Abstract
Purpose
The purpose of the study was whether the use of a tourniquet increases cement mantle thickness in primary total knee arthroplasty and influences the calculated blood loss and postoperative pain.
Methods
Ninety patients with a primary total knee arthroplasty (TKA) were enroled in this prospective randomised trial and divided into a group with (n = 45) and without tourniquet (n = 45). The radiological tibial cement mantle thickness was evaluated postoperatively in four zones on anteroposterior and two zones on lateral radiographs, and values were cumulated. Additionally, the calculated blood loss and postoperative pain levels were recorded.
Results
There was a median cumulative cement mantle thickness of 13 mm (range 8–19 mm) without tourniquet and of 14.2 mm (range 9–18 mm) with tourniquet (p = 0.009). The median calculated blood loss was 0.6 L (range 0.2–2.0 L) without and 0.9 L (range 0.3–1.5 L) (p = 0.02) with tourniquet. Patient-reported postoperative pain levels were significantly higher in the tourniquet group during mobilisation (p = 0.01) and at rest (p = 0.001).
Conclusions
The use of a tourniquet in primary TKA increased the tibial cement mantle thickness but also increased the postoperative calculated blood loss and postoperative pain. Surgeons might take this into consideration for decision-making whether to use a tourniquet during TKA.
Level of evidence
II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cemented implants in primary total knee arthroplasty (TKA) have demonstrated excellent results and long-term implant survival [4, 14]. However, aseptic loosening is still one of the most frequent causes of implant failure [33]. The tourniquet, which is commonly used by many surgeons during TKA, has the proposed benefits of creating clean and blood-poor surfaces and improved visualisation due to reduced bleeding [41]. However, considerations for cementing techniques are based on assumptions from total hip arthroplasty. Therefore, it is still unknown if this reduced bleeding, caused by the tourniquet, also increases cement penetration into the trabecular bone and consequently implant stability. In two recent studies, the tourniquet showed no effect on early micromotion measured by radiostereometric analysis (RSA) between the tibial component and the tibia, but it remains unknown if this method is valid to predict long-term implant stability [23, 29]. In contrast, implant stability was found to be dependent on cement mantle thickness [8, 30, 38]. However, to the author’s knowledge, this is the first study investigating the influence of the tourniquet on cement mantle thickness in TKA. Regarding the perioperative blood loss, the literature is still inconsistent, caused by the inhomogeneity of the surgical and perioperative protocol of the studies [3, 24, 25, 36, 40]. The hypothesis of this study was that the use of a tourniquet results in an increased cement mantle thickness at the tibial component in primary TKA. Therefore, the cement mantle thickness of the tibial component with and without the use of a tourniquet during TKA was determined. Secondary goals were an evaluation of the perioperative blood loss and postoperative pain levels.
Materials and methods
A randomised clinical trial was performed in which 90 patients with primary end-stage osteoarthritis received a unilateral total knee arthroplasty (TKA). Patients were excluded if they received any anticoagulation prior to surgery (e.g., acetylsalycylic acid, phenprocoumon, warfarin, clopidogrel, dabigatran, rivaroxaban and low molecular weight heparin) and had the diagnosis of liver dysfunction/coagulation dysfunction or a history of peripheral arterial obstructive disease or thromboembolic events. A total of 114 patients with primary end-stage osteoarthritis were identified for this study, and 94 patients met the inclusion criteria. Four patients declined participation. Finally, 90 knees (90 patients) undergoing cemented TKA were randomised into two groups: use of a tourniquet (Group 1, n = 45) and no use of a tourniquet (Group 2, n = 45) (Fig. 1). Patients were assigned to the two branches of the study at the start of the operation, by opening a sealed envelope. All operations were performed in a single centre by a single senior surgeon (RH) who had completed more than 1,000 primary TKAs.
Flow diagram illustrating patient enrolment, allocation, follow-up and analysis [28]
Surgical technique
All patients received a cemented, posterior-stabilised primary TKA (Nexgen LPS Flex, Zimmer, Warsaw, IN, USA) with a fixed bearing design without patella resurfacing. A total amount of 40 g of bone–cement (Palacos R®, Heraeus, Hanau, Germany) was used with a fourth-generation cementing technique including pulsatile lavage, vacuum mixture, double cementing technique and cement gun pressurisation. The tibial component was cemented first, and so independently from the used implant size, always the same amount/thickness of cement was applied to the implant and to the bony surface. A medial mini-midvastus approach was used in all patients [17]. According to the randomisation, a pneumatic tourniquet was applied prior surgery at the proximal thigh and inflated from skin incision until skin closure without the use of an esmarch bandage for exsanguination of the leg before inflation. The inflation pressure was 350 mmHg in the respective cases. An intra-articular drainage was used in every patient and removed after 24 h. Every patient received the same standardised postoperative pain medication protocol.
Radiological evaluation
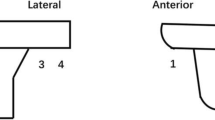
On the fourth postoperative day, a standardised digital anteroposterior and lateral radiograph of the operated knee was taken. All radiographs were analysed independently by two investigators (NV, PvR). Measurements were performed two times with an interval of 2 weeks within the local picture archiving and communication system (PACS) blinded whether a tourniquet was used. Despite the high accuracy of the PACS, the thickness of the cement mantle was analysed in millimetres with one decimal because of the decreased reliability and doubtful clinical relevance of more than one decimal. Measurements were performed in six zones based on the Knee Society scoring system and according to the technique of Kopec et al. [13, 19]. After standard magnification of the radiograph and identification of the bone–cement interface with the contrast tool, four zones at the tibial baseplate on the anteroposterior view and two zones on the lateral view were measured (Fig. 2). Cement mantle measurements were only performed at the tibial baseplate (Fig. 3), because of the ongoing discussion about full tibial stem cementation and the inconsistent recommendations from implant manufacturers [15]. The penetration of cement is following a random distribution affected by the individual structure of the host bone. To reduce bias in single measurements due to these individual variations within the cement mantle, the thickness of the six zones was cumulated and compared between the groups.
Blood loss and pain level
On the second postoperative day, the pain at rest on a visual analogue scale (VAS) was documented [9, 12]. On the fourth postoperative day, the pain at rest and during mobilisation was again documented on a VAS. To determine the overall blood loss, peripheral blood was taken preoperatively and on the first postoperative day. Total blood loss was calculated using the method of Bourke and Smith [10] to take both obvious and hidden blood loss into account. The study was approved by the institutional review board (Charité-Universitätsmedizin Berlin; ID-Number: EA1/078/11), and informed consent was obtained from every participant.
Statistical analysis
An a priori power analysis was performed to calculate the sample size. Based on a power of 0.80 (1−β) with α = 0.05 to detect a difference of 1 mm between two groups, a total required sample size of n = 88 was identified. An overall study sample size of n = 90 was assumed as appropriate to include possible study dropout with this short follow-up. Data were not-normally distributed and presented as median with ranges. A nonparametric approach was used to compare the cement mantle thickness. Significant differences between the groups were evaluated by the Mann–Whitney U test for independent samples. Inter- and intraobserver reliability testing was performed by calculation of the intraclass correlation coefficients. For statistical analysis SPSS® (version 19; IBM Corp; Somers, NY, USA) was used. The level of significance was set with an alpha of 0.05.
Results
The study sample included 32 men and 58 women, with a median age of 70 years (range 47–90 years). The groups were comparable with regards to age, gender and BMI (Table 1).
The mean correlation coefficient of the interobserver reliability was 0.93 [95 % confidence interval (CI) 0.89–0.97], and the correlation of the intraobserver reliability was 0.96 (95 % CI 0.94–0.99) and 0.94 (95 % CI 0.90–0.97). The cumulative cement mantle thickness was 14.2 mm (range 9–18 mm) with the use of a tourniquet and 13 mm (range 8–19 mm) without the use of a tourniquet (p = 0.009) (Table 2) (Fig. 4).
The calculated blood loss was 0.9 L (range 0.3–1.5 L) with the use of a tourniquet and 0.6 L (range 0.2–2.0 L) without the use of a tourniquet (Table 2). Comparing both groups, the use of the tourniquet showed an increased calculated total blood loss (p = 0.02).
In the tourniquet group (Group 1), the preoperative pain level at rest on the VAS was 3 points (0–8 points) compared to 2 points (0–8 points) without a tourniquet (Group 2) (n.s.). On the second postoperative day, the pain at rest was 3 points (0–7 points) on the VAS in Group 1 and 3 points (1–8 points) in Group 2 (n.s.). Looking at the pain at rest on the fourth postoperative day, Group 1 showed a decrease to 2 points (0–6 points) on the VAS and Group 2 to 1 points (0–3 points) (p = 0.001). The pain during mobilisation on the fourth postoperative day was 3 points (0–7 points) on the VAS in Group 1 and 2 points (0–4 points) in Group 2 (p = 0.01) (Table 2).
Discussion
The most important findings of the present study were that the use of a tourniquet increased the cement mantle thickness at the tibial component in primary TKA. Furthermore, the use of a tourniquet showed an increased total blood loss and increased postoperative pain. To our knowledge, this prospective study is the first to investigate the influence of the tourniquet on the cement mantle thickness in TKA.
There is a lack of data available in the literature whether the use of a tourniquet increases implant fixation and survival in TKA. The only studies investigating the influence of the tourniquet on implant fixation in TKA using radiostereometric analysis (RSA) found no difference between the groups [23, 29]. However, there is little evidence whether this technique predicts implant survival. Ledin et al. [23] mentioned that the capability of the RSA method to predict loosening is mostly based on beliefs and theoretical reasoning. The only study supporting this found early micromotion with RSA to be a risk factor for loosening mostly in noncemented tibial components [32]. Accordingly, Ledin et al. [23] referred the weakness of the RSA method to predict clinical outcomes as major limitation of their study. In contrast, an increased cement mantle thickness was found to increase implant stability and survival [8, 30, 38]. Aseptic loosening is mostly localised at the bone–cement interface [25]. Preparation of the bone surface is therefore most important [31]. Pulsatile lavage such as use of “low-viscosity” cement showed an increased cement penetration depth [11, 20]. Residual blood at the bony interface reduces the adhesive/tensile strength by up to 50 % [6, 16, 26]. In the comparative study of Vandenbussche et al. [37], no difference in radiological signs of loosening was observed at 3-month follow-up between TKAs performed with or without tourniquet. However, the short follow-up does not allow an evaluation of differences in implant longevity.
In addition, the calculated total blood loss was found to be increased with the use of a tourniquet. This is in line with other prospective studies that showed an increased blood loss with the use of a tourniquet [24, 34, 36]. Causes are an increased “hidden blood loss” by using a tourniquet due to an increased haemolysis [24] and postischaemic activated fibrinolysis [2, 18], such as hyperperfusion after deflation [5, 22]. In contrast, the blood loss was reduced with the use of a tourniquet in other studies [3, 24, 36, 40]. These different results might be caused by the different methods of blood loss calculation used, such as the different protocols of tourniquet application and duration of inflation (whole procedure vs. only during cementation) [21, 35]. Alcelik et al. [3] mentioned this inconsistent use of the tourniquet in the included studies as major shortcoming of their meta-analysis. Furthermore, the early postoperative pain was increased in the tourniquet group. There is distinct evidence that the use of the tourniquet increases postoperative pain and impairs function [1, 23, 37]. Causes are the greater hidden blood loss with postischaemic swelling of the soft tissue envelope and the direct trauma of the tourniquet to nerval structures and soft tissues [1, 24, 37, 42]. This is supported by the data, which show that a lower inflation pressure and a shorter tourniquet time result in reduced postoperative pain [7, 27, 39].
This study has limitations. Only the cement mantle thickness of the tibial component was analysed. Evaluation of the femoral cement mantle thickness is only possible on lateral radiographs with potential inaccuracy due to overlay of the medial and lateral condyle. Moreover, a posterior stabilised design was used in every case. A measurement of the cement mantle thickness at the most relevant parts of the femur is not possible with the intercondylar box. Second, bone mineral density was not measured in the study patients, but groups were equal regarding age, gender and BMI. However, this might affect cement penetration, even if there is no evidence that osteoporosis is a risk factor for loosening in cemented TKA. Third, the surgeon was not blinded. It seems to be impossible to blind a surgeon whether a tourniquet is inflated during surgery or not. To reduce possible bias, the application of the cement, pressurisation and implantation of the component was performed in a standardised way in every patient.
Even if an increased cement mantle thickness was found to improve implant stability [8, 30, 38], there is no evidence in the literature if an increase of 1 mm in cement mantle thickness or the use of a tourniquet improves long-term implant survival. The differences of tibial cement mantle thickness between the tourniquet and nontourniquet group in this study were statistically identifiable but small and therefore of questionable clinical relevance. Further prospective studies are necessary to evaluate the influence of the tourniquet and cement mantle thickness on long-term implant survival.
Conclusion
The use of a tourniquet in primary TKA increased the tibial cement mantle thickness but also increased the postoperative calculated blood loss and postoperative pain. Surgeons might take this into consideration for decision-making whether to use a tourniquet during TKA.
References
Abdel-Salam A, Eyres KS (1995) Effects of tourniquet during total knee arthroplasty: a prospective randomised study. J Bone Joint Surg Br 77(2):250–253
Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M (2000) Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res 371:169–177
Alcelik I, Pollock RD, Sukeik M, Bettany-Saltikov J, Armstrong PM, Fismer P (2012) A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplast 27(3):331–340
Argenson JN, Parratte S, Ashour A, Saintmard B, Aubaniac JM (2012) The outcome of rotating-platform total knee arthroplasty with cement at a minimum of 10 years of follow-up. J Bone Joint Surg Am 94(7):638–644
Authier B (1988) Reactive hyperemia monitored on rat muscle using perfluorocarbons and 19F NMR. Magn Reson Med 8(1):80–83
Bannister GC, Miles AW (1988) The influence of cementing technique and blood on the strength of the bone-cement interface. Eng Med 17(3):131–133
Barwell J, Anderson G, Hassan A, Rawlings I (1997) The effects of early tourniquet release during total knee arthroplasty: a prospective randomized double-blind study. J Bone Joint Surg Br 79(2):265–268
Bert JM, McShane M (1998) Is it necessary to cement the tibial stem in cemented total knee arthroplasty? Clin Orthop Relat Res 356:73–78
Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y (2001) The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology 95(6):1356–1361
Bourke DL, Smith TC (1974) Estimating allowable hemodilution. Anesthesiology 41(6):609–612
Clarius M, Hauck C, Seeger JB, James A, Murray DW, Aldinger PR (2009) Pulsed lavage reduces the incidence of radiolucent lines under the tibial tray of Oxford unicompartmental knee arthroplasty: pulsed lavage versus syringe lavage. Int Orthop 33(6):1585–1590
Coll AM, Ameen JR, Mead D (2004) Postoperative pain assessment tools in day surgery: literature review. J Adv Nurs 46(2):124–133
Ewald FC (1989) The knee society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 248:9–12
Feng B, Weng X, Lin J, Jin J, Wang W, Qiu G (2013) Long-term follow-up of cemented fixed-bearing total knee arthroplasty in a Chinese population: a survival analysis of more than 10 years. J Arthroplast 28(10):1701–1706
Galasso O, Jenny JY, Saragaglia D, Miehlke RK (2013) Full versus surface tibial baseplate cementation in total knee arthroplasty. Orthopedics 36(2):151–158
Gruen TA, Markolf KL, Amstutz HC (1976) Effects of laminations and blood entrapment on the strength of acrylic bone cement. Clin Orthop Relat Res 119:250–255
Hube R, Keim M, Mayr HO (2009) The mini-midvastus approach for total knee arthroplasty. Oper Orthop Traumatol 21(1):3–13
Klenerman L, Chakrabarti R, Mackie I, Brozovic M, Stirling Y (1977) Changes in haemostatic system after application of a tourniquet. Lancet 1(8019):970–972
Kopec M, Milbrandt JC, Duellman T, Mangan D, Allan DG (2009) Effect of hand packing versus cement gun pressurization on cement mantle in total knee arthroplasty. Can J Surg 52(6):490–494
Krause WR, Krug W, Miller J (1982) Strength of the cement-bone interface. Clin Orthop Relat Res 163:290–299
Kvederas G, Porvaneckas N, Andrijauskas A, Svensen CH, Ivaskevicius J, Mazunaitis J, Marmaite U, Andrijauskas P (2013) A randomized double-blind clinical trial of tourniquet application strategies for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(12):2790–2799
Larsson J, Lewis DH, Liljedahl SO, Lofstrom JB (1977) Early biochemical and hemodynamic changes after operation in a bloodless field. Eur Surg Res 9(5):311–320
Ledin H, Aspenberg P, Good L (2012) Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 83(5):499–503
Li B, Wen Y, Wu H, Qian Q, Lin X, Zhao H (2009) The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop 33(5):1263–1268
Maistrelli GL, Antonelli L, Fornasier V, Mahomed N (1995) Cement penetration with pulsed lavage versus syringe irrigation in total knee arthroplasty. Clin Orthop Relat Res 312:261–265
Majkowski RS, Bannister GC, Miles AW (1994) The effect of bleeding on the cement-bone interface: an experimental study. Clin Orthop Relat Res 299:293–297
Manen Berga F, Novellas Canosa M, Angles Crespo F, Bernal Dzekonski J (2002) Effect of ischemic tourniquet pressure on the intensity of postoperative pain. Rev Esp Anestesiol Reanim 49(3):131–135
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG (2010) Consolidated standards of reporting trials CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63(8):1–37
Molt M, Harsten A, Toksvig-Larsen S (2014) The effect of tourniquet use on fixation quality in cemented total knee arthroplasty a prospective randomized clinical controlled RSA trial. Knee 21(2):396–401
Peters CL, Craig MA, Mohr RA, Bachus KN (2003) Tibial component fixation with cement: full- versus surface-cementation techniques. Clin Orthop Relat Res 409:158–168
Ritter MA, Herbst SA, Keating EM, Faris PM (1994) Radiolucency at the bone-cement interface in total knee replacement: the effects of bone-surface preparation and cement technique. J Bone Joint Surg Am 76(1):60–65
Ryd L, Albrektsson BE, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S (1995) Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 77(3):377–383
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM (2002) Insall award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res 404:7–13
Tai TW, Lin CJ, Jou IM, Chang CW, Lai KA, Yang CY (2011) Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 19(7):1121–1130
Tarwala R, Dorr LD, Gilbert PK, Wan Z, Long WT (2014) Tourniquet use during cementation only during total knee arthroplasty: a randomized trial. Clin Orthop Relat Res 472(1):169–174
Tetro AM, Rudan JF (2001) The effects of a pneumatic tourniquet on blood loss in total knee arthroplasty. Can J Surg 44(1):33–38
Vandenbussche E, Duranthon LD, Couturier M, Pidhorz L, Augereau B (2002) The effect of tourniquet use in total knee arthroplasty. Int Orthop 26(5):306–309
Walker PS, Soudry M, Ewald FC, McVickar H (1984) Control of cement penetration in total knee arthroplasty. Clin Orthop Relat Res 185:155–164
Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE (1997) Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplast 12(8):848–852
Zhang FJ, Xiao Y, Liu YB, Tian X, Gao ZG (2010) Clinical effects of applying a tourniquet in total knee arthroplasty on blood loss. Chin Med J (Engl) 123(21):3030–3033
Zhang W, Li N, Chen S, Tan Y, Al-Aidaros M, Chen L (2014) The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 9(1):13
Zhang Y, Li L, Wang J, Li ZH, Shi ZJ (2013) Do patients benefit from tourniquet in arthroscopic surgeries of the knee? Knee Surg Sports Traumatol Arthrosc 21(5):1125–1130
Conflict of interest
Tilman Pfitzner, Philipp von Roth, Ninja Voerkelius, Hermann Mayr and Carsten Perka declare that they have no conflicts of interest. Robert Hube is a consultant for Zimmer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfitzner, T., von Roth, P., Voerkelius, N. et al. Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24, 96–101 (2016). https://doi.org/10.1007/s00167-014-3341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3341-6