysiology of stress urinary incontinence in women and its implications for surgical treatment. World J Urol. 1997;15:268–74.
DeLancey JOL, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179:286.
Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: the results in symptom-free women and in those with genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:919–33.
Rud T. Urethral pressure profile in continent women from childhood to old age. Acta Obstet Gynecol Scand. 1980;59:331–5.
Perucchini D, DeLancey JOL, Ashton-Miller JA, Galecki A, Scaer G. Age effects on urethral striated muscle. II. Anatomic location of muscle loss. Am J Obstet Gynecol. 2002;186(3):356–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Aparna Hegde was funded by IUGA International Fellowship Grant Award, 2011. G.W. Davila: consultant and honoraria: Astellas, Watson, American Medical System, Novasys Medical, CL Medical; research funding: American Medical Systems, Astellas. Other authors: no disclosures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegde, A., Rostaminia, G., Quiroz, L.H. et al. Are there age-related changes in the measurements of the urethral sphincter complex in nulliparous women? A three-dimensional ultrasound assessment. Int Urogynecol J 32, 653–659 (2021). https://doi.org/10.1007/s00192-020-04530-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04530-y