Abstract
Purpose
Graft tensioning during medial patellofemoral ligament (MPFL) reconstruction typically allows for lateral patellar translation within the trochlear groove. Computational simulation was performed to relate the allowed patellar translation to patellofemoral kinematics and contact pressures.
Methods
Multibody dynamic simulation models were developed to represent nine knees with patellar instability. Dual limb squatting was simulated representing the pre-operative condition and simulated MPFL reconstruction. The graft was tensioned to allow 10, 5, and 0 mm of patellar lateral translation at 30° of knee flexion. The patellofemoral contact pressure distribution was quantified using discrete element analysis.
Results
For the 5 and 10 mm conditions, patellar lateral shift decreased significantly at 0° and 20°. The 0 mm condition significantly decreased lateral shift for nearly all flexion angles. All graft conditions significantly decreased lateral tilt at 0°, with additional significant decreases for the 5 and 0 mm conditions. The 0 mm condition significantly increased the maximum medial pressure at multiple flexion angles, increasing by 57% at 30°, but did not alter the maximum lateral pressure.
Conclusions
Allowing 5 to 10 mm of patellar lateral translation limits lateral maltracking, thereby decreasing the risk of post-operative recurrent instability. Allowing no patellar translation during graft tensioning reduces maltracking further, but can overconstrain the patella, increasing the pressure applied to medial patellar cartilage already fibrillated or eroded from an instability episode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medial patellofemoral ligament (MPFL) reconstruction is performed to improve patellar stability in patients with recurrent instability related to lateral maltracking or trauma. Intra-operatively, fixation points on the femur and patella are chosen based on an anatomometric criteria to approximate the native MPFL attachment and minimize variations in graft force as the knee flexes. The resting length of the graft is also set intra-operatively, typically allowing some lateral translation of the patella within the trochlear groove before the graft is loaded in tension to avoid overconstraining the patella. Allowing one quadrant of lateral translation while tensioning the graft with the knee at 30° is common [6, 30], although techniques have also been described that do not quantify lateral translation or allow less translation [21, 22, 33].
Biomechanical studies performed with cadavers and computational models have generally shown that MPFL reconstruction limits lateral patellar maltracking [12, 25, 27, 37]. Elevated levels of graft tension due to initial over tensioning or non-anatomic femoral fixation have been shown to overconstrain the patella and increase pressure applied to cartilage on the medial facet of the patella [1, 11, 12, 27, 38, 39]. With the exception of one study [12], the previous studies were performed with knees that did not include pathology causing patellar instability. None of the previous studies focused on the intra-operative procedure of setting an allowable patellar lateral translation during graft tensioning. Therefore, the influence of the patellar translation allowed during MPFL graft tensioning on post-operative patellofemoral function is currently unknown.
The current study was performed to characterize how the lateral patellar translation allowed while tensioning an MPFL graft influences patellar kinematics and contact pressures. Quantifying patellar kinematics and contact pressures allows assessment of patellar maltracking that could lead to instability and pressure levels that could lead to cartilage degradation. The study is based on dynamic simulation of knee squatting for knees with recurrent patellar instability, and includes a separate accuracy assessment for pre-operative and post-operative simulated kinematics.
Materials and methods
Nine computational models were constructed to represent subjects with recurrent patellar instability. The subjects previously participated in studies focused on computational reconstruction of in vivo knee motion [4, 10, 14]. Each treated subject had a history of symptomatic recurrent lateral patellar dislocation episodes related to trauma or maltracking with unsuccessful conservative treatment. One subject had a previous medial imbrication and lateral release, but was included based on the assumption that the medial imbrication failed with continued instability. No other subject had previous surgical treatment for patellar instability. The subjects included 7 females. The average age was 16 years (range: 12 to 19 years). The subjects were treated with MPFL reconstruction (3 subjects), tibial tuberosity anteromedialization (4 subjects), MPFL reconstruction combined with tibial tuberosity anteromedialization (1 subject), and MPFL reconstruction combined with tibial tuberosity anteromedialization and distalization (1 subject). Lateral retinacular release was performed in combination with the other procedures for four subjects. Based on the models developed from MRI scans, the average tibial tuberosity-to-trochlear groove (TT–TG) distance and lateral trochlear inclination were 12 mm (range 9 to 18 mm) and 13° (range 0° to 26°), respectively. With the knees flexed to approximately 30°, the average Insall–Salvati index for the subjects was 1.3 (range 1.0 to 1.6).
Computational reconstruction of knee motion for accuracy assessment
Pre-operative and post-operative patellofemoral kinematics were characterized for each knee. For two subjects, knee kinematics were characterized based on dynamic CT imaging (Aquilion ONE scanner, Toshiba Medical Systems) as subjects extended their knee against gravity [10, 14]. For the other seven subjects, knee kinematics were characterized based on MRI scans (Magnetom Skyra, Siemens) acquired with the knee loaded and positioned at multiple positions of knee flexion [4]. The flexed and loaded scans were performed with a load frame applying a patient-selected force of 60–85 N to the foot along the axis of the scanner.
A computational model of each knee was reconstructed (3D Doctor, Able Software Corp and Mimics, Materialise) from an MRI scan of the extended and unloaded knee (3.0 T, proton density weighted, slice thickness ranging from 0.6 to 1.5 mm). Models of the femur, tibia, and patella were also reconstructed for 3 to 5 positions spanning the flexion range from the CT or MRI scans acquired during knee function. A single model of the femur, patella, and tibia from the unloaded MRI scan was transferred to all pre-operative and post-operative positions using an iterative closest point algorithm [3]. A local coordinate system was created for the femur based on the transepicondylar axis and long axis, with similar coordinate systems created for the patella and tibia [10, 12], allowing characterization of knee kinematics based on the floating axis convention [18].
Simulation conditions
The technique for computational simulation of knee motion has previously been shown to produce patellar tracking patterns representative of motions recorded from subjects with patellar instability, with root-mean-square errors for patellar lateral shift and tilt of 3.3 mm and 5.8°, respectively [12]. The models were further assessed to determine the accuracy of simulated changes in patellar kinematics due to patellar stabilization. Attachment points for each MPFL graft were identified on the patella and femur based on post-operative imaging. The graft tensioning process, in terms of the knee flexion angle and allowed patellar lateral translation, was individualized for each subject based on consultation with the surgeon. For subjects treated with tibial tuberosity osteotomy and realignment, the post-operative orientation of the patellar tendon was determined from post-operative imaging. For subjects treated with lateral retinacular release, the lateral retinaculum was not represented for the post-operative simulation.
With the kinematic accuracy for each model established, the influence of the MPFL graft tensioning technique on patellofemoral mechanics was assessed by applying loading and boundary conditions to represent a dual limb knee squat. The motion was simulated in a pre-operative condition, and for three graft tensioning techniques. Graft tension was set with the knee flexed to 30°. One patellar quadrant of lateral translation [6, 30] was represented by allowing the patella to displace 10 mm laterally from the deepest point of the trochlear groove with no tension in the graft (range of patellar width: 36 to 43.5 mm). Grafts tensioned to allow 5 mm (0.5 quadrants) and 0 mm of lateral translation were also simulated to represent more restrictive surgical approaches.
Simulation of knee motion and patellofemoral contact
Multibody dynamic simulation of motion for knees with patellar instability has been described in detail previously [12]. Simplified Hertzian contact governs reaction forces developed at the patellofemoral and tibiofemoral joints [19, 28]. Ligaments, tendons, joint capsule, and retinacular structures are represented by tension-only springs, with stiffness, damping, and pre-strain at full extension assigned based on the previous studies [2, 5, 8, 32, 36] (Fig. 1). Forces are applied to represent the quadriceps and hamstring muscles. Patellofemoral and tibiofemoral kinematics are quantified based on the same coordinate axes used for computational reconstruction of in vivo function for each knee. Force within an MPFL graft is determined from the springs representing the graft. Patellofemoral contact pressures are quantified at knee flexion angles ≥ 15°, when the patella is within the trochlear groove, using a previously validated discrete element analysis approach [13] (Fig. 1). Discrete element analysis relates overlap of cartilage surfaces to contact forces and pressure [5], with the position of the patella iteratively adjusted from the position determined with multibody dynamic simulation to balance articular contact forces and moments with the forces and moments applied to the patella.
The modeling technique was further developed for the current study. All models were developed based on reconstruction of structures from 3.0 T MRI scans, as opposed to the lower resolution MRI scans used previously. Anatomical structures reconstructed from the high-resolution MRI scans determined the shape of the cartilage surfaces, orientation and attachment points for the quadriceps and hamstrings muscles, and attachment points for the anterior and posterior cruciate ligaments and patellar tendon. Another update was representation of the residual medial retinaculum, considering rupture of the MPFL due to recurrent dislocation, with a spring with stiffness equal to 2 N/mm [8]. The contact pressure distribution was updated to balance the total articular compression force, medial/lateral force, and lateral tilt moment applied to the patella, as determined from discrete element analysis, with the corresponding patellofemoral contact forces and moments determined from multibody dynamic simulation.
The models were developed to represent the motion of each subject (pre-operative and post-operative accuracy assessment) and the simulated knee squat (comparing graft tensioning techniques). Knee extension within the dynamic CT scanner (2 subjects) was simulated with the femur fixed in space and mass added to the tibia to represent the moment of the lower limb about the flexion axis [12]. The total quadriceps force was set to initiate and maintain extension from maximum flexion in the scanner (approximately 50°) to 0° for each knee, with the total force decreasing as the knee extended. For simulation of isometric knee extension (7 subjects), models were set at each flexion angle from the MRI scans, with the femur fixed in place and a force applied proximally at the foot to match the force applied to each subject. The quadriceps force that maintained the flexion angle was applied. For simulation of a dual limb knee squat with all models (Fig. 1), an ankle joint was represented with 3 rotational degrees of freedom and a hip joint was represented allowing flexion/extension, varus/valgus rotation, and proximal/distal translation. A 200 N body weight was applied at the hip. The total quadriceps force increased from 42 N at full extension to 300 N at 50° of flexion. The distance from the femoral to patellar attachment of an MPFL graft typically decreases at deeper flexion angles [35, 42], unloading the graft. An initial hip flexion moment was applied over the first few degrees of flexion to initiate motion.
MPFL reconstruction was simulated with two springs representing a dual strand gracilis tendon graft with a total stiffness of 20 N/mm [31]. For representation of standard MPFL reconstruction (not patient-specific based on post-operative imaging), the graft was attached at the Schöttle point on the femur [34] and between the medial edge of the VMO attachment and the medial edge of the patella [20, 40]. The graft wrapped around the medial femoral condyle, with the portion from femoral attachment to the wrapping surface rigid.
The study was approved by the IRB’s of the Johns Hopkins Medical Institutions (ID #NA00022624) and Akron Children’s Hospital (ID #110908), where the subjects were treated.
Statistical analysis
Assessment of the simulated kinematics focused on patellar lateral shift and tilt, as the parameters most relevant to patellar instability. For the accuracy assessment, each data point for pre-operative patellar lateral shift and tilt from reconstruction of in vivo motion was compared to the corresponding simulation data from the same knee at the same flexion angle, and root-mean-square errors were quantified. The change in kinematics from the pre-operative condition to the post-operative condition at the closest flexion angle was also compared between data from the subjects and simulations, with average differences and root-mean-square errors quantified.
For simulation of knee squatting with multiple MPFL conditions, patellar lateral shift and tilt, graft force, and maximum pressure applied to the medial and lateral facets of the patella were compared between the pre-operative and MPFL reconstruction conditions at every 5° of knee flexion with nonparametric repeated-measures Friedman tests. Nonparametric analyses were used due to Shapiro–Wilk tests indicating that residuals from repeated-measures comparisons were not normally distributed. Post hoc comparisons were performed with a nonparametric Dunnett’s comparison against a control, using the pre-operative condition as the control for all data but the graft force, which used the 10 mm condition as the control. Because computational precision allows quantification and ranking of small differences between the conditions for statistical analysis, a conservative level of p < 0.01 was set for significance. With 9 models, the study was designed for a statistical power of 0.9 based on an estimated effect size of 1.8 [16] for repeated-measures changes in patellar tracking and contact pressure related to elevated MPFL graft tension [12].
Results
For pre-operative motion, root-mean-square errors comparing simulated to subject-derived kinematics were 3.7° and 2.7 mm for patellar tilt and shift, respectively. For differences between pre-operative and post-operative motion, root-mean-square errors were 4.8° and 2.9 mm for patellar tilt and shift, respectively. The average (± standard deviation) kinematics changes from pre-operative to post-operative motion were a 2.5° ± 4.1° decrease in tilt and a 1.4 ± 3.8 mm decrease in shift for the subject-derived kinematics, compared to a 2.3° ± 5.9° decrease in tilt and a 2.4 ± 4.8 mm decrease in shift for the simulated motions.
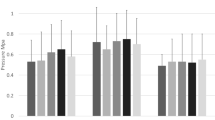
For simulated knee squatting, the graft force increased and patellar lateral shift and tilt decreased as the amount of lateral translation allowed during graft tensioning decreased from 10 to 0 mm. The graft tension tended to be largest at low flexion angles (Fig. 2). For the 10 and 5 mm conditions, the graft force was approximately 0 N at 50° of flexion. For the 0 mm condition, the graft force increased significantly at 20°, 30°, 35°, and 40°. For the 10 and 5 mm conditions, the graft only significantly decreased patellar lateral shift at 0° and 20° of flexion (Fig. 3). The 0 mm condition significantly decreased lateral shift for all flexion angles except 15° and 25°. The trends were similar for patellar tilt, although the change in patellar tilt for the 0 mm condition was only significant at 0°, 5°, and 15° (Fig. 4).
Average (± standard deviation) patellar lateral shift for the pre-operative condition and MPFL graft conditions allowing 10, 5, and 0 mm of lateral patellar translation during graft fixation. Open symbols represent data points that are significantly (p < 0.01) different from the pre-operative condition
Average (± standard deviation) patellar lateral tilt for the pre-operative condition and MPFL graft conditions allowing 10, 5, and 0 mm of lateral patellar translation during graft fixation. Open symbols represent data points that are significantly (p < 0.01) different from the pre-operative condition
For the simulated pressure distribution, the graft only influenced the maximum pressure applied to medial cartilage. The maximum lateral pressure tended to exceed the maximum medial pressure and was not significantly influenced by any of the graft conditions (Fig. 5). The 0 mm condition significantly increased the maximum medial pressure from 30° to 40° (Fig. 6).
Average (± standard deviation) maximum medial pressure for the pre-operative condition and MPFL graft conditions allowing 10, 5, and 0 mm of lateral patellar translation during graft fixation. Open symbols represent data points that are significantly (p < 0.01) different from the pre-operative condition
Discussion
The most important finding of the present study was that allowing up to one quadrant of patellar lateral translation while tensioning an MPFL graft limits lateral maltracking without overconstraining the patella. For simulated knee squatting, allowing one quadrant of lateral translation while setting the graft tension at 30° limited lateral patellar tracking. Reducing the allowable patellar translation during graft tensioning by 50% further decreased lateral tracking. Allowing no lateral translation decreased lateral tracking even further, with significant changes compared to the pre-operative condition at nearly all flexion angles, but also increased the maximum pressure applied to medial cartilage. Although the maximum medial pressure still tended to be lower than the maximum lateral pressure, the increase is a concern due to medial cartilage fibrillation and erosion caused by contact between the medial facet of the patella and the lateral condyle of the femur with recurrent instability [24, 29]. Previous computational simulation [11, 12] and in vitro experimental studies [1, 27, 38, 39] have shown that elevated levels of graft tension can overconstrain the patella and increase cartilage pressures. The previous studies focused on malpositioning of the graft attachment on the femur or magnitude of applied graft tension, as opposed to the current approach simulating variations in the intra-operative graft tensioning strategy with knees being treated for recurrent instability.
Loads carried by the MPFL graft and the resulting influence on patellar kinematics depend on graft isometry for a normally tracking patella, the level of lateral maltracking, and varying anatomical constraints as the patella enters the trochlear groove. Patellar lateral tracking for the knee squatting motion was typically largest with the knee in full extension, and decreased as the patella entered the trochlear groove, as noted when characterizing patellar tracking based on reconstruction of in vivo motion [4, 10]. Therefore, graft forces and the influence on patellar kinematics tend to be largest at low flexion angles. In addition, for a normally tracking patella, the previous studies have indicated that the length of the MPFL tends to decrease beyond 30° to 50° of flexion [35, 42], also contributing to the decrease in graft force with increasing flexion noted for the current study. When a graft helps the trochlear groove capture the patella, increased articular constraints can influence patellar tracking, as indicated by lower average lateral patellar shift and tilt values for the 5 and 10 mm graft conditions than for the pre-operative condition at deeper flexion angles with the grafts unloaded. For the 0 mm graft condition, the grafts were in tension from 30° to 50° of flexion due to the patella tracking more laterally during squatting than for the unloaded condition for which the graft length was set.
The current study further established the multibody dynamic simulation technique for producing patellar tracking patterns typical of patients with patellar instability. With the improved representation of knee anatomy based on high-resolution imaging, the accuracy assessment of pre-operative motion provided root-mean-square errors of 2.7 mm and 3.7° for patellar shift and tilt, respectively, which is an improvement compared to the previous values of 3.3 mm and 5.8° for patellar lateral shift and tilt [12]. Simulated changes in kinematics due to surgical stabilization were assessed for accuracy for the first time. The simulations replicated the subject-derived trends for decreases in patellar lateral shift and tilt with root-mean-square errors of 2.9 mm and 4.8°, respectively. The discrete element analysis technique, which has been previously validated against in vitro experimental measurements of the patellofemoral pressure distribution [13], was updated to more closely align the pressure distribution with the patellofemoral reaction forces and moments obtained from multibody dynamic simulation. The simulation technique was also advanced to represent knee squatting, which is more representative of in vivo function than the previous unresisted knee extension.
Several anatomical factors can contribute to patellar instability, resulting in diverse patellar tracking patterns for unstable knees. The predominate anatomical contributors to recurrent patellar instability are trochlear dysplasia, a lateralized tibial tuberosity, and patella alta [7]. Published outcomes studies for MPFL reconstruction typically include a limited range of these pathological conditions [43]. Trochlear dysplasia and a lateralized tibial tuberosity have been specifically correlated with lateral patellar tracking based on computational reconstruction of in vivo motion [4, 14]. Multiple tracking patterns have been identified for recurrent instability, ranging from the patella tracking along the center of the trochlear groove to the patella maltracking laterally throughout motion [41]. Instability can occur due to consistent lateral maltracking or primarily with traumatic episodes. The nine models used for the current study also vary in pathologic anatomy and tracking patterns. The ranges for the Insall–Salvati index, TT–TG distance, and lateral trochlear inclination show the variation in pathologic anatomy. The tracking variations between knees are demonstrated by residuals from repeated-measures comparisons that were not normally distributed, leading to use of a conservative p value of 0.01 to avoid overstating significant differences between conditions of MPFL reconstruction. While the aggregated data are relevant to the influence of graft tensioning approaches on patellar mechanics, future studies should more specifically relate the anatomical and tracking patterns of individual knees to the variations caused by MPFL reconstruction to help surgeons optimize MPFL reconstruction techniques for individual patients. In addition, techniques to simulate more traumatic loading conditions need to be developed to evaluate MPFL reconstruction for knees with nearly normal tracking patterns during simulated squatting.
Limitations of the modeling technique should be noted. While high-resolution imaging of the knees with instability improved representation of knee anatomy, many properties of the knees are still generalized based on previously published data, rather than individualized for the subjects. Generalized properties include elastic properties and resting lengths for springs representing ligaments, tendons and retinacular structures, and applied muscle forces. The assumed properties emphasize the importance of the updated accuracy assessment. Graft attachments and properties do not account for graft elongation or tunnel widening [21] that can occur post-operatively. The data also specifically apply to tensioning a hamstrings tendon graft at 30° of knee flexion, while some studies described tensioning at higher or lower flexion angles [9, 15, 26, 44] and other graft options, such as the quadriceps tendon [17, 23], are available.
Conclusion
The current computational simulation of dynamic knee squatting indicates that tensioning a MPFL graft while allowing 0.5 to 1 patellar quadrant of lateral translation at 30° generally improves post-operative knee function. The approach limits lateral maltracking without overconstraining the patella. Limiting lateral maltracking can reduce the risk of patellar instability following MPFL reconstruction. Allowing no patellar lateral translation while tensioning the graft more dramatically limits lateral maltracking, but can also overconstrain the patella, including increasing the pressure applied to medial patellofemoral cartilage. If the medial cartilage is fibrillated or eroded due to the previous instability episodes, the increased pressure could lead to patellofemoral pain. Additional investigations are needed to evaluate options for MPFL reconstruction as a function of anatomy and pre-operative tracking of individual knees.
References
Beck P, Brown NA, Greis PE, Burks RT (2007) Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med 35:1557–1563
Besier TF, Gold GE, Delp SL, Fredericson M, Beaupré GS (2008) The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J Orthop Res 26:1627–1635
Besl PJ, McKay HD (1992) A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intel 14:239–256
Biyani R, Elias JJ, Saranathan A, Feng H, Guseila LM, Morscher MA, Jones KC (2014) Anatomical factors influencing patellar tracking in the unstable patellofemoral joint. Knee Surg Sports Traumatol Arthrosc 22:2334–2341
Blankevoort L, Kuiper JH, Huiskes R, Grootenboer HJ (1991) Articular contact in a three-dimensional model of the knee. J Biomech 24:1019–1031
Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ (2010) Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med 38:2248–2254
Christensen TC, Sanders TL, Pareek A, Mahan R, Dahm DL, Krych AJ (2017) Risk factors and time to recurrent ipsilateral and contralateral patellar dislocations. Am J Sports Med. https://doi.org/10.1177/0363546517704178
Conlan T, Garth WP Jr, Lemons JE (1993) Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am 75:682–693
Damasena I, Blythe M, Wysocki D, Kelly D, Annear P (2017) Medial patellofemoral ligament reconstruction combined with distal realignment for recurrent dislocations of the patella. Am J Sports Med 45:369–376
Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ (2014) Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc 22:2350–2356
Elias JJ, Cosgarea AJ (2006) Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med 34:1478–1485
Elias JJ, Kelly MJ, Smith KE, Gall KA, Farr J (2016) Dynamic simulation of the effects of graft fixation errors during medial patellofemoral ligament reconstruction. Orthop J Sports Med 4:2325967116665080
Elias JJ, Saranathan A (2013) Discrete element analysis for characterizing the patellofemoral pressure distribution: model evaluation. J Biomech Eng 135:81011
Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ (2016) Dynamic tracking influenced by anatomy in patellar instability. Knee 23:450–455
Fabricant PD, Ladenhauf HN, Salvati EA, Green DW (2014) Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee 21:1180–1184
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Goyal D (2015) The “superficial quad technique” for medial patellofemoral ligament reconstruction: The surgical video Technique. Arthrosc Tech 4:e569-75
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105:136–144
Guess TM, Liu H, Bhashyam S, Thiagarajan G (2013) A multibody knee model with discrete cartilage prediction of tibio-femoral contact mechanics. Comput Methods Biomech Biomed Eng 16:256–270
Kita K, Horibe S, Toritsuka Y, Nakamura N, Tanaka Y, Yonetani Y, Mae T, Nakata K, Yoshikawa H, Shino K (2012) Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc 20:829–837
Kita K, Tanaka Y, Toritsuka Y, Amano H, Uchida R, Shiozaki Y, Takao R, Horibe S (2017) 3D computed tomography evaluation of morphological changes in the femoral tunnel after medial patellofemoral ligament reconstruction with hamstring tendon graft for recurrent patellar dislocation. Am J Sports Med 45:1599–1607
Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M (2014) Reconstruction of the medial patellofemoral ligament: clinical outcomes and return to sports. Am J Sports Med 42:1661–1668
Nelitz M, Dreyhaupt J, Williams SRM (2017) Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents using a pedicled quadriceps tendon graft shows favourable results at a minimum of 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4597-4
Nomura E, Inoue M (2004) Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med 32:498–502
Ostermeier S, Holst M, Bohnsack M, Hurschler C, Stukenborg-Colsman C, Wirth CJ (2007) In vitro measurement of patellar kinematics following reconstruction of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc 15:276–285
Parikh SN, Nathan ST, Wall EJ, Eismann EA (2013) Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med 41:1030–1038
Philippot R, Boyer B, Testa R, Farizon F, Moyen B (2012) Study of patellar kinematics after reconstruction of the medial patellofemoral ligament. Clin Biomech 27:22–26
Purevsuren T, Elias JJ, Kim K, Kim YH (2015) Dynamic simulation of tibial tuberosity realignment: model evaluation. Comput Methods Biomech Biomed Engin 18:1606–1610
Salonen EE, Magga T, Sillanpää PJ, Kiekara T, Mäenpää H, Mattila VM (2017) Traumatic patellar dislocation and cartilage injury: A follow-up study of long-term cartilage deterioration. Am J Sports Med 45:1376–1382
Sampatacos NE, Getelman MH (2013) Medial patellofemoral ligament reconstruction using a modified “reverse-loop” technique. Arthrosc Tech 2:e175–e181
Saper MG, Meijer K, Winnier S, Popovich J Jr, Andrews JR, Roth C (2017) Biomechanical evaluation of classic solid and all-soft suture anchors for medial patellofemoral ligament reconstruction. Am J Sports Med 45:1622–1626
Shin CS, Chaudhari AM, Andriacchi TP (2007) The influence of deceleration forces on ACL strain during single-leg landing: a simulation study. J Biomech 40:1145–1152
Schiphouwer L, Rood A, Tigchelaar S, Koëter S (2017) Complications of medial patellofemoral ligament reconstruction using two transverse patellar tunnels. Knee Surg Sports Traumatol Arthrosc 25:245–250
Schöttle PB, Schmeling A, Rosenstiel N, Weiler A (2007) Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med 35:801–804
Smirk C, Morris H (2003) The anatomy and reconstruction of the medial patellofemoral ligament. Knee 10:221–227
Stäubli HU, Schatzmann L, Brunner P, Rincón L, Nolte LP (1996) Quadriceps tendon and patellar ligament: cryosectional anatomy and structural properties in young adults. Knee Surg Sports Traumatol Arthrosc 4:100–110
Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA (2015) The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med 43:2198–2207
Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA (2014) The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med 42:364–372
Stephen JM, Kittl C, Williams A, Zaffagnini S, Marcheggiani Muccioli GM, Fink C, Amis AA (2016) Effect of medial patellofemoral ligament reconstruction method on patellofemoral contact pressures and kinematics. Am J Sports Med 44:1186–1194
Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ (2012) Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am 94:e87
Tanaka MJ, Elias JJ, Williams AA, Demehri S, Cosgarea AJ (2016) Characterization of patellar maltracking using dynamic kinematic CT imaging in patients with patellar instability. Knee Surg Sports Traumatol Arthrosc 24:3634–3641
Tischer T, Geier A, Lenz R, Woernle C, Bader R (2016) Impact of the patella height on the strain pattern of the medial patellofemoral ligament after reconstruction: a computer model-based study. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-016-4190-2
Tompkins MA, Arendt EA (2015) Patellar instability factors in isolated medial patellofemoral ligament reconstructions—what does the literature tell us? A systematic review. Am J Sports Med 43:2318–2327
Zhao J, Huangfu X, He Y (2012) The role of medial retinaculum plication versus medial patellofemoral ligament reconstruction in combined procedures for recurrent patellar instability in adults. Am J Sports Med 40:1355–1364
Acknowledgements
Research reported in this publication was supported by the National Institute Of Arthritis And Musculoskeletal And Skin Diseases of the National Institutes of Health under Award Number R21AR069150 and a Basic Science Research Grant from the Pediatric Orthopaedic Society of North America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
John Elias has received a research grant from MedShape and is the PI of the grant from the NIH. Andrew Cosgarea has been a committee member for the American Orthopaedic Society for Sports Medicine and the Patellofemoral Foundation, has received grant funding from the Arthroscopy Association of North America, and textbook royalties from Elsevier. Kerwyn Jones is the PI of the grant from the Pediatric Orthopaedic Society of North America. Joseph Gabra, Molly Lalonde, and Cyrus Rezvanifar report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Elias, J.J., Jones, K.C., Lalonde, M.K. et al. Allowing one quadrant of patellar lateral translation during medial patellofemoral ligament reconstruction successfully limits maltracking without overconstraining the patella. Knee Surg Sports Traumatol Arthrosc 26, 2883–2890 (2018). https://doi.org/10.1007/s00167-017-4799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4799-9