Abstract
Purpose
One of the causes of aseptic loosening is marked tibial bone resorption (TR) at the tibial bone–component interface after total knee arthroplasty (TKA). It was hypothesized that insufficient coverage of the tibial component and improper cementing technique would cause increased TR after cemented TKA.
Methods
One hundred thirty-four primary TKAs in 107 patients with varus osteoarthritis were included in this study. The relationships between the TRs at 2 years after TKA and the tibial component underhang (TUH), the thickness of the cement mantle around the keel, and clinical parameters were evaluated.
Results
The widths of TRs on anteroposterior radiographs were significantly larger on the medial side than on the lateral side (p = 0.001), whereas the difference between the anterior and posterior sides on lateral radiographs was relatively small. Multiple regression analyses showed that medial TR was positively related to medial TUH (p = 0.006), and lateral TR was positively related to a thicker distal cement mantle (p = 0.027). On the lateral view, stepwise selection indicated that postoperative knee flexion angle was the most significant risk factor (p = 0.005) for anterior TR, and posterior TUH was the strongest predictor (p = 0.001) of posterior TR.
Conclusions
To avert postoperative progressive TR, surgeons should perfectly fit a suitably sized tibial component to the medial edge of the tibia. Also, care should be taken to avoid an excessive cement mantle at the distal portion of the keel during TKA.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) has become increasingly common as a reliable treatment for patients with severe osteoarthritis of the knee, and previous studies including the data of arthroplasty registries reported a survival rate of more than 90% at 10-year follow-up [28, 40]. The number of revision TKAs has also risen steadily [6]. While one of the most common reasons for late failure in primary TKA is aseptic loosening, the underlying mechanism is still debatable [6, 31, 33, 36, 37].
A previous study summarized the various factors that contribute to aseptic loosening, including the effect of wear particles on activated macrophages, micromotion of the tibial component, and stress shielding along the bone–component interface [36]. Of these, wear-related loosening has been reduced because of improvements in polyethylene manufacturing and material properties [37]. However, patient- and operation-related factors are still problematic, especially on the tibial side [6, 31, 35]. Appropriate tibial preparation and component fixation are crucial to achieve long-term durability in cemented TKA [14, 30, 31]. Previous studies reported that the development of tibial bone resorption (TR) could induce component loosening, and generally occurred within 2 years postoperatively [15, 24, 36]. Tibial component coverage on the tibial cutting surface theoretically affects stress distribution, and a thicker cement mantle might cause stress shielding at the tibial bone–component interface. This study was the first to investigate these issues, specifically assessing what tibial component coverage and cement mantle thickness could best prevent increased TR.
The purpose of this study was to evaluate TR using early and 2-year postoperative anteroposterior (AP) and lateral radiographs after cemented primary TKA. It was hypothesized that insufficient coverage of the tibial component on the tibial cutting surface and a thick cement mantle around the tibial keel might increase TR.
Materials and methods
This study retrospectively analyzed 134 knees (right, 68 knees; left, 66 knees) in 107 patients (88 women, 19 men) who underwent cemented primary TKA with a single design and who were followed up for at least 2 years between December 2010 and June 2015. The preoperative diagnosis was medial osteoarthritis in all knees. The exclusion criteria were metal tibial augmentations due to severe bone defects or other component designs such as a more constrained TKA (41 knees), valgus knees (17 knees), revision TKA (21 knees), TKA after failed high tibial osteotomy (5 knees), other inflammatory arthritides such as rheumatoid arthritis (73 knees), posttraumatic osteoarthritis (4 knees), postoperative infections or wounds (2 knees), and lost to follow-up (5 knees). The mean age and body mass index (BMI) of patients at the time of surgery were 74.4 ± 6.5 years (range 58–86 years) and 26.0 ± 4.0 kg/m² (range 16.8–38.9 kg/m²), respectively. The mean time to follow-up was 40.3 ± 13.1 months (range 24–72 months). The knee flexion angles were examined using a goniometer at 2-year postoperative follow-up.
Surgical procedure
The Bi-Surface Knee System (Kyocera, Kyoto, Japan), which is a fixed-bearing posterior cruciate-substituting prosthesis, was used in all patients [21, 27]. The tibial component of this prosthesis is made of titanium alloy (Ti) that is designated by the American Society for Testing Materials (ASTM) as F136. This titanium alloy contains 6% aluminium and 4% vanadium (Ti-6Al-4V), and the bone-side surface is manufactured using shot blasting. The diameter of the tibial keel was 13 mm, and its length ranged from 42 to 48 mm depending on the tibial component size.
A pneumatic tourniquet was applied [29] and the medial parapatellar approach was performed in all patients. To align the components in the coronal plane, the tibial component was set perpendicular to the mechanical axis of the tibia, which connected the centre of the knee to the centre of the ankle joint. For the sagittal alignment, the tibial component was aligned with the mechanical axis of the tibia, and the posterior tibial slope was adjusted to 5°. To improve sagittal bone-cutting accuracy, the angle between the anterior border of the tibial axis and the sagittal mechanical axis was checked preoperatively using lateral radiographs, and the angle was used to intraoperatively facilitate the appropriate placement of extramedullary guides relative to the anterior border of the tibia during TKA [13]. The rotation of the tibial components was aligned with the tibial AP axis; this axis connected the medial one-third of the tibial tubercle and the geometric centre, the latter defined as the centre of the cutting surface. The rotational direction of the preparation instrument was set relative to the tibial AP axis, and then the keel hole of the tibia was prepared.
The medium-viscosity cement Simplex P (Stryker, NJ, USA), which was manually mixed without the use of a vacuum-mixing device, was used for fixing the tibial component to the tibial cutting surface. The cement on the tibial cutting surface was manually spread with a knife after high-pressure lavage so that it covered the tibial component, including the tibial keel, because the manufacturer advises cementing the keel. In the single-stage cementing technique, the tibial component was pressed against the tibial cutting surface first, followed by the femoral component. Following a trial of a tibial insert, the knee was maintained in knee extension to exert pressure against the bone–component interface until the cement was hardened [14]. The thickness of polyethylene inserts ranged from 9 to 17 mm.
Radiographic assessments
The hip–knee–ankle (HKA) angle was measured on pre- and postoperative, long-standing, weight-bearing AP radiographs. The mean preoperative HKA angle was 11.8 ± 5.2° in varus (range 28.3° in varus to 2.8° in valgus). Component positioning was measured on postoperative AP and lateral radiographs in accordance with the Knee Society Radiographic Evaluation System: the medial angle between the tibial plate and the coronal mechanical axis of the tibial shaft (β angle), and the posterior tilting angle between the tibial plate and the sagittal mechanical axis of the tibial shaft (δ angle). True AP and lateral radiographs of the knee were obtained in the following manner [18]. The patella was directed upward by moving the knee through a full range of flexion and extension. Subsequently, the knee was positioned at knee extension or at approximately 90° knee flexion, and AP or lateral radiographs were taken, respectively. If adequate views were not obtained, radiographs were retaken. Aseptic loosening of the tibial component was defined radiographically when the radiolucent line was seen in all zones or when the tibial component had migrated. The same radiographic measurements of each parameter were performed three times by the same examiner with iRad-OT (infocom, Tokyo, Japan) to minimize measurement errors, and the mean value of the three measurements was used throughout this study. The results in this study are presented to one decimal place because our system could measure each value with this degree of accuracy.
Evaluation of TR
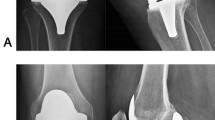
This study evaluated TR using early (within 2 weeks postoperatively) and 2-year postoperative AP and lateral radiographs (Fig. 1), which were divided into five zones according to the modernized Knee Society Radiographic Evaluation System [26] (Fig. 2). The 2-year TR in each zone was defined as the change in TR from early to 2-year postoperative radiographs. Figure 1 shows the TR measurement method using zones 1 (medial) and 2 (lateral) on AP radiographs. On 2-year AP radiographs, the horizontal width between the edge of the tibial cutting surface and the end of extended bone absorption was defined as TR (Fig. 1). In terms of vertical measurement, TR was considered to be present only when the width was > 1 mm, because a width of ≤ 1 mm can be caused by insufficient cement penetration. The edge of the tibial cutting surface on each 2-year AP radiograph was determined by referring to that on the corresponding early AP radiograph. A positive value indicated the progression of TR, whereas a negative value indicated the presence of new bone formation at 2 years. On lateral radiographs, TRs in zones 1 (anterior) and 2 (posterior) were measured in the same way as on AP radiographs (Fig. 1).
Evaluation of over- or underhang of the tibial baseplate
Medial and lateral tangent lines of the tibial baseplate were defined on early AP radiographs, along with the edges of the medial and lateral tibial cutting surfaces, respectively. The width between each tangent line and the corresponding edge was defined as tibial component overhang or underhang (TOH or TUH), which represented over- and undercoverage, respectively, of the tibial component on the tibial cutting surface on early postoperative AP radiographs (Fig. 1). The TOH or TUH from the edge of the medial or lateral tibial cutting surface was recorded as a positive or negative value, respectively. On lateral radiographs, the TOH or TUH in zones 1 and 2 was also measured in the same way as on AP radiographs (Fig. 1).
Evaluation of the cement mantle thickness
The maximum thickness of the cement mantle was measured in five zones of the keel area, including zones 3M (medial), 3L (lateral), and 5 (distal) on the AP view, and zones 3A (anterior), 3P (posterior), and 5 (distal) on the lateral view, calibrated to 0.1 mm (Fig. 2).
The relationships of various parameters with the widths of 2-year postoperative TRs in zones 1 and 2 on AP radiographs, and zones 1 and 2 on lateral radiographs were evaluated. The dependent variables that were evaluated to verify our hypothesis were 2-year postoperative TRs in zones 1 and 2 on AP and lateral radiographs. The independent variables were sex, age, BMI, postoperative knee flexion angle, postoperative residual knee joint pain, postoperative HKA angle, postoperative β or δ angles, TOH or TUH, and the thickness of the cement mantle in each zone. This study was approved by the Institutional Review Board of Kyoto University (registration number R1144), and all patients provided informed consent regarding pre- and postoperative radiographic measurements and risks of radiation exposure.
Statistical analysis
Stata software (version 13; Stata Corporation, College Station, TX, USA) was used. As for the sample size calculation, a previous study with 100 TKA cases reported that a 4-mm-thick cobalt–chromium (CoCr) tibial tray was associated with increased medial TR compared with a standard tray on AP views, and the mean medial bone loss was significantly higher in the thick tray cohort than in the standard tray cohort (1.1 ± 1.3 mm vs. 0.2 ± 0.5 mm; p < 0.01) [24]; a bone loss difference of about 1 mm was considered clinically significant. With α and power set at 0.05 and 0.90, respectively, a minimum of 50 cases were needed to obtain a sufficient sample size, and over 100 patients were recruited in this study. Differences between the measured TR widths in zones 1 and 2 of AP or lateral radiographs were analyzed using the two-tailed Student’s t test. The thickness of the ideal cement mantle was set at 5.0 mm [7, 39], and the difference in 2-year TR between the ideal cement mantle group (≤ 5.0 mm) and the thicker cement mantle group (> 5.0 mm) in each zone was evaluated. The intraclass correlation coefficient (ICC) was used to assess intra-observer test–retest reliability in obtaining TR measurements. A value > 0.75 indicates excellent test–retest reproducibility. Pearson correlation coefficients were calculated for the relationships between the area of the 2-year postoperative TR in each zone, and the postoperative TOH or TUH and the thickness of the cement mantle. Correlation coefficients (R) were characterized as very weak (0.00–0.20), weak (0.21–0.40), moderate (0.41–0.70), or strong (0.71–1.00). Simple and stepwise multiple linear regression analyses were also calculated to determine optimum predictors of the dependent variables based on the independent variables. The statistical significance level was set at p < 0.05.
Results
The mean postoperative HKA angles, β and δ angles, angle of knee flexion, and rate of residual knee joint pain at 2-year postoperative follow-up are shown in Table 1. There was an excellent intra-observer test–retest reproducibility of TR measurements, with an ICC of 0.999. The mean widths of TRs in zones 1 and 2 on AP and lateral radiographs at 2 years postoperatively are also shown in Table 1. The widths of TRs on AP radiographs were significantly larger in zone 1 than in zone 2 (p = 0.001). On the other hand, there was also a significant difference between the TRs in zones 1 and 2 on lateral radiographs (p = 0.003), but the difference was relatively small.
The TOH and TUH values on early postoperative AP and lateral radiographs are shown in Table 2. There was a tendency to choose smaller tibial component sizes, and consequently TUH occurred in zones 1 and 2 on both AP radiographs (48% and 34%, respectively) and lateral radiographs (51% and 69%, respectively). The TUH was positively correlated with 2-year TR in zone 1 on the AP view, and zones 1 and 2 on the lateral view (Table 2).
As shown in Table 3, the mean thickness of the cement mantle was around 3.0 mm in each zone, except for zone 5. On the AP view, there was a very weak positive correlation between the thickness of the cement mantle in zone 5 and 2-year TR in zone 2. On the lateral view, the correlations between the thickness of the cement mantle in zones 3A and 5, and the 2-year TR in zone 1 were very weak and weak, respectively, and there was a negative correlation between the thickness of the cement mantle in zone 5 and 2-year TR in zone 2 (Table 3). As for zone 5, on the AP view the group with a cement mantle ≤ 5.0 mm had a smaller 2-year TR in zone 1 (2.8 ± 2.6 mm) than the group with a cement mantle > 5.0 mm (4.1 ± 3.7 mm) (p = 0.017); on the lateral view, the ≤ 5.0 mm group also had a smaller TR in zone 1 (− 0.8 ± 1.4 mm) than the > 5.0 mm group (− 0.2 ± 1.4 mm) (p = 0.011), whereas the thicknesses of the cement mantle in the other zones were not associated with TR.
Simple linear regression analyses between 2-year TR and postoperative variables in each zone are shown in Tables 4 and 5 for AP and lateral radiographs, respectively. Sex, BMI, postoperative residual pain, HKA angle, and β and δ angles were not related to 2-year TR on AP and lateral radiographs. On the other hand, TRs in zones 1 and 2 on the AP view were positively related only to TUH in zone 1 and to a thicker cement mantle in zone 5, respectively (Table 4). On the lateral view, TR in zone 1 was positively related to postoperative knee flexion angle, TUH, and a thicker cement mantle in zones 3A and 5, whereas TR in zone 2 was positively related to age and TUH, and negatively related to a thicker cement mantle in zone 5 (Table 5).
Based on multiple regression analysis with stepwise variable selection on the AP view, the TUH in zone 1 was the strongest risk factor for increased TR in zone 1, and a thicker cement mantle in zone 5 was the strongest risk factor in zone 2 (Table 6). On the lateral view, postoperative knee flexion angle was the strongest risk factor for increased TR in zone 1, and TUH was the strongest risk factor in zone 2, because the t values of age and TUH were 3.7 and 6.8, respectively (Table 7). However, no gross loosening was observed in any cases at 2-year follow-up.
Discussion
The most important finding of this study was that TUH and a thicker cement mantle at the distal part of the tibial keel on AP radiographs at 2-year follow-up after TKA resulted in progressive medial and lateral TRs, respectively. This study also found that the most important predictive factors for increased anterior and posterior TRs on the lateral view were the postoperative knee flexion angle and TUH, respectively.
Few studies have focused on TOH or TUH in TKA [34]. In regard to component over- or underhang, a previous study of unicompartmental knee arthroplasty (UKA) reported that medial TOH of ≥ 3 mm caused worse postoperative patient outcomes, but significant TUH should be avoided because of tibial component loosening and subsidence [9]. However, UKA generally aims for a slight varus alignment, whereas TKA, including that performed in this study, largely aims for a neutral alignment. Therefore, the biomechanics at the tibial bone–component interface differ between UKA and TKA. Berend et al. [4, 5] found that tibial component loosening and migration in TKA was related to a BMI greater than 33 kg/m² and an undersized tibial component. Oversizing the tibial component has also not been recommended because it results in local pain in the posterolateral corner after TKA with TOH in the posterolateral lesion [34]. To avoid tibial oversizing and malpositioning [3, 8], when TOH occurred intraoperatively in this study, surgeons tended to choose a tibial component one size smaller than that predicted during preoperative planning [10, 19, 20, 25]; this raised the likelihood of TUH, which would subsequently increase TR due presumably to lack of weight bearing at the uncovered bone. The findings of this study suggest that surgeons should perfectly fit an appropriately sized tibial component to the medial edge of the tibial bone to avoid extensive 2-year postoperative TR. Our clinical outcome at 2 years after TKA, including postoperative residual knee joint pain, was not affected by TR [2, 16, 22].
It is very important to achieve a strong bone–cement interface in TKA through appropriate cementation techniques [14, 35]. In this study, the group with a cement mantle thickness of > 5.0 mm in zone 5 had a significantly increased TR compared with the group with an ideal cement mantle thickness of ≤ 5.0 mm. This difference probably occurred due to stress shielding in the former group, because the tibial component interlocked with the more distal part of the tibia. Previous studies also showed that a longer tibial keel caused greater stress shielding and increased the risk of tibial component loosening [17, 32]. Previous finite element (FE) analyses showed that metaphyseal strain shielding of the tibia was greater with a fully cemented standard stem than with a proximally cemented standard stem [7, 32]. In addition, proximal tibial strain shielding was greater with a fully cemented longer stem than with a fully cemented standard stem, while strain concentration of the distal portion was greatest with the fully cemented longer stem [32]. Another biomechanical study found that load sharing at the tibial cortical rim was lower when using a fully cemented longer stem than when using fully cemented short stem or a press-fit longer stem because of distal stress distribution [12]. The present study found no residual postoperative knee joint pain due to stress concentration at the distal keel.
This is the first study to confirm the relationship between 2-year anterior TR and postoperative flexion angle after TKA. Regarding the femoral side, one study using an FE simulation reported that the highest shear stresses were found at the cement–implant interface behind the anterior flange of a high-flex femoral component [38]. Another study also showed that the anterior flange was at greatest risk of failure, especially in patients with high flexion angles [41]. However, no studies thus far have examined the relationship between failure of the tibial bone–component interface and the postoperative knee flexion angle. A greater postoperative flexion angle with increased femoral rollback would induce a larger lifting force at the anterior bone–component interface. Attention should be paid to anterior excessive TR in patients with high postoperative flexion angles. The clinical relevance of this study is that surgeons should avoid both medial TUH from the edge of the medial tibial cutting surface and an excessive cement mantle (i.e., > 5.0 mm) at the distal portion of the keel during TKA. This knowledge can be useful in daily clinical settings to achieve both suitable preparation of the tibial cutting surface and appropriate cementation techniques to avert postoperative progressive TR.
This study has several limitations. First, it used a single component design with a tibial component made of Ti. Previous studies reported that CoCr tibial baseplates were associated with significantly more medial tibial resorption than Ti baseplates, because stiffer tibial component materials had more stress shielding; the modulus of elasticity of CoCr is higher than that of Ti, resulting in greater medial tibial stress shielding [11, 23, 24]. Second, a previous study reported that higher BMI and overall limb varus were risk factors associated with aseptic loosening, but the mean BMI of the participants in this study was relatively low at 26.0 kg/m², and the mean postoperative HKA angle was slightly valgus [1, 5]. Third, in this study a single examiner performed each radiographic measurement three times. The intra-observer reproducibility was excellent, but the reliability might be higher if measurements were made by three examiners. Fourth, a relatively small number of participants were chosen over a long recruitment period. This was because many knees met the exclusion criteria since we were only investigating the influence of insufficient coverage of the tibial component on the tibial cutting surface and that of a thick cement mantle around the tibial keel on tibial bone resorption. Finally, the follow-up time was 2 years, while previous studies reported that progressive TR and bone loss could occur within 2 years postoperatively [15]. However, it is still difficult to determine whether or not massive TR leads to aseptic loosening, and longer-term research is needed.
Conclusions
Medial TUH caused increased medial TR, and a thicker distal cement mantle around the tibial keel resulted in lateral TR on 2-year AP radiographs. On the lateral view, the postoperative knee flexion angle and TUH were the most significant risk factors for increased anterior and posterior TRs, respectively. There was no gross loosening or residual knee joint pain related to TR in any cases at 2-year follow-up.
References
Abdel MP, Bonadurer GF 3rd, Jennings MT, Hanssen AD (2015) Increased aseptic tibial failures in patients with a BMI ≥ 35 and well-aligned total knee arthroplasties. J Arthroplast 30:2181–2184
Al-Nabhani K, Michopoulou S, Allie R, Alkalbani J, Saad Z, Sajjan R et al (2014) Painful knee prosthesis: can we help with bone SPECT/CT? Nucl Med Commun 35:182–188
Awengen R, Rasch H, Amsler F, Hirschmann MT (2016) Symptomatic versus asymptomatic knees after bilateral total knee arthroplasty: what is the difference in SPECT/CT? Eur J Nucl Med Mol Imaging 43:762–772
Berend ME, Ritter MA, Hyldahl HC, Meding JB, Redelman R (2008) Implant migration and failure in total knee arthroplasty is related to body mass index and tibial component size. J Arthroplast 23:104–109
Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R et al (2004) Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res 428:26–34
Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP et al (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468:45–51
Cawley DT, Kelly N, McGarry JP, Shannon FJ (2013) Cementing techniques for the tibial component in primary total knee replacement. Bone Joint J 95:295–300
Cerquiglini A, Henckel J, Hothi H, Rotigliano N, Hirschmann MT, Hart AJ (2018) 3D patient imaging and retrieval analysis help understand the clinical importance of rotation in knee replacements. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-4891-9
Chau R, Gulati A, Pandit H, Beard DJ, Price AJ, Dodd CAF et al (2009) Tibial component overhang following unicompartmental knee replacement—Does. it matter? Knee 16:310–313
Clary C, Aram L, Deffenbaugh D, Heldreth M (2014) Tibial base design and patient morphology affecting tibial coverage and rotational alignment after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22:3012–3018
Completo A, Fonseca F, Simões JA (2008) Strain shielding in proximal tibia of stemmed knee prosthesis: experimental study. J Biomech 41:560–566
Completo A, Simões JA, Fonseca F, Oliveira M (2008) The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA. Knee 15:227–232
Fukagawa S, Matsuda S, Mitsuyasu H, Miura H, Okazaki K, Tashiro Y et al (2011) Anterior border of the tibia as a landmark for extramedullary alignment guide in total knee arthroplasty for varus knees. J Orthop Res 29:919–924
Guha AR, Debnath UK, Graham NM (2008) Radiolucent lines below the tibial component of a total knee replacement (TKR)—a comparison between single-and two-stage cementation techniques. Int Orthop 32:453–457
Hazelwood KJ, O’Rourke M, Stamos VP, McMillan RD, Beigler D, Robb WJ (2015) Case series report: early cement-implant interface fixation failure in total knee replacement. Knee 22:424–428
Hirschmann MT, Amsler F, Rasch H (2015) Clinical value of SPECT/CT in the painful total knee arthroplasty (TKA): a prospective study in a consecutive series of 100 TKA. Eur J Nucl Med Mol Imaging 42:1869–1882
Jazrawi LM, Bai B, Kummer FJ, Hiebert R, Stuchin SA (2001) The effect of stem modularity and mode of fixation on tibial component stability in revision total knee arthroplasty. J Arthroplasty 16:759–767
Kuriyama S, Hyakuna K, Inoue S, Kawai Y, Tamaki Y, Ito H et al (2018) Bone-femoral component interface gap after sagittal mechanical axis alignment is filled with new bone after cementless total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 26:1478–1484
Kuriyama S, Hyakuna K, Inoue S, Tamaki Y, Ito H, Matsuda S (2014) Tibial rotational alignment was significantly improved by use of a CT-navigated control device in total knee arthroplasty. J Arthroplast 29:2352–2356
Kuriyama S, Ishikawa M, Furu M, Ito H, Matsuda S (2014) Malrotated tibial component increases medial collateral ligament tension in total knee arthroplasty. J Orthop Res 32:1658–1666
Kuriyama S, Ishikawa M, Nakamura S, Furu M, Ito H, Matsuda S (2017) Noise generation with good range of motion but without femorotibial instability has small effect on patient satisfaction after total knee arthroplasty. J Arthroplast 32:407–412
Lim HA, Song EK, Seon JK, Park KS, Shin YJ, Yang HY (2017) Causes of aseptic persistent pain after total knee arthroplasty. Clin Orthop Surg 9:50–56
Martin JR, Watts CD, Levy DL, Kim RH (2017) Medial tibial stress shielding: a limitation of cobalt chromium tibial baseplates. J Arthroplast 32:558–562
Martin JR, Watts CD, Levy DL, Miner TM, Springer BD, Kim RH (2017) Tibial tray thickness significantly increases medial tibial bone resorption in cobalt–chromium total knee arthroplasty implants. J Arthroplast 32:79–82
Martin S, Saurez A, Ismaily S, Ashfaq K, Noble P, Incavo SJ (2014) Maximizing tibial coverage is detrimental to proper rotational alignment. Clin Orthop Relat Res 472:121–125
Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR (2015) Development of a modern knee society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplast 30:2311–2314
Nakamura S, Ito H, Nakamura K, Kuriyama S, Furu M, Matsuda S (2017) Long-term durability of ceramic tri-condylar knee implants: a minimum 15-year follow-up. J Arthroplast 32:1874–1879
Nakamura S, Kobayashi M, Ito H, Nakamura K, Ueo T, Nakamura T (2010) The bi-surface total knee arthroplasty: minimum 10-year follow-up study. Knee 17:274–278
Pfitzner T, von Roth P, Voerkelius N, Mayr H, Perka C, Hube R (2016) Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24:96–101
Pijls BG, Valstar ER, Nouta K-A, Plevier JW, Fiocco M, Middeldorp S et al (2012) Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop 83:614–624
Ritter MA, Keating EM, Sueyoshi T, Davis KE, Barrington JW, Emerson RH (2016) Twenty-five-years and greater, results after nonmodular cemented total knee arthroplasty. J Arthroplast 31:2199–2202
Scott CE, Biant LC (2012) The role of the design of tibial components and stems in knee replacement. J Bone Joint Surg Br 94:1009–1015
Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J (2014) Why are total knee arthroplasties failing today—Has anything changed after 10 years? J Arthroplast 29:1774–1778
Simsek ME, Akkaya M, Gursoy S, Isik C, Zahar A, Tarabichi S et al (2018) Posterolateral overhang affects patient quality of life after total knee arthroplasty. Arch Orthop Trauma Surg 138:409–418
Staats K, Wannmacher T, Weihs V, Koller U, Kubista B, Windhager R (2018) Modern cemented total knee arthroplasty design shows a higher incidence of radiolucent lines compared to its predecessor. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-5130-0
Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C (2006) Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop 77:177–197
Thiele K, Perka C, Matziolis G, Mayr HO, Sostheim M, Hube R (2015) Current failure mechanisms after knee arthroplasty have changed: Polyethylene wear is less common in revision surgery. J Bone Joint Surg Am 97:715–720
van de Groes, de Waal-Malefijt, V (2014) Probability of mechanical loosening of the femoral component in high flexion total knee arthroplasty can be reduced by rather simple surgical techniques. Knee 21:209–215
Vanlommel J, Luyckx JP, Labey L, Innocenti B, De Corte R, Bellemans J (2011) Cementing the tibial component in total knee arthroplasty: which technique is the best? J Arthroplasty 26:492–496
Wang H, Lou H, Zhang H, Jiang J, Liu K (2014) Similar survival between uncemented and cemented fixation prostheses in total knee arthroplasty: a meta-analysis and systematic comparative analysis using registers. Knee Surg Sports Traumatol Arthrosc 22:3191–3197
Zelle J, Janssen D, Van Eijden J, De Waal Malefijt M, Verdonschot N (2011) Does high-flexion total knee arthroplasty promote early loosening of the femoral component? J Orthop Res 29:976–983
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Ethical approval
All procedures were in accordance with the ethical standards of our institutional research committee.
Informed consent
Informed consent was obtained from all study participants.
Rights and permissions
About this article
Cite this article
Gu, S., Kuriyama, S., Nakamura, S. et al. Underhang of the tibial component increases tibial bone resorption after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 27, 1270–1279 (2019). https://doi.org/10.1007/s00167-018-5309-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5309-4